An Analysis of the Correlation between the Asymmetry of Different EEG-Sensor Locations in Diverse Frequency Bands and Short-Term Subjective Well-Being Changes
Abstract
:1. Introduction
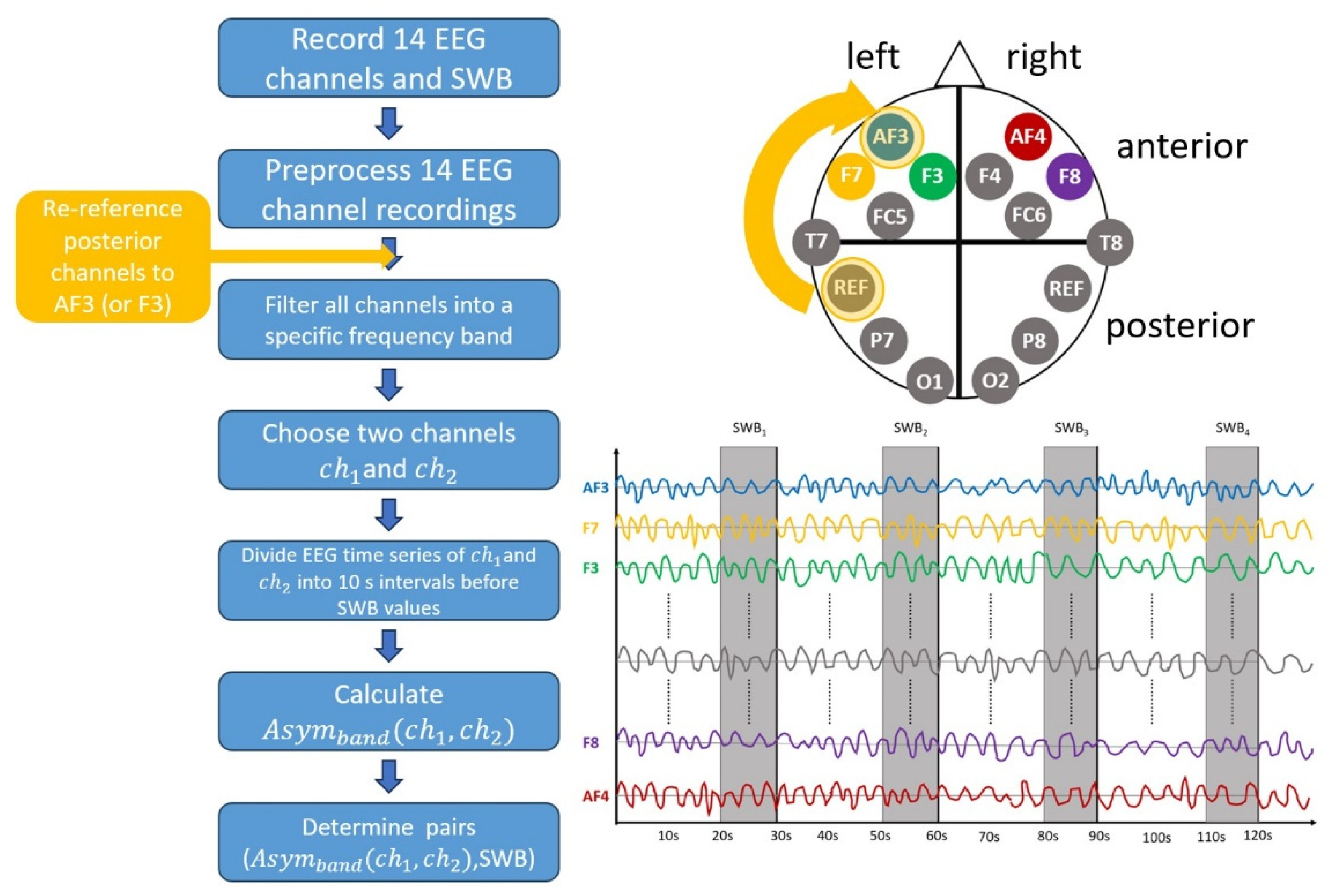
2. Materials and Methods
2.1. Experiment
2.2. Asymmetry Calculation
2.3. Re-Referencing the Data
2.4. Averaging over Different Sensors
2.5. Correlation between Asym and SWB
2.6. Overall Statistical Analysis
3. Results
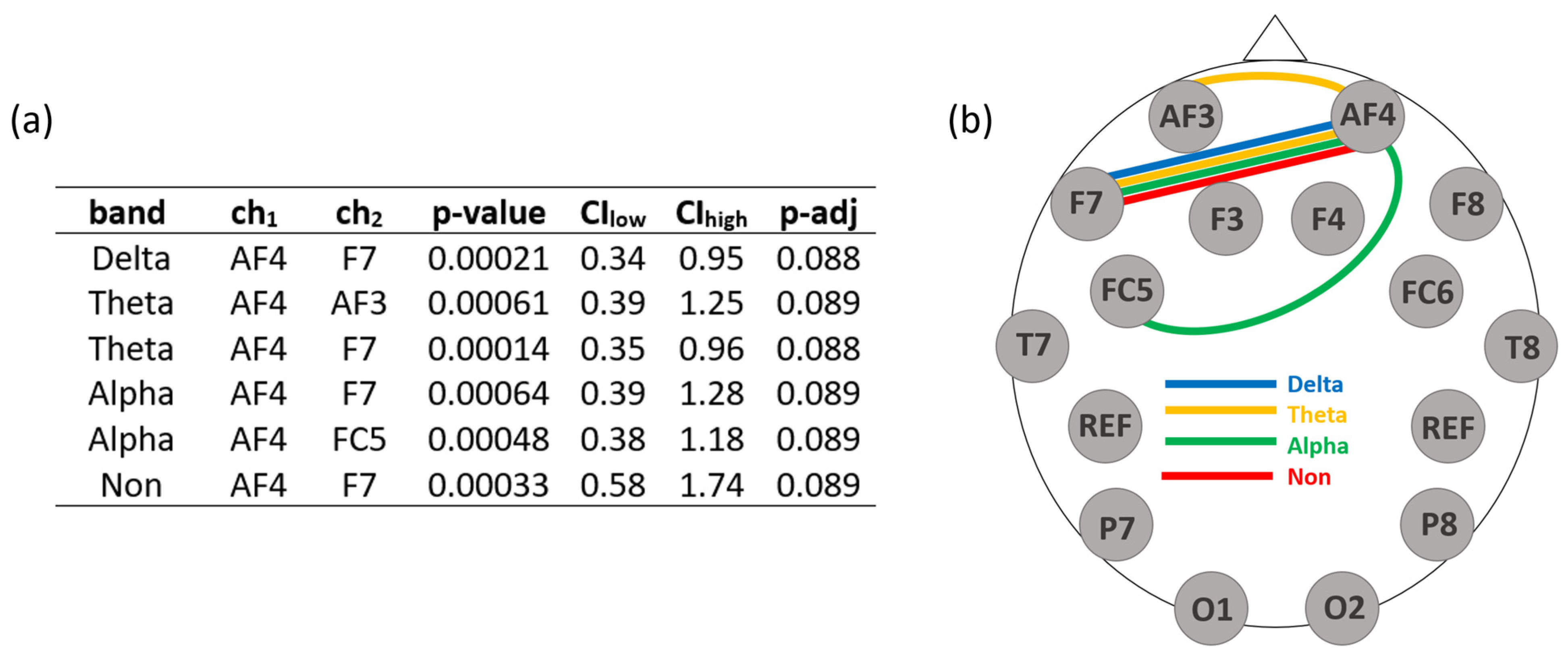
3.1. Results with the Original Reference Electrode
3.2. Results after Re-Referencing the EEG Data
3.3. Results from Signals Averaged over Brain Quadrants
4. Discussion
4.1. Discussion of the Results with the Original Reference Electrode
4.2. Discussion of Results after Re-Referencing the EEG Data
4.3. Discussion of Results from Signals Averaged over Quadrants
4.4. General Discussion
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, H. Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. 2019, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. Motivation, Emotion, and Their Inhibitory Control Mirrored in Brain Oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Fries, P. Beta-Band Oscillations—Signalling the Status Quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Laufs, H.; Holt, J.L.; Elfont, R.; Krams, M.; Paul, J.S.; Krakow, K.; Kleinschmidt, A. Where the BOLD Signal Goes When Alpha EEG Leaves. NeuroImage 2006, 31, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; Vallesi, A. Asymmetry in Prefrontal Resting-State EEG Spectral Power Underlies Individual Differences in Phasic and Sustained Cognitive Control. NeuroImage 2016, 124, 843–857. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G. When and Why Did Brains Break Symmetry? Symmetry 2015, 7, 2181–2194. [Google Scholar] [CrossRef]
- Güntürkün, O.; Ocklenburg, S. Ontogenesis of Lateralization. Neuron 2017, 94, 249–263. [Google Scholar] [CrossRef]
- Corballis, M.C. Bilaterally Symmetrical: To Be or Not to Be? Symmetry 2020, 12, 326. [Google Scholar] [CrossRef]
- Travis, L.E.; Knott, J.R. Bilaterally Recorded Brain Potentials from Normal Speakers and Stutterers. J. Speech Disord. 1937, 2, 239–241. [Google Scholar] [CrossRef]
