Differential Cortical and Subcortical Activations during Different Stages of Muscle Control: A Functional Magnetic Resonance Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Collection and Analysis
3. Results
4. Discussion
4.1. Cortical Network
4.1.1. M1/PMA
4.1.2. SMA
4.1.3. Insula
4.2. Subcortical Network
4.2.1. Striatum
4.2.2. STN
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, S.L.; Raichlen, D.A.; Clark, A.E. What moves us? How mobility and movement are at the center of human evolution. Evol. Anthropol. Issues News Rev. 2016, 25, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Vogt, T.; Kanosue, K. Brain activity underlying muscle relaxation. Front. Physiol. 2019, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Fagergren, A.; Ehrsson, G.O.; Forssberg, H. Holding an object: Neural activity associated with fingertip force adjustments to external perturbations. J. Neurophysiol. 2007, 97, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Macauda, G.; Schrafl-Altermatt, M.; Wirz, M.; Kloter, E.; Michels, L. Neural coupling of cooperative hand movements: A reflex and fMRI Study. Cereb. Cortex 2015, 25, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.K.; Yamamoto, T.; Nakayama, Y.; Hamano, Y.H.; Fukunaga, M.; Sadato, N.; Nishimura, Y. Premovement activity in the mesocortical system links peak force but not initiation of force generation under incentive motivation. Cereb. Cortex 2023, 33, 11408–11419. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Sakamoto, M.; Matsumoto, A.; Okusaki, T.; Sasaya, R.; Irie, K.; Liang, N. Accuracy of force generation and preparatory prefrontal oxygenation in ballistic hand power and precision grips. J. Mot. Behav. 2024, 56, 226–240. [Google Scholar] [CrossRef]
- Derosière, G.; Alexandre, F.; Bourdillon, N.; Mandrick, K.; Ward, T.E.; Perrey, S. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. Neuroimage 2014, 85, 471–477. [Google Scholar] [CrossRef]
- Spraker, M.B.; Corcos, D.M.; Vaillancourt, D.E. Cortical and subcortical mechanisms for precisely controlled force generation and force relaxation. Cereb. Cortex 2009, 19, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Kuhtz-Buschbeck, J.P.; Ehrsson, H.H.; Forssberg, H. Human brain activity in the control of fine static precision grip forces: An fMRI study. Eur. J. Neurosci. 2001, 14, 382–390. [Google Scholar] [CrossRef]
- Mayhew, S.D.; Porcaro, C.; Tecchio, F.; Bagshaw, A.P. fMRI characterisation of widespread brain networks relevant for behavioural variability in fine hand motor control with and without visual feedback. Neuroimage 2017, 148, 330–342. [Google Scholar] [CrossRef]
