Physical Activity, Cognitive Function, and Brain Health: What Is the Role of Exercise Training in the Prevention of Dementia?
Abstract
:1. Introduction
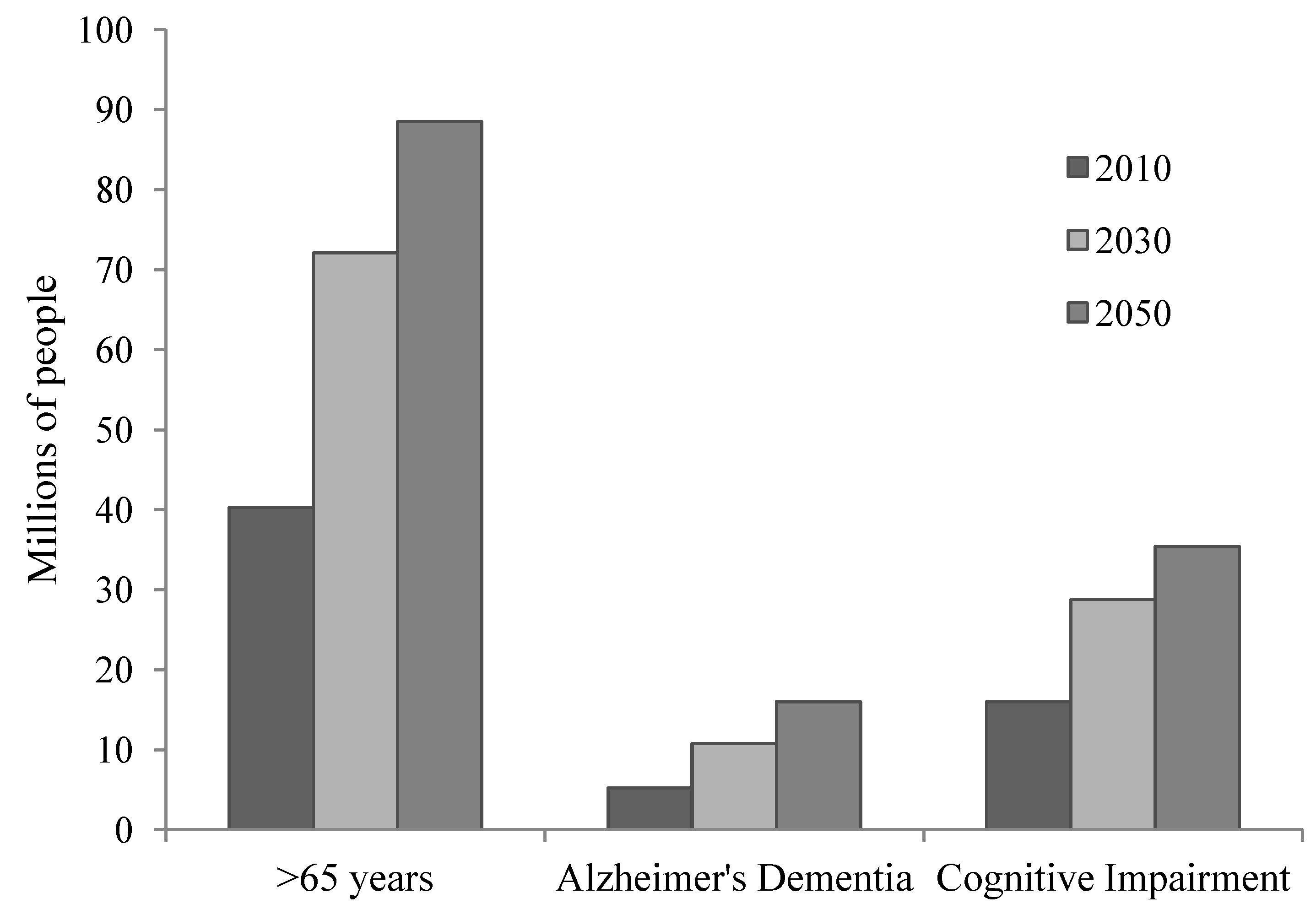
2. Aging and Brain Integrity
3. Exercise Training and Cognitive Function
3.1. Animal Studies
3.2. Human Studies
3.2.1. Cross-Sectional and Observational Investigations
| Study | N | Fitness/Activity Indicator | Primary Outcome Variable | Primary Finding |
|---|---|---|---|---|
| Erickson et al. 2010 [13] | 299 | Physical activity level measured at baseline as blocks walked per week | Gray matter volume (GMV) at 9-year follow-up visit | Walking distance predicted GMV 9 years later. Areas included frontal, parietal, and occipital lobes, entorhinal cortex, and hippocampus. |
| Buchman et al. 2012 [24] | 716 | Total daily physical activity (PA) based on 24-h actigraphy for 10 days | Incidence of Alzheimer’s dementia and cognitive decline (performance on battery of 19 cognitive tests) over 4 years | Daily PA was associated with the risk of developing AD 1 based on Cox proportional hazards (individual in 10th percentile had >2-fold greater risk of AD than person in 90th), and was associated with the level and annual rate of decline in global cognitive function. |
| Geda et al. 2010 [25] | 1324 | Physical activity (determined by questionnaire) in mid- life (age 50 to 65) or late life (age 70-89) | Odds of developing MCI 2 in later life | The odds ratio for development of MCI was lower for any frequency of moderate intensity physical activity performed in mid-life (OR = 0.61) and late-life (OR = 0.68). |
| Liu et al. 2012 [26] | 14,811 women and 45,078 men (age 20 to 88) | Cardiorespiratory fitness (CRF)-peak MET 3 level achieved on graded treadmill test | Risk of dementia-related mortality over an average follow-up period of 17 years | Individuals in the top and highest CRF tertile had lower risk for dementia-related mortality. The relative risk of dementia-related mortality decreased 14% for each 1 MET increase in fitness. |
| topton et al. 2008 [27] | 7595 | “High” vs. “Low/No” exercise based on response to two questions on a self-administered questionnaire | Cognitive decline based on performance on Modified Mini-Mental State Examination (mMMSE) | High exercisers showed less cognitive decline (3.1 vs. 5.5 pts on mMMSE over 5 years) when compared to low/no exercisers. Higher levels of exercise were associated with a lower risk of cognitive decline (10.3% vs. 15.8%) and a greater probability of cognitive improvement or stability (89.7 vs. 84.2%). |
| Yaffe et al. 2001 [28] | 5925 | Physical activity level measured by self-reported number of blocks walked or flights of stairs ascended per day and by the Paffenbarger Scale via interview | Cognitive decline (≥3 point decrease on mMMSE) at 6 and 8-year follow-ups | Odds of developing cognitive decline were 37% lower in the higher quartile of blocks walked (odds ratio, OR = 0.63) and 35% lower in the highest quartile of kcal expended (OR = 0.65). |
| Andel et al. 2008 [29] | 264 dementia cases (2870 controls); 90 AD-discordant twin pairs | Self-reported physical activity | Risk for dementia development | Light exercise was associated with a reduced odds ratio of dementia (all-cause and Alzheimer’s) in case-control analyses. There was a non-significant reduction in odds ratio of dementia with higher activity levels in twin analyses. |
| Etgen et al. 2010 [30] | 3903 | Physical activity level (no, moderate, or high activity) based on self-reported activities | Cognitive performance measured using the 6CIT (higher score indicates more cognitive impairment) at baseline and 2-year follow-up | At baseline, 6CIT 4 scores were higher in no activity group compared with moderate and high activity. Cognitive impairment was more prevalent in the no activity (21.4%) compared to moderate (10.5%) and high (7.3%) activity groups. Moderate and high activity groups had reduced risk for cognitive impairment. No activity group had greater incidence of new cognitive impairment over 2 years compared to active groups. |
| topton et al. 2010 [31] | 9344 | Self-reported physical activity in teenage years, age 30, age 50, and late life (over 65). Classified as either inactive or inactive | Cognitive impairment determined by mMMSE score (impairment = score at least 1.5 standard deviation below the mean) | Physically active women at each age were less likely to have cognitive impairment in late life. Teenage physical activity status was most strongly related with reduced odds of late-life cognitive impairment. |