- Lindsley, D.B. Bilateral Differences in Brain Potentials From the Two Cerebral Hemispheres in Relation to Laterality and Stuttering. J. Exp. Psychol. 1940, 26, 211. [Google Scholar] [CrossRef]
- Tomarken, A.J.; Davidson, R.J.; Wheeler, R.E.; Kinney, L. Psychometric Properties of Resting Anterior EEG Asymmetry: Temporal Stability and Internal Consistency. Psychophysiology 1992, 29, 576–592. [Google Scholar] [CrossRef]
- Debener, S.; Beauducel, A.; Nessler, D.; Brocke, B.; Heilemann, H.; Kayser, J. Is Resting Anterior EEG Alpha Asymmetry a Trait Marker for Depression?: Findings for Healthy Adults and Clinically Depressed Patients. Neuropsychobiology 2000, 41, 31–37. [Google Scholar] [CrossRef]
- Hagemann, D.; Naumann, E.; Thayer, J.F.; Bartussek, D. Does Resting Electroencephalograph Asymmetry Reflect a Trait? An Application of Latent State-Trait Theory. J. Personal. Soc. Psychol. 2002, 82, 619–641. [Google Scholar] [CrossRef]
- Vuga, M.; Fox, N.A.; Cohn, J.F.; George, C.J.; Levenstein, R.M.; Kovacs, M. Long-Term Stability of Frontal Electroencephalographic Asymmetry in Adults with a History of Depression and Controls. Int. J. Psychophysiol. 2006, 59, 107–115. [Google Scholar] [CrossRef]
- Metzen, D.; Genç, E.; Getzmann, S.; Larra, M.F.; Wascher, E.; Ocklenburg, S. Frontal and Parietal EEG Alpha Asymmetry: A Large-Scale Investigation of Short-Term Reliability on Distinct EEG Systems. Brain Struct. Funct. 2022, 227, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Friedrich, P.; Schmitz, J.; Schlüter, C.; Genc, E.; Güntürkün, O.; Peterburs, J.; Grimshaw, G. Beyond Frontal Alpha: Investigating Hemispheric Asymmetries over the EEG Frequency Spectrum as a Function of Sex and Handedness. Laterality 2019, 24, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Reznik, S.J.; Allen, J.J.B. Frontal Asymmetry as a Mediator and Moderator of Emotion: An Updated Review. Psychophysiology 2018, 55, e12965. [Google Scholar] [CrossRef] [PubMed]
- Urry, H.L.; Nitschke, J.B.; Dolski, I.; Jackson, D.C.; Dalton, K.M.; Mueller, C.J.; Rosenkranz, M.A.; Ryff, C.D.; Singer, B.H.; Davidson, R.J. Making a Life Worth Living: Neural Correlates of Well-Being. Psychol. Sci. 2004, 15, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Y.; Feng, Z.-Q.; Xie, Y.-J.; Zhang, J.; Peng, S.-H.; Yu, Y.-J.; Li, M. Frontal Alpha EEG Asymmetry Before and After Positive Psychological Interventions for Medical Students. Front. Psychiatry 2018, 9, 432. [Google Scholar] [CrossRef]