- Michely, J.; Volz, L.J.; Hoffstaedter, F.; Tittgemeyer, M.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Network connectivity of motor control in the ageing brain. Neuroimage Clin. 2018, 18, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Davare, M.; Andres, M.; Fadiga, L. Precision grasping in humans: From motor control to cognition. Curr. Opin. Neurobiol. 2007, 17, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Farina, D.; Gandevia, S. The neural control of movement: A century of in vivo motor unit recordings is the legacy of Adrian and Bronk. J. Physiol. 2023, 602, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural correlates of action: Comparing meta-analyses of imagery, observation, and execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Spraker, M.B.; Prodoehl, J.; Corcos, D.M.; Comella, C.L.; Vaillancourt, D.E. Basal Ganglia hypoactivity during grip force in drug naive Parkinson’s Disease. Hum. Brain Mapp. 2010, 31, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Pope, P.A.; Holton, A.; Hassan, S.; Kourtis, D.; Praamstra, P. Cortical control of muscle relaxation: A lateralized readiness potential (LRP) investigation. Clin. Neurophysiol. 2007, 118, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Honda, M.; Hanakawa, T.; Okada, T.; Fukuyama, H.; Ikeda, A.; Nishizawa, S.; Konishi, J.; Shibasaki, H. Activities of the primary and supplementary motor areas increase in preparation and execution of voluntary muscle relaxation: An event-related fMRI study. J. Neurosci. 1999, 19, 3527–3534. [Google Scholar] [CrossRef] [PubMed]
- Labyt, E.; Cassim, F.; Szurhaj, W.; Bourriez, J.L.; Derambure, P. Oscillatory cortical activity related to voluntary muscle relaxation: Influence of normal aging. Clin. Neurophysiol. 2006, 117, 1922–1930. [Google Scholar] [CrossRef]
- Labyt, E.; Cassim, F.; Devos, D.; Bourriez, J.L.; Destée, A.; Guieu, J.D.; Defebvre, L.; Derambure, P. Abnormal cortical mechanisms in voluntary muscle relaxation in de novo Parkinsonian patients. J. Clin. Neurophysiol. 2005, 22, 192–203. [Google Scholar]
- Oga, T.; Honda, M.; Toma, K.; Murase, N.; Okada, T.; Hanakawa, T.; Sawamoto, N.; Nagamine, T.; Konishi, J.; Fukuyama, H.; et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer’s cramp: An fMRI study. Brain 2002, 125, 895–903. [Google Scholar] [CrossRef]
- Vaillancourt, D.E.; Yu, H.; Mayka, M.A.; Corcos, D.M. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage 2007, 36, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Purves, D. Neuroscience; Sinauer Associates, Incorporated: Sunderland, MA, USA, 2004. [Google Scholar]
- DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.; Wichmann, T. Update on models of basal ganglia function and dysfunction. Park. Relat. Disord. 2009, 15, S237–S240. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Glover, G.H.; Pfefferbaum, A. Differential activation of dorsal basal ganglia during externally and self paced sequences of arm movements. Neuroreport 1998, 9, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Wasson, P.; Prodoehl, J.; Coombes, S.A.; Corcos, D.M.; Vaillancourt, D.E. Predicting grip force amplitude involves circuits in the anterior basal ganglia. Neuroimage 2010, 49, 3230–3238. [Google Scholar] [CrossRef]