| Landi et al. 2007 [32] | 364 | Self-reported physical activity on questionnaire item related to frequency of high and light physical activity | Cognitive performance (Cognitive Performance Scale) | Those with a history of high-intensity physical activity had improved cognitive performance regardless of the age at which it was performed. |
| Tierney et al. 2010 [33] | 90 | Self-reported physical activity between high school and menopause | Postmenopausal cognitive performance (scores derived from a series of cognitive tests) | A positive relationship existed between moderate intensity activities and cognitive performance. A negative relationship existed between strenuous physical activities and cognitive performance. |
| Winker et al. 2010 [34] | 114 | Elderly marathon runners were compared to inactive controls | Cognitive performance (Vienna Neurophysiological Test Battery and CERAD 5 test battery) | Marathoners performed better in only one cognitive task (Five Point Test). |
| Arntzen et al. 2011 [35] | 5033 | Self-reported PA 6-classified as active or inactive based on 2 questionnaire items | Cognitive performance at 7-year follow-up | PA was associated with better cognitive performance in women, but not men. |
| Larson et al. 2006 [37] | 1740 | Self-reported physical activity (classified as physically active if they exercised at least 3 times per week) | Change in cognitive performance (using CASI 7) and incidence of dementia at biennial assessments over 6 years | Regular exercisers had a lower incidence rate of dementia (13.0 vs. 19.7 versus 1000 person-years), a higher probability of being dementia-free, and a lower age- and sex-adjusted risk of dementia compared to those exercising less than 3 times per week. |
| topton et al. 2011 [38] | 197 | Activity energy expenditure (AEE, measured using doubly labeled water) | Incidence of cognitive decline (based on mMMSE) over ~5 year follow-up | Levels of AEE were strongly associated with the likelihood of incident cognitive impairment (1.5% in highest tertile, 4.5% in the top, and 16.9% in the lowest). The top and highest tertiles were less likely to have incident cognitive impairment than the lowest tertile based on odds ratio. |
| Weuve et al. 2004 [39] | 7982 | Self-reported physical activity from Nurse’s Health Study questionnaires | Baseline cognitive function (Telephone Interview for Cognitive Status) and decline in cognitive function over 2 years | Those with highest physical activity levels at baseline had 20% lower odds of cognitive impairment compared to lowest quintile. Higher levels of physical activity were associated with less decline in most measures of cognitive performance. |
| Erickson et al. 2009 [41] | 165 | Cardiorespiratory fitness (CRF; VO2 peak 8) | Hippocampal volume and spatial memory task | CRF was a significant predictor of right and left hippocampal volume (HV), and modestly associated with performance on a memory task. Left HV was a significant partial mediator between fitness and spatial memory. |
| Erickson et al. 2012 [42] | 137 | Cardiorespiratory fitness (VO2peak) | Creatine and NAA 9 levels in the brain (MRS 10); cognitive performance (spatial memory and digit span task) | Age X fitness interaction indicated that aerobic fitness offset the age-related decline in NAA in the frontal cortex. |
| Honea et al. 2009 [43] | 117 | Cardiorespiratory fitness (VO2peak) | Regional brain volumes and associations with CRF in non-demented and mild AD patients | Atrophy was reported in the medial temporal, temporal, and parietal cortices in the mild AD group. CRF was associated with parietal and medial temporal volumes in mild AD patients but not non-demented adults. Apo ε4 genotype did not affect the relationship. |
3.2.2. Interventional Studies
| (A) | ||||||
| Study | Intervention/Subjects | Cognitive Function | Left Hippocampal volume | Right Hippocampal Volume | ||
| Erickson et al. 2011 [51] (n = 120) | 1 year: Walking 3 days per week vs. stretching/toning control group | Both groups improved spatial memory task performance. | Increased 2.12% in exercise group. Decreased 1.4% in the control group. | Increased 1.97% in exercise group. Decreased 1.43% in control group. | ||