- van der Vinne, N.; Vollebregt, M.A.; van Putten, M.J.A.M.; Arns, M. Frontal Alpha Asymmetry as a Diagnostic Marker in Depression: Fact or Fiction? A Meta-Analysis. NeuroImage Clin. 2017, 16, 79–87. [Google Scholar] [CrossRef]
- Grimshaw, G.M.; Carmel, D. An Asymmetric Inhibition Model of Hemispheric Differences in Emotional Processing. Front. Psychol. 2014, 5, 489. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, S.R.; Fogler, J.; Braverman, Y.; Gilbert, R.; Faja, S. Resting Frontal Alpha Asymmetry as a Predictor of Executive and Affective Functioning in Children with Neurodevelopmental Differences. Front. Psychol. 2023, 13, 1065598. [Google Scholar] [CrossRef]
- Garrison, K.; Schmeichel, B.; Baldwin, C. Meta-Analysis of the Relationship between Frontal EEG Asymmetry and Approach/Avoidance Motivation. PsyArXiv 2024, preprint. [Google Scholar] [CrossRef]
- Barros, C.; Pereira, A.R.; Sampaio, A.; Buján, A.; Pinal, D. Frontal Alpha Asymmetry and Negative Mood: A Cross-Sectional Study in Older and Younger Adults. Symmetry 2022, 14, 1579. [Google Scholar] [CrossRef]
- Davidson, R.J. EEG Measures of Cerebral Asymmetry: Conceptual and Methodological Issues. Int. J. Neurosci. 1988, 39, 71–89. [Google Scholar] [CrossRef]
- Henriques, J.B.; Davidson, R.J. Regional Brain Electrical Asymmetries Discriminate between Previously Depressed and Healthy Control Subjects. J. Abnorm. Psychol. 1990, 99, 22–31. [Google Scholar] [CrossRef]
- Bruder, G.E.; Tenke, C.E.; Warner, V.; Nomura, Y.; Grillon, C.; Hille, J.; Leite, P.; Weissman, M.M. Electroencephalographic Measures of Regional Hemispheric Activity in Offspring at Risk for Depressive Disorders. Biol. Psychiatry 2005, 57, 328–335. [Google Scholar] [CrossRef]
- Bruder, G.E.; Tenke, C.E.; Warner, V.; Weissman, M.M. Grandchildren at High and Low Risk for Depression Differ in EEG Measures of Regional Brain Asymmetry. Biol. Psychiatry 2007, 62, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Metzger, L.J.; Paige, S.R.; Carson, M.A.; Lasko, N.B.; Paulus, L.A.; Pitman, R.K.; Orr, S.P. PTSD Arousal and Depression Symptoms Associated With Increased Right-Sided Parietal EEG Asymmetry. J. Abnorm. Psychol. 