- Penney, J.B.; Young, A.B. Striatal inhomogeneities and basal ganglia function. Mov. Disord. 1986, 1, 3–15. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.X. Cortical and subcortical contributions to non-motor inhibitory control: An fMRI study. Cereb. Cortex 2023, 33, 10909–10917. [Google Scholar] [CrossRef]
- Kou, W.Y.; Wang, X.M.; Zheng, Y.C.; Zhao, J.J.; Cai, H.H.; Chen, H.M.; Sui, B.B.; Feng, T. Freezing of gait in Parkinson’s disease is associated with the microstructural and functional changes of globus pallidus internus. Front. Aging Neurosci. 2022, 14, 975068. [Google Scholar] [CrossRef] [PubMed]
- Rolinski, M.; Griffanti, L.; Szewczyk-Krolikowski, K.; Menke, R.A.L.; Wilcock, G.K.; Filippini, N.; Zamboni, G.; Hu, M.T.M.; Mackay, C.E. Aberrant functional connectivity within the basal ganglia of patients with Parkinson’s disease. Neuroimage Clin. 2015, 8, 126–132. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-analysis of Go/No-go tasks, demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef]
- Aron, A.R.; Poldrack, R.A. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J. Neurosci. 2006, 26, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.R.; Dolan, M.; Elliott, R.; Deakin, J.F.W.; Woodruff, P.W.R. Response inhibition and impulsivity: An fMRI study. Neuropsychologia 2003, 41, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Steele, V.R.; Aharoni, E.; Munro, G.E.; Calhoun, V.D.; Nyalakanti, P.; Stevens, M.C.; Pearlson, G.; Kiehl, K.A. A large scale (N = 102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav. Brain Res. 2013, 256, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Ross, T.J.; Stein, E.A. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proc. Natl. Acad. Sci. USA 1999, 96, 8301–8306. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, O.; Sakamoto, M.; Usui, S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J. Neurophysiol. 1989, 61, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.P.; Frith, C.D.; Frackowiak, R.S. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Lieberman, M.D.; Cunningham, W.A. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009, 4, 423–428. [Google Scholar] [CrossRef]
- Ruan, J.H.; Bludau, S.; Palomero-Gallagher, N.; Caspers, S.; Mohlberg, H.; Eickhoff, S.B.; Seitz, R.J.; Amunts, K. Cytoarchitecture, probability maps, and functions of the human supplementary and pre-supplementary motor areas. Brain Struct. Funct. 2018, 223, 4169–4186. [Google Scholar] [CrossRef]