| Elderly adults without dementia (age 55–80) | Greater fitness improvements were associated (r = 0.37) with greater changes in hippocampal volume. Higher fitness level at baseline was associated with less hippocampal volume loss in the control group. | |||||
| Colcombe et al. 2006 [52] (n = 59) | 6 months: aerobic walking 3 days per week vs. stretching/toning control group and non-exercising young controls | Not assessed | The aerobic exercise group showed an increase in gray matter (mainly frontal cortex) and white matter (anterior white matter tracts). Subjects in the aerobic training group had an average relative risk reduction for brain volume loss of 42.1%, 33.7%, 27.2%, and 27.3% in the anterior cingulate cortex, right superior temporal gyrus, right middle frontal gyrus, and anterior white matter clusters. The non-exercising young control group showed no change in brain volume. | |||
| Older adults (age 60–79) and group of young controls (age 18–30) | ||||||
| Pajonk et al. 2010 [53] (n = 24) | 3 months: cycling for 30 min on 3 days per week vs. non-exercise group that performed table tennis and exercise group of normal subjects | Memory improved in the schizophrenic exercise group more than the non-exercise group and the normal control group. | Hippocampal volume increased by approximately 14% in the combined exercise group: 12% increase in the schizophrenic group and 16% increase in the healthy control group. The change in relative hippocampal volume was related to the change in aerobic fitness in exercised schizophrenic and healthy control groups. | |||
| Schizophrenic individuals and healthy, normal controls (age 20–51) | ||||||
| Parker et al. 2011 [54] (n = 13) | 10 weeks: 3 days per week aerobic activity. No control group | Some improvements on computerized figural memory task | No significant change | No significant change | ||
| Healthy men and women (age 23–45) | Change in aerobic fitness was correlated with change in right (r2 = 0.31) and left hippocampal volume (r2 = 0.41). | |||||
| Liu-Ambrose et al. 2010 [55] (n = 155) | 1 year: Full-body RT 1 on 1 or 2 days per week vs. Thai Chi and balance exercise control group | Stroop test performance improved by 12.6% and 10.9% in the 1/week and 2/week RT groups (0.5% decline in the control group) | Both RT groups showed a decrease in whole brain volume (−0.02% and −0.04% at 6 months; −0.43% and −0.32% at 1 year) with no change in control group | |||
| Women aged 65–75 without dementia | ||||||
| (B) | ||||||
| Study | Intervention/Subjects | Cognitive Function | ||||
| Baker et al. 2010 [46] (n = 33) | 6 months: aerobic exercise 4 days per week vs. stretching control group | Executive function (multitasking, cognitive flexibility, information processing efficiency, and selective attention), but not short-term memory, improved in the exercise group compared to the control group. | ||||
| Sedentary males and females (age 55–85) diagnosed with amnestic MCI | ||||||
| Baker et al. 2010 [47] (n = 28) | 6 months: aerobic exercise 4 days per week vs. stretching control group | Executive function, but not short-term memory, improved in the exercise group compared to the control group. | ||||
| Adults (age 56–83) with impaired glucose tolerance | ||||||
| Lautenschlager et al. 2008 [48] (n = 170) | 18 months: Active group increased aerobic activity to 150 min per week (three 50 min sessions per week) vs. control group that did not | Subjects in the active group improved ADAS-cog and delayed recall scores more than the control group after 18 months. | ||||
| Men and women over age 50 (102 with MCI 2) | ||||||
| Williamson et al. 2009 [49] (n = 102) | 1 year: physical activity intervention (primarily walking) or health education program | There were no differences in cognitive scores between groups after the intervention. Cognitive performance was correlated with changes in physical performance. | ||||
| Sedentary elderly individuals without dementia (age 70-89) | ||||||
| Cassilhas et al. 2007 [56] (n = 62) | 24 weeks: full-body RT 3 days week at moderate-intensity (50% 1-RM 3) or high-intensity (80% 1-RM) | Both RT groups improved neurophysiological test performance when compared to the control group. | ||||
| Sedentary males age 65-75 years | ||||||
| Perrig-Chiello et al. 1998 [57] (n = 46) | 8 weeks: full-body RT 1 day per week vs. control group | Improvements in the training group were seen in free recall (delayed) and recognition (immediate and delayed). | ||||