2004, 113, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Towers, D.N.; Coan, J.A.; Allen, J.J.B. The Oft-Neglected Role of Parietal EEG Asymmetry and Risk for Major Depressive Disorder. Psychophysiology 2011, 48, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, G.M.; Foster, J.J.; Corballis, P.M. Frontal and Parietal EEG Asymmetries Interact to Predict Attentional Bias to Threat. Brain Cogn. 2014, 90, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.S.; Smalley, S.L.; Walshaw, P.D.; Hanada, G.; Macion, J.; McCracken, J.T.; McGough, J.J.; Loo, S.K. Atypical EEG Beta Asymmetry in Adults with ADHD. Neuropsychologia 2010, 48, 3532–3539. [Google Scholar] [CrossRef] [PubMed]
- Hale, T.S.; Kane, A.M.; Tung, K.L.; Kaminsky, O.; McGough, J.J.; Hanada, G.; Loo, S.K. Abnormal Parietal Brain Function in ADHD: Replication and Extension of Previous EEG Beta Asymmetry Findings. Front. Psychiatry 2014, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Hofman, D.; Schutter, D.J.L.G. Asymmetrical Frontal Resting-State Beta Oscillations Predict Trait Aggressive Tendencies and Behavioral Inhibition. Soc. Cogn. Affect. Neurosci. 2012, 7, 850–857. [Google Scholar] [CrossRef] [PubMed]
- van Bochove, M.E.; Ketel, E.; Wischnewski, M.; Wegman, J.; Aarts, E.; de Jonge, B.; Medendorp, W.P.; Schutter, D.J.L.G. Posterior Resting State EEG Asymmetries Are Associated with Hedonic Valuation of Food. Int. J. Psychophysiol. 2016, 110, 40–46. [Google Scholar] [CrossRef]
- Park, D.-H.; Shin, C.-J. Asymmetrical Electroencephalographic Change of Human Brain During Sleep Onset Period. Psychiatry Investig. 2017, 14, 839–843. [Google Scholar] [CrossRef]
- Cannard, C.; Wahbeh, H.; Delorme, A. Electroencephalography Correlates of Well-Being Using a Low-Cost Wearable System. Front. Hum. Neurosci. 2021, 15, 745135. [Google Scholar] [CrossRef]
- Lyubomirsky, S.; Lepper, H.S. A Measure of Subjective Happiness: Preliminary Reliability and Construct Validation. Soc. Indic. Res. 1999, 46, 137–155. [Google Scholar] [CrossRef]
- Ryff, C.D. Happiness Is Everything, or Is It? Explorations on the Meaning of Psychological Well-Being. J. Personal. Soc. Psychol. 1989, 57, 1069. [Google Scholar] [CrossRef]