- Dum, R.P.; Strick, P.L. Medial wall motor areas and skeletomotor control. Curr. Opin. Neurobiol. 1992, 2, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Chikazoe, J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr. Opin. Psychiatry 2010, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.O.; Baier, B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Struct. Funct. 2010, 214, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Tinaz, S.; Para, K.; Vives-Rodriguez, A.; Martinez-Kaigi, V.; Nalamada, K.; Sezgin, M.; Scheinost, D.; Hampson, M.; Louis, E.D.; Constable, R.T. Insula as the interface between body awareness and movement: A neurofeedback-guided kinesthetic motor imagery study in Parkinson’s Disease. Front. Hum. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Tamietto, M.; Cauda, F.; Celeghin, A.; Diano, M.; Costa, T.; Cossa, F.M.; Sacco, K.; Duca, S.; Geminiani, G.C.; de Gelder, B. Once you feel it, you see it: Insula and sensory-motor contribution to visual awareness for fearful bodies in parietal neglect. Cortex 2015, 62, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Perri, R.L.; Berchicci, M.; Bianco, V.; Quinzi, F.; Spinelli, D.; Di Russo, F. Awareness of perception and sensory-motor integration: ERPs from the anterior insula. Brain Struct. Funct. 2018, 223, 3577–3592. [Google Scholar] [CrossRef] [PubMed]
- Baier, B.; Karnath, H.O. Tight link between our sense of limb ownership and self-awareness of actions. Stroke 2008, 39, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, N. Confirming the role of the insula in coordinating complex but not simple articulatory movements. Brain Lang. 2004, 91, 23–24. [Google Scholar] [CrossRef]
- Zhang, R.B.; Geng, X.J.; Lee, T.M.C. Large-scale functional neural network correlates of response inhibition: An fMRI meta-analysis. Brain Struct. Funct. 2017, 222, 3973–3990. [Google Scholar] [CrossRef]
- Steele, V.R.; Claus, E.D.; Aharoni, E.; Harenski, C.; Calhoun, V.D.; Pearlson, G.; Kiehl, K.A. A large scale (N = 102) functional neuroimaging study of error processing in a Go/NoGo task. Behav. Brain Res. 2014, 268, 127–138. [Google Scholar] [CrossRef]
- Jacopo, P.; Rory, D.; Louise, W.; Morten Gersel, S.; Lynn, R.; David James, B.; David, B.; Nicola, P. Clinical implications of early caudate dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1098. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Fagergren, A.; Jonsson, T.; Westling, G.; Johansson, R.S.; Forssberg, H. Cortical activity in precision- versus power-grip tasks: An fMRI study. J. Neurophysiol. 2000, 83, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.G.; Kim, J.S.; Chung, C.K. Brain mechanisms in motor control during reaching movements: Transition of functional connectivity according to movement states. Sci. Rep. 2020, 10, 567. [Google Scholar] [CrossRef] [PubMed]

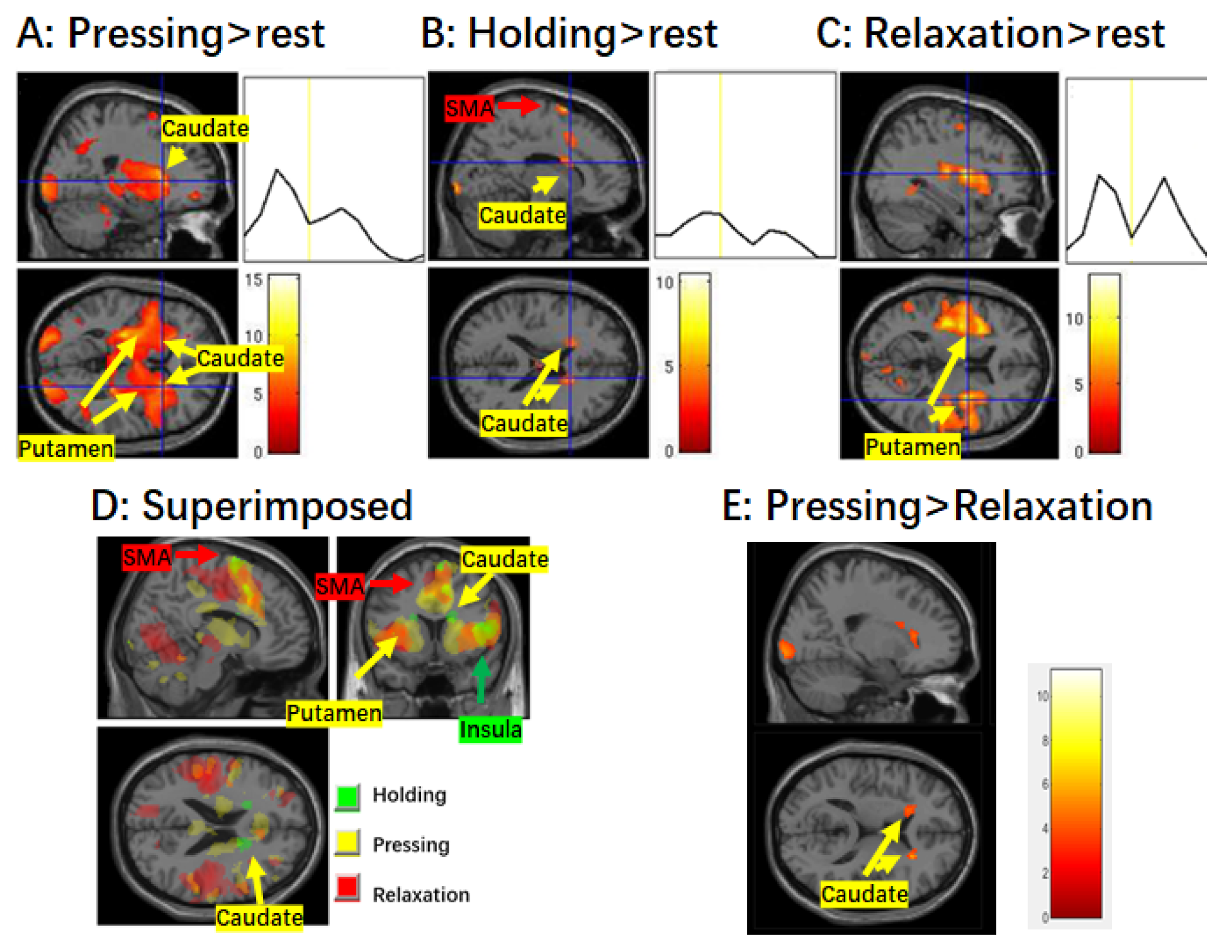
| Conditions | Location | Side | MNI Coordinates | Cluster Size | T Score | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| Pressing > rest | |||||||
| M1/PMA | L | −34 | −34 | 36 | 790 | 6.42 ** | |
| R | 58 | −34 | 24 | 1417 | 8.04 *** | ||
| SMA | L/R | 0 | 16 | 44 | 2026 | 12.28 *** | |
| ACC | L/R | 8 | 24 | 28 | 1813 | 6.69 * | |
| R | 6 | −38 | 50 | 215 | 4.79 | ||