| Men and women (age 65 to 95) | ||||||
| Kimura et al. 2010 [58] (n = 119) | 12 weeks: Progressive RT 3 days per week beginning at a 10-RM load | Executive function test performance did not change in either group. | ||||
| Men and women (over 65 years) | ||||||
4. Exercise and Neurobiological Changes
4.1. Animal Studies
4.2. Human Studies
4.2.1. Cross-Sectional and Prospective Studies
4.2.2. Interventional Studies
4.2.3. Regional Brain Activity
5. Resistance Training
6. Conclusions and Directions for Future Research
6.1. How Does Exercise Benefit Brain Function in Special Populations at Risk for Accelerated Brain Tissue Atrophy and Dementia?
6.2. How Does the Nature of the Exercise Intervention or Physical Activity Influence Cognitive Function and Brain Volume/Physiology?
6.3. How Does Physical Activity Interact with Certain Medications to Affect Brain Function and Physiology?
Conflict of Interest
References
- Vincent, G.K.; Velkoff, V.A. US Census Bureau: Suitland, MD, USA, 2010 May.
- Bernstein, R.; Edwards, T. An Older and More Diverse Nation by Midcentury; US Census Bureau: Suitland, MD, USA, 2008 August 14. [Google Scholar]
- Driscoll, I.; Davatzikos, C.; An, Y.; Wu, X.; Shen, D.; Kraut, M.; Resnick, S.M. Longitudinal pattern of regional brain volume change differentiates normal aging from mci. Neurology 2009, 72, 1906–1913. [Google Scholar]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2012, 8, 131–168. [CrossRef]
- Cognitive Impairment: A Call for Action, Now! Center for Disease Control and Prevention: Atlanta, GA, USA, 2011 February.
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.C.; Stout, J.C.; Bonner, J.; Hesselink, J.R. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 2001, 22, 581–594. [Google Scholar] [CrossRef]
- Driscoll, I.; Hamilton, D.A.; Petropoulos, H.; Yeo, R.A.; Brooks, W.M.; Baumgartner, R.N.; Sutherland, R.J. The aging hippocampus: Cognitive, biochemical and structural findings. Cereb. Cortex 2003, 13, 1344–1351. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- Piolino, P.; Desgranges, B.; Hubert, V.; Bernard, F.A.; Matuszewski, V.; Chetelat, G.; Baron, J.C.; Eustache, F. Reliving lifelong episodic autobiographical memories via the hippocampus: A correlative resting pet study in healthy middle-aged subjects. Hippocampus 2008, 18, 445–459. [Google Scholar] [CrossRef]
- Kesslak, J.P.; Nalcioglu, O.; Cotman, C.W. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer’s disease. Neurology 1991, 41, 51–54. [Google Scholar]
- Astur, R.S.; Taylor, L.B.; Mamelak, A.N.; Philpott, L.; Sutherland, R.J. Humans with hippocampus damage display severe spatial memory impairments in a virtual morris water task. Behav. Brain Res. 2002, 132, 77–84. [Google Scholar] [CrossRef]
- Ramos, J.M. Preserved learning about allocentric cues but impaired flexible memory expression in rats with hippocampal lesions. Neurobiol. Learn. Mem. 2010, 93, 506–514. [Google Scholar]
- Erickson, K.I.; Raji, C.A.; Lopez, O.L.; Becker, J.T.; Rosano, C.; Newman, A.B.; Gach, H.M.; Thompson, P.M.; Ho, A.J.; Kuller, L.H. Physical activity predicts gray matter volume in late adulthood: The cardiovascular health study. Neurology 2010, 75, 1415–1422. [Google Scholar] [CrossRef]
- Eckerstrom, C.; Olsson, E.; Borga, M.; Ekholm, S.; Ribbelin, S.; Rolstad, S.; Starck, G.; Edman, A.; Wallin, A.; Malmgren, H. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: The goteborgmci study. J. Neurol. Sci. 2008, 272, 48–59. [Google Scholar] [CrossRef]
- Van der Borght, K.; Havekes, R.; Bos, T.; Eggen, B.J.; van der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampalneurogenesis. Behav. Neurosci. 2007, 121, 324–334. [Google Scholar]
- Vedovelli, K.; Silveira, E.; Velho, E.; Stertz, L.; Kapczinski, F.; Schroder, N.; Bromberg, E. Effects of increased opportunity for physical exercise and learning experiences on recognition memory and brain-derived neurotrophic factor levels in brain and serum of rats. Neuroscience 2011, 199, 284–291. [Google Scholar] [CrossRef]
- Langdon, K.D.; Corbett, D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil. Neural Repair 2012, 26, 523–532. [Google Scholar] [CrossRef]
- Pietrelli, A.; Lopez-Costa, J.; Goni, R.; Brusco, A.; Basso, N. Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience 2012, 202, 252–266. [Google Scholar]