- Frisch, M.B.; Cornell, J.; Villanueva, M.; Retzlaff, P.J. Clinical Validation of the Quality of Life Inventory. A Measure of Life Satisfaction for Use in Treatment Planning and Outcome Assessment. Psychol. Assess. 1992, 4, 92–101. [Google Scholar] [CrossRef]
- Pinto, S.; Fumincelli, L.; Mazzo, A.; Caldeira, S.; Martins, J.C. Comfort, Well-Being and Quality of Life: Discussion of the Differences and Similarities among the Concepts. Porto Biomed. J. 2017, 2, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Dolcos, S.; Moore, M.; Katsumi, Y. Neuroscience and Well-Being. In Handbook of Well-Being; DEF Publishers: Salt Lake City, UT, USA, 2018. [Google Scholar]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A Systematic Review of the Literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Likert, R. A Technique for the Measurement of Attitudes. Arch. Psychol. 1932, 22, 55. [Google Scholar]
- Wutzl, B.; Leibnitz, K.; Kominami, D.; Ohsita, Y.; Kaihotsu, M.; Murata, M. Analysis of the Correlation between Frontal Alpha Asymmetry of Electroencephalography and Short-Term Subjective Well-Being Changes. Sensors 2023, 23, 7006. [Google Scholar] [CrossRef]
- Peper, F.; Leibnitz, K.; Tanaka, C.; Honda, K.; Hasegawa, M.; Theofilis, K.; Li, A.; Wakamiya, N. High-Density Resource-Restricted Pulse-Based IoT Networks. IEEE Trans. Green Commun. Netw. 2021, 5, 1856–1868. [Google Scholar] [CrossRef]
- MATLAB; Version: 9.12.0.1884302 (R2022a); The MathWorks Inc.: Natick, MA, USA, 2022.
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Gabard-Durnam, L.J.; Mendez Leal, A.S.; Wilkinson, C.L.; Levin, A.R. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized Processing Software for Developmental and High-Artifact Data. Front. Neurosci. 2018, 12, 97. [Google Scholar] [CrossRef]
- Winkler, I.; Haufe, S.; Tangermann, M. Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behav. Brain Funct. 2011, 7, 30. [Google Scholar] [CrossRef]
- Winkler, I.; Brandl, S.; Horn, F.; Waldburger, E.; Allefeld, C.; Tangermann, M. Robust Artifactual Independent Component Classification for BCI Practitioners. J. Neural Eng. 2014, 11, 035013. [Google Scholar] [CrossRef]
- Tesař, M. Frontal Alpha Asymmetry Toolbox. Available online: https://github.com/michtesar/asymmetry_toolbox (accessed on 8 June 2023).