| Insula/IFG | L | −32 | 2 | 14 | 1187 | 15.22 *** | |
| R | 36 | 8 | 12 | 1259 | 8.06 *** | ||
| Caudate | L | −16 | 6 | 20 | 326 | 6.6 † | |
| R | 22 | 18 | 6 | 595 | 8.86 * | ||
| Putamen | L | −28 | −20 | 4 | 995 | 9.43 ** | |
| R | 24 | 16 | 8 | 1000 | 8.11 ** | ||
| Pallidum | L | −26 | −6 | −2 | 248 | 7.67 | |
| R | 24 | −2 | −6 | 277 | 5.53 | ||
| MOG/IOG | L | −22 | −90 | −6 | 1386 | 9.17 *** | |
| R | 38 | −72 | −12 | 842 | 7.09 ** | ||
| FG | L | −32 | −72 | −18 | 566 | 6.59 * | |
| R | 44 | −62 | −22 | 530 | 7.46 * | ||
| Thalamus | L/R | −22 | −18 | 0 | 1335 | 7.16 *** | |
| MFG | L | −30 | 46 | 28 | 144 | 5.56 | |
| R | 38 | 44 | 30 | 343 | 4.85 | ||
| Cerebellum | L | −32 | −74 | −20 | 589 | 6.55 * | |
| R | 38 | −66 | −24 | 1723 | 9.20 *** | ||
| Holding > rest | |||||||
| SMA | R | 14 | 10 | 72 | 182 | 5.81 | |
| ACC | R | 12 | 12 | 48 | 139 | 4.65 | |
| Insula/IFG | R | 50 | 8 | 10 | 647 | 10.19 ** | |
| L | −40 | 6 | 14 | 181 | 4.3 † | ||
| PoCG | R | 64 | −30 | 46 | 166 | 5.11 | |
| Caudate | R | 24 | 24 | 10 | 321 | 5.51 † | |
| L | −18 | 16 | 22 | 91 | 4.68 † | ||
| MOG | L | −24 | −90 | −4 | 1128 | 10.43 *** | |
| R | 26 | −92 | 0 | 924 | 8.59 ** | ||
| Relaxation > rest | |||||||
| M1/PMA | L | −48 | −22 | 26 | 2082 | 8.86 *** | |
| R | 58 | 14 | 32 | 938 | 6.68 ** | ||
| SMA | L/R | −10 | −8 | 52 | 2835 | 11.26 *** | |
| ACC | L/R | 12 | −8 | 50 | 1411 | 13.13 ** | |
| Insula/IFG | L | −40 | 14 | 8 | 1702 | 10.22 *** | |
| R | 34 | −2 | 12 | 1845 | 9.06 *** | ||
| Putamen | L | 32 | 2 | 10 | 563 | 8.58 ‡ | |
| R | −30 | 8 | −6 | 543 | 8.64 ‡ | ||
| STN | L/R | 4 | −14 | −4 | 194 | 7.39 ** | |
| LG/PhG/FG | R | 20 | −70 | −2 | 1803 | 7.27 *** | |
| L | −22 | −70 | −4 | 1138 | 8.87 ** | ||
| PCG | L | −20 | −10 | 66 | 949 | 6.01 ** | |
| R | 26 | −18 | 16 | 1262 | 7.55 ** | ||
| MTG | L | −52 | −62 | 4 | 656 | 5.61 * | |
| Cuneus | L | −14 | −76 | 20 | 219 | 5.25 | |
| MFG | L | −24 | 38 | 26 | 354 | 4.58 | |
| R | 34 | 38 | 24 | 132 | 4.41 | ||
| Cerebellum | L | −34 | −38 | −30 | 132 | 4.49 | |
| R | 14 | −52 | −18 | 1114 | 5.98 ** | ||
| STG | L | −46 | 32 | 22 | 1283 | 7.46 ** | |
| L | 52 | −24 | 16 | 1186 | 6.88 ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wang, Z. Differential Cortical and Subcortical Activations during Different Stages of Muscle Control: A Functional Magnetic Resonance Imaging Study. Brain Sci. 2024, 14, 404. https://doi.org/10.3390/brainsci14040404
Peng Y, Wang Z. Differential Cortical and Subcortical Activations during Different Stages of Muscle Control: A Functional Magnetic Resonance Imaging Study. Brain Sciences. 2024; 14(4):404. https://doi.org/10.3390/brainsci14040404
Chicago/Turabian StylePeng, Yu, and Zhaoxin Wang. 2024. "Differential Cortical and Subcortical Activations during Different Stages of Muscle Control: A Functional Magnetic Resonance Imaging Study" Brain Sciences 14, no. 4: 404. https://doi.org/10.3390/brainsci14040404