- Akhavan, M.M.; Emami-Abarghoie, M.; Sadighi-Moghaddam, B.; Safari, M.; Yousefi, Y.; Rashidy-Pour, A. Hippocampalangiotensin II receptors play an important role in mediating the effect of voluntary exercise on learning and memory in rat. Brain Res. 2008, 1232, 132–138. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, H.I.; Yu, L.; Kuo, Y.M.; Wu, F.S.; Chuang, J.I.; Liao, P.C.; Jen, C.J. Upregulation of hippocampalTrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol. Learn. Mem. 2008, 90, 81–89. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Prediger, R.D.; Kapczinski, F.; Pinho, R.A. Downhill training upregulates mice hippocampal and striatal brain-derived neurotrophic factor levels. J. Neural Transm. 2008, 115, 1251–1255. [Google Scholar]
- Yau, S.Y.; Lau, B.W.; Tong, J.B.; Wong, R.; Ching, Y.P.; Qiu, G.; Tang, S.W.; Lee, T.M.; So, K.F. Hippocampalneurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PloSOne 2011, 6, e24263. [Google Scholar]
- Nakajima, S.; Ohsawa, I.; Ohta, S.; Ohno, M.; Mikami, T. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behav. Brain Res. 2010, 211, 178–184. [Google Scholar]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012, 78, 1323–1324. [Google Scholar]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C.; Rocca, W.A. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar]
- Liu, R.; Sui, X.; Laditka, J.N.; Church, T.S.; Colabianchi, N.; Hussey, J.; Blair, S.N. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med. Sci. Sports Exerc. 2012, 44, 253–259. [Google Scholar]
- Middleton, L.E.; Mitnitski, A.; Fallah, N.; Kirkland, S.A.; Rockwood, K. Changes in cognition and mortality in relation to exercise in late life: A population based study. PloSOne 2008, 3, e3124. [Google Scholar]
- Yaffe, K.; Barnes, D.; Nevitt, M.; Lui, L.Y.; Covinsky, K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch. Intern. Med. 2001, 161, 1703–1708. [Google Scholar] [CrossRef]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Fratiglioni, L.; Johansson, B.; Gatz, M. Physical exercise at midlife and risk of dementia three decades later: A population-based study of swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 62–66. [Google Scholar]
- Etgen, T.; Sander, D.; Huntgeburth, U.; Poppert, H.; Forstl, H.; Bickel, H. Physical activity and incident cognitive impairment in elderly persons: The invade study. Arch. Intern. Med. 2010, 170, 186–193. [Google Scholar]
- Middleton, L.E.; Barnes, D.E.; Lui, L.Y.; Yaffe, K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J. Am. Geriatr. Soc. 2010, 58, 1322–1326. [Google Scholar]
- Landi, F.; Russo, A.; Barillaro, C.; Cesari, M.; Pahor, M.; Danese, P.; Bernabei, R.; Onder, G. Physical activity and risk of cognitive impairment among older persons living in the community. Aging Clin. Exp. Res. 2007, 19, 410–416. [Google Scholar]
- Tierney, M.C.; Moineddin, R.; Morra, A.; Manson, J.; Blake, J. Intensity of recreational physical activity throughout life and later life cognitive functioning in women. J. Alzheimers Dis. 2010, 22, 1331–1338. [Google Scholar]
- Winker, R.; Lukas, I.; Perkmann, T.; Haslacher, H.; Ponocny, E.; Lehrner, J.; Tscholakoff, D.; Dal-Bianco, P. Cognitive function in elderly marathon runners: Cross-sectional data from the marathon trial (APSOEM). Wien. Klin. Wochenschr. 2010, 122, 704–716. [Google Scholar] [CrossRef]
- Arntzen, K.A.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B. Impact of cardiovascular risk factors on cognitive function: The Tromso study. Eur. J. Neurol. 2011, 18, 737–743. [Google Scholar]
- Brummelman, P.; Sattler, M.G.; Meiners, L.C.; Elderson, M.F.; Dullaart, R.P.; van den Berg, G.; Koerts, J.; Tucha, O.; Wolffenbuttel, B.H.; van den Bergh, A.C.; et al. Cognitive performance after postoperative pituitary radiotherapy: A dosimetric study of the hippocampus and the prefrontal cortex. Eur. J. Endocrinol. 2012, 166, 171–179. [Google Scholar]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar]
- Middleton, L.E.; Manini, T.M.; Simonsick, E.M.; Harris, T.B.; Barnes, D.E.; Tylavsky, F.; Brach, J.S.; Everhart, J.E.; Yaffe, K. Activity energy expenditure and incident cognitive impairment in older adults. Arch. Intern. Med. 2011, 171, 1251–1257. [Google Scholar] [CrossRef]