- Hall, E.E.; Ekkekakis, P.; Petruzzello, S.J. Predicting Affective Responses to Exercise Using Resting EEG Frontal Asymmetry: Does Intensity Matter? Biol. Psychol. 2010, 83, 201–206. [Google Scholar] [CrossRef]
- Smith, E.E.; Reznik, S.J.; Stewart, J.L.; Allen, J.J.B. Assessing and Conceptualizing Frontal EEG Asymmetry: An Updated Primer on Recording, Processing, Analyzing, and Interpreting Frontal Alpha Asymmetry. Int. J. Psychophysiol. 2017, 111, 98–114. [Google Scholar] [CrossRef]
- Reid, S.A.; Duke, L.M.; Allen, J.J. Resting Frontal Electroencephalographic Asymmetry in Depression: Inconsistencies Suggest the Need to Identify Mediating Factors. Psychophysiology 1998, 35, 389–404. [Google Scholar] [CrossRef]
- Hagemann, D.; Naumann, E.; Thayer, J.F. The Quest for the EEG Reference Revisited: A Glance from Brain Asymmetry Research. Psychophysiology 2001, 38, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.L.; Bismark, A.W.; Towers, D.N.; Coan, J.A.; Allen, J.J.B. Resting Frontal EEG Asymmetry as an Endophenotype for Depression Risk: Sex-Specific Patterns of Frontal Brain Asymmetry. J. Abnorm. Psychol. 2010, 119, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Velo, J.R.; Stewart, J.L.; Hasler, B.P.; Towers, D.N.; Allen, J.J.B. Should It Matter When We Record? Time of Year and Time of Day as Factors Influencing Frontal EEG Asymmetry. Biol. Psychol. 2012, 91, 283–291. [Google Scholar] [CrossRef]
- Stewart, J.L.; Coan, J.A.; Towers, D.N.; Allen, J.J.B. Resting and Task-Elicited Prefrontal EEG Alpha Asymmetry in Depression: Support for the Capability Model. Psychophysiology 2014, 51, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Lemaître, G.; Nogueira, F.; Aridas, C.K. Imbalanced-Learn: A Python Toolbox to Tackle the Curse of Imbalanced Datasets in Machine Learning. J. Mach. Learn. Res. 2017, 18, 559–563. [Google Scholar]
- Python; 3.9.18; Python Software Foundation: Wilmington, DE, USA, 2022.
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Zakzouk, A.; Menzel, K. Brain-Computer-Interface (BCI) Based Smart Home Control Using EEG Mental Commands. In Working Conference on Virtual Enterprises, Proceedings of the 24th IFIP WG 5.5 Working Conference on Virtual Enterprises, PRO-VE 2023, Valencia, Spain, 27–29 September 2023; Springer: Cham, Switzerland, 2023; pp. 720–732. ISBN 978-3-031-42621-6. [Google Scholar]
- Kim, B.-H.; Seo, S.-H. Development of Smart Home System Based on EEG. In Machine Learning and Optimization for Engineering Design; Shastri, A.S., Shaw, K., Singh, M., Eds.; Engineering Optimization: Methods and Applications; Springer Nature: Singapore, 2023; pp. 153–164. ISBN 978-981-9974-56-6. [Google Scholar]
- Jackson, P.; Sirgy, M.; Medley, G. Neurobiology of Well-Being. In Research Anthology on Mental Health Stigma, Education, and Treatment; IGI Global: Hershey, PA, USA, 2021; pp. 32–52. ISBN 978-1-79988-599-3. [Google Scholar]