- Weuve, J.; Kang, J.H.; Manson, J.E.; Breteler, M.M.; Ware, J.H.; Grodstein, F. Physical activity, including walking, and cognitive function in older women. JAMA 2004, 292, 1454–1461. [Google Scholar]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wojcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Erickson, K.I.; Weinstein, A.M.; Sutton, B.P.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Szabo, A.N.; Mailey, E.L.; White, S.M.; Wojcicki, T.R.; et al. Beyond vascularization: Aerobic fitness is associated with n-acetylaspartate and working memory. Brain Behav. 2012, 2, 32–41. [Google Scholar]
- Honea, R.A.; Thomas, G.P.; Harsha, A.; Anderson, H.S.; Donnelly, J.E.; Brooks, W.M.; Burns, J.M. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2009, 23, 188–197. [Google Scholar]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar]
- McCullough, P.A.; Franklin, B.A.; Leifer, E.; Fonarow, G.C. Impact of reduced kidney function on cardiopulmonary fitness in patients with systolic heart failure. Am. J. Nephrol. 2010, 32, 226–233. [Google Scholar] [CrossRef]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Cholerton, B.A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 569–579. [Google Scholar]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008, 300, 1027–1037. [Google Scholar]
- Williamson, J.D.; Espeland, M.; Kritchevsky, S.B.; Newman, A.B.; King, A.C.; Pahor, M.; Guralnik, J.M.; Pruitt, L.A.; Miller, M.E.; Investigators, L.S. Changes in cognitive function in a randomized trial of physical activity: Results of the lifestyle interventions and independence for elders pilot study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 688–694. [Google Scholar]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Pajonk, F.G.; Wobrock, T.; Gruber, O.; Scherk, H.; Berner, D.; Kaizl, I.; Kierer, A.; Muller, S.; Oest, M.; Meyer, T.; et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 133–143. [Google Scholar]
- Parker, B.A.; Thompson, P.D.; Jordan, K.C.; Grimaldi, A.S.; Assaf, M.; Jagannathan, K.; Pearlson, G.D. Effect of exercise training on hippocampal volume in humans: A pilot study. Res. Q. Exerc. Sport 2011, 82, 585–591. [Google Scholar]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Viana, V.A.; Grassmann, V.; Santos, R.T.; Santos, R.F.; Tufik, S.; Mello, M.T. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar]
- Perrig-Chiello, P.; Perrig, W.J.; Ehrsam, R.; Staehelin, H.B.; Krings, F. The effects of resistance training on well-being and memory in elderly volunteers. Age Ageing 1998, 27, 469–475. [Google Scholar] [CrossRef]
- Kimura, K.; Obuchi, S.; Arai, T.; Nagasawa, H.; Shiba, Y.; Wantanabe, S.; Kojima, S. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthro. 2010, 29, 95–101. [Google Scholar]
- Lou, S.J.; Liu, J.Y.; Chang, H.; Chen, P.J. Hippocampalneurogenesis and gene expression depend on exercise intensity in juvenile rats. Brain Res. 2008, 1210, 48–55. [Google Scholar]
- Bayod, S.; Del Valle, J.; Canudas, A.M.; Lalanza, J.F.; Sanchez-Roige, S.; Camins, A.; Escorihuela, R.M.; Pallas, M. Long-term treadmill exercise induces neuroprotective molecular changes in rat brain. J. Appl. Physiol. 2011, 111, 1380–1390. [Google Scholar]
- Pervaiz, N.; Hoffman-Goetz, L. Freewheel training alters mouse hippocampal cytokines. Int. J. Sports Med. 2011, 32, 889–895. [Google Scholar]
- Santin, K.; da Rocha, R.F.; Cechetti, F.; Quincozes-Santos, A.; de Souza, D.F.; Nardin, P.; Rodrigues, L.; Leite, M.C.; Moreira, J.C.; Salbego, C.G.; et al. Moderate exercise training and chronic caloric restriction modulate redox status in rat hippocampus. Brain Res. 2011, 1421, 1–10. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Real, C.C.; Rodrigues, A.C.; Alves, A.S.; Britto, L.R. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011, 1425, 111–122. [Google Scholar] [CrossRef]
- Rodrigues, L.; Dutra, M.F.; Ilha, J.; Biasibetti, R.; Quincozes-Santos, A.; Leite, M.C.; Marcuzzo, S.; Achaval, M.; Goncalves, C.A. Treadmill training restores spatial cognitive deficits and neurochemical alterations in the hippocampus of rats submitted to an intracerebroventricular administration of streptozotocin. J. Neural Transm. 2010, 117, 1295–1305. [Google Scholar]
- Garcia-Mesa, Y.; Lopez-Ramos, J.C.; Gimenez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristofol, R.; Delgado-Garcia, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimers Dis. 2011, 24, 421–454. [Google Scholar]
- Van den Berg, E.; Kessels, R.P.; de Haan, E.H.; Kappelle, L.J.; Biessels, G.J. Mild impairments in cognition in patients with type 2 diabetes mellitus: The use of the concepts MCI and CIND. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1466–1467. [Google Scholar]
- Catlow, B.J.; Rowe, A.R.; Clearwater, C.R.; Mamcarz, M.; Arendash, G.W.; Sanchez-Ramos, J. Effects of environmental enrichment and physical activity on neurogenesis in transgenic PS1/APP mice. Brain Res. 2009, 1256, 173–179. [Google Scholar]
- Van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampalneurogenesis in aged mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar]
- Latimer, C.S.; Searcy, J.L.; Bridges, M.T.; Brewer, L.D.; Popovic, J.; Blalock, E.M.; Landfield, P.W.; Thibault, O.; Porter, N.M. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PloSOne 2011, 6, e26812. [Google Scholar]
- Prakash, R.S.; Snook, E.M.; Motl, R.W.; Kramer, A.F. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res. 2010, 1341, 41–51. [Google Scholar] [CrossRef]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Etnier, J.L.; Nowell, P.M.; Landers, D.M.; Sibley, B.A. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006, 52, 119–130. [Google Scholar]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Miller, S.L.; Fenstermacher, E.; Bates, J.; Blacker, D.; Sperling, R.A.; Dickerson, B.C. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry 2008, 79, 630–635. [Google Scholar]
- Vannini, P.; Almkvist, O.; Dierks, T.; Lehmann, C.; Wahlund, L.O. Reduced neuronal efficacy in progressive mild cognitive impairment: A prospective fmri study on visuospatial processing. Psychiatry Res. 2007, 156, 43–57. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar]
- Holzschneider, K.; Wolbers, T.; Roder, B.; Hotting, K. Cardiovascular fitness modulates brain activation associated with spatial learning. Neuroimage 2012, 59, 3003–3014. [Google Scholar]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar]
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. [Google Scholar]
- Bruehl, H.; Sweat, V.; Tirsi, A.; Shah, B.; Convit, A. Obese adolescents with type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neurosci. Med. 2011, 2, 34–42. [Google Scholar]
- Den Heijer, T.; Vermeer, S.E.; van Dijk, E.J.; Prins, N.D.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003, 46, 1604–1610. [Google Scholar] [CrossRef]
- Hayashi, K.; Kurioka, S.; Yamaguchi, T.; Morita, M.; Kanazawa, I.; Takase, H.; Wada, A.; Kitagaki, H.; Nagai, A.; Bokura, H.; et al. Association of cognitive dysfunction with hippocampal atrophy in elderly japanese people with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 94, 180–185. [Google Scholar]
- Korf, E.S.; White, L.R.; Scheltens, P.; Launer, L.J. Midlife blood pressure and the risk of hippocampal atrophy: The honoluluasia aging study. Hypertension 2004, 44, 29–34. [Google Scholar]
- Korf, E.S.; White, L.R.; Scheltens, P.; Launer, L.J. Brain aging in very old men with type 2 diabetes: The Honolulu-Asia aging study. Diabetes Care 2006, 29, 2268–2274. [Google Scholar] [CrossRef]
- Qiu, A.; Taylor, W.D.; Zhao, Z.; MacFall, J.R.; Miller, M.I.; Key, C.R.; Payne, M.E.; Steffens, D.C.; Krishnan, K.R. Apoe related hippocampal shape alteration in geriatric depression. Neuroimage 2009, 44, 620–626. [Google Scholar]
- Steffens, D.C.; McQuoid, D.R.; Payne, M.E.; Potter, G.G. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry 2011, 19, 4–12. [Google Scholar] [CrossRef]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar]
- Zhao, Z.; Taylor, W.D.; Styner, M.; Steffens, D.C.; Krishnan, K.R.; MacFall, J.R. Hippocampus shape analysis and late-life depression. PloSOne 2008, 3, e1837. [Google Scholar]
- Barnes, D.E.; Yaffe, K.; Byers, A.L.; McCormick, M.; Schaefer, C.; Whitmer, R.A. Midlife vs. late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Arch. Gen. Psychiatry 2012, 69, 493–498. [Google Scholar]
- Sachs-Erisson, N.; Sawyer, K.; Corsentino, E.; Collins, N.; Steffens, D.C. The moderating effect of the apoe small element of 4 allele on the relationship between hippocampal volume and cognitive decline in older depressed patients. Am. J. Geriatr. Psychiatry 2011, 19, 23–32. [Google Scholar]
- Steffens, D.C.; Payne, M.E.; Greenberg, D.L.; Byrum, C.E.; Welsh-Bohmer, K.A.; Wagner, H.R.; MacFall, J.R. Hippocampal volume and incident dementia in geriatric depression. Am. J. Geriatr. Psychiatry 2002, 10, 62–71. [Google Scholar]