- Bishop, P.A.; Herron, R.L. Use and Misuse of the Likert Item Responses and Other Ordinal Measures. Int. J. Exerc. Sci. 2015, 8, 297–302. [Google Scholar]
| Band | ch1 | ch2 | p-Value | CIlow | CIhigh |
|---|---|---|---|---|---|
| Theta | T8 | P7 | 0.067 | −0.47 | 0.02 |
| Theta | P8 | O1 | 0.063 | −0.72 | 0.02 |
| Beta | O2 | P7 | 0.054 | −0.95 | 0.01 |
| Gamma | O2 | P7 | 0.042 | −1.05 | −0.02 |
| Gamma | T8 | O1 | 0.046 | 0.01 | 1.43 |
| Non | O2 | P7 | 0.031 | −1.25 | −0.07 |
| Non | T8 | O1 | 0.052 | −0.00 | 1.05 |
| Band | ch1 | ch2 | p-Value | CIlow | CIhigh |
|---|---|---|---|---|---|
| Delta | O2 | T7 | 0.048 | −0.55 | −0.00 |
| Theta | P8 | P7 | 0.096 | −0.66 | 0.06 |
| Theta | P8 | O1 | 0.026 | −0.62 | −0.04 |
| Alpha | O2 | P7 | 0.011 | −0.93 | −0.13 |
| Alpha | T8 | P7 | 0.026 | −0.71 | −0.05 |
| Beta | T8 | T7 | 0.094 | −1.08 | 0.09 |
| Beta | O2 | P7 | 0.014 | −1.52 | −0.19 |
| Gamma | O2 | T7 | 0.078 | −0.92 | 0.05 |
| Gamma | O2 | P7 | 0.047 | −1.30 | −0.01 |
| Non | O2 | T7 | 0.056 | −1.10 | 0.02 |
| Non | O2 | P7 | 0.024 | −1.84 | −0.14 |
| Band | ch1 | ch2 | p-Value | CIlow | CIhigh |
|---|---|---|---|---|---|
| Delta | O2 | T7 | 0.033 | −0.54 | −0.02 |
| Delta | O2 | P7 | 0.046 | −0.64 | −0.01 |
| Delta | P8 | P7 | 0.060 | −0.57 | 0.01 |
| Delta | P8 | O1 | 0.079 | −0.52 | 0.03 |
| Theta | P8 | O1 | 0.048 | −0.62 | −0.00 |
| Alpha | O2 | P7 | 0.050 | −0.86 | 0.00 |
| Beta | O2 | P7 | 0.009 | −1.54 | −0.25 |
| Gamma | O2 | T7 | 0.053 | −1.58 | 0.01 |
| Non | O2 | T7 | 0.094 | −1.15 | 0.10 |
| Non | O2 | P7 | 0.023 | −2.12 | −0.17 |
| Band | Area | Re-Ref | p-Value | CIlow | CIhigh |
|---|---|---|---|---|---|
| Theta | anterior | - | 0.016 | 0.15 | 1.33 |
| Alpha | anterior | - | 0.019 | 0.16 | 1.64 |
| Non | anterior | - | 0.058 | −0.05 | 2.51 |
| Beta | posterior | F3 | 0.073 | −1.24 | 0.06 |
| Non | posterior | F3 | 0.074 | −2.26 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wutzl, B.; Leibnitz, K.; Murata, M. An Analysis of the Correlation between the Asymmetry of Different EEG-Sensor Locations in Diverse Frequency Bands and Short-Term Subjective Well-Being Changes. Brain Sci. 2024, 14, 267. https://doi.org/10.3390/brainsci14030267
Wutzl B, Leibnitz K, Murata M. An Analysis of the Correlation between the Asymmetry of Different EEG-Sensor Locations in Diverse Frequency Bands and Short-Term Subjective Well-Being Changes. Brain Sciences. 2024; 14(3):267. https://doi.org/10.3390/brainsci14030267
Chicago/Turabian StyleWutzl, Betty, Kenji Leibnitz, and Masayuki Murata. 2024. "An Analysis of the Correlation between the Asymmetry of Different EEG-Sensor Locations in Diverse Frequency Bands and Short-Term Subjective Well-Being Changes" Brain Sciences 14, no. 3: 267. https://doi.org/10.3390/brainsci14030267