- Ho, A.J.; Raji, C.A.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Lee, S.; Hibar, D.; Dinov, I.D.; Stein, J.L.; et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 2010, 31, 1326–1339. [Google Scholar]
- Jagust, W.; Harvey, D.; Mungas, D.; Haan, M. Central obesity and the aging brain. Arch. Neurol. 2005, 62, 1545–1548. [Google Scholar]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar]
- Taki, Y.; Kinomura, S.; Sato, K.; Inoue, K.; Goto, R.; Okada, K.; Uchida, S.; Kawashima, R.; Fukuda, H. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity 2008, 16, 119–124. [Google Scholar]
- Den Heijer, T.; Launer, L.J.; Prins, N.D.; van Dijk, E.J.; Vermeer, S.E.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005, 64, 263–267. [Google Scholar]
- Di Paola, M.; Caltagirone, C.; Fadda, L.; Sabatini, U.; Serra, L.; Carlesimo, G.A. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus 2008, 18, 719–728. [Google Scholar]
- Fotuhi, M.; Do, D.; Jack, C. Modifiable factors that alter the size of the hippocampus with ageing. Nat. Rev. Neurol. 2012, 8, 189–202. [Google Scholar]
- Fujioka, M.; Nishio, K.; Miyamoto, S.; Hiramatsu, K.I.; Sakaki, T.; Okuchi, K.; Taoka, T.; Fujioka, S. Hippocampal damage in the human brain after cardiac arrest. Cerebrovasc. Dis. 2000, 10, 2–7. [Google Scholar]
- Horstmann, A.; Frisch, S.; Jentzsch, R.T.; Muller, K.; Villringer, A.; Schroeter, M.L. Resuscitating the heart but losing the brain: Brain atrophy in the aftermath of cardiac arrest. Neurology 2010, 74, 306–312. [Google Scholar]
- Briones, T.L.; Woods, J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011, 12, 124. [Google Scholar]
- Winocur, G.; Henkelman, M.; Wojtowicz, J.M.; Zhang, H.; Binns, M.A.; Tannock, I.F. The effects of chemotherapy on cognitive function in a mouse model: A prospective study. Clin. Cancer Res. 2012, 18, 3112–3121. [Google Scholar] [CrossRef]
- Davis, J.C.; Marra, C.A.; Beattie, B.L.; Robertson, M.C.; Najafzadeh, M.; Graf, P.; Nagamatsu, L.S.; Liu-Ambrose, T. Sustained cognitive and economic benefits of resistance training among community-dwelling senior women: A 1-year follow-up study of the brain power study. Arch. Intern. Med. 2010, 170, 2036–2038. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E.; Cavanna, A.E. The cognitive impact of antiepileptic drugs. Ther. Adv. Neurol. Disord. 2011, 4, 385–407. [Google Scholar] [CrossRef]
- Fox, C.; Richardson, K.; Maidment, I.D.; Savva, G.M.; Matthews, F.E.; Smithard, D.; Coulton, S.; Katona, C.; Boustani, M.A.; Brayne, C. Anticholinergic medication use and cognitive impairment in the older population: The medical research council cognitive function and ageing study. J. Am. Geriatr. Soc. 2011, 59, 1477–1483. [Google Scholar] [CrossRef]
- Nebes, R.D.; Pollock, B.G.; Perera, S.; Halligan, E.M.; Saxton, J.A. The greater sensitivity of elderly APOE epsilon4 carriers to anticholinergic medications is independent of cerebrovascular disease risk. Am. J. Geriatr. Pharmacother. 2012, 10, 185–192. [Google Scholar]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-associated memory loss: Analysis of 60 case reports and review of the literature. Pharmacotherapy 2003, 23, 871–880. [Google Scholar]
- King, D.S.; Wilburn, A.J.; Wofford, M.R.; Harrell, T.K.; Lindley, B.J.; Jones, D.W. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy 2003, 23, 1663–1667. [Google Scholar]
- Muldoon, M.F.; Barger, S.D.; Ryan, C.M.; Flory, J.D.; Lehoczky, J.P.; Matthews, K.A.; Manuck, S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000, 108, 538–546. [Google Scholar]
- Muldoon, M.F.; Ryan, C.M.; Sereika, S.M.; Flory, J.D.; Manuck, S.B. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am. J. Med. 2004, 117, 823–829. [Google Scholar]
- Ancelin, M.L.; Carriere, I.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Fourlanos, S.; Berr, C.; Dupuy, A.M.; Ritchie, K. Lipid lowering agents, cognitive decline, and dementia: The three-city study. J. Alzheimers Dis. 2012, 30, 629–637. [Google Scholar]
- Bettermann, K.; Arnold, A.M.; Williamson, J.; Rapp, S.; Sink, K.; Toole, J.F.; Carlson, M.C.; Yasar, S.; Dekosky, S.; Burke, G.L. Statins, risk of dementia, and cognitive function: Secondary analysis of the ginkgo evaluation of memory study. J. Stroke Cerebrovasc. Dis. 2011, 21, 436–444. [Google Scholar]
- Levine, D.A.; Langa, K.M. Vascular cognitive impairment: Disease mechanisms and therapeutic implications. Neurotherapeutics 2011, 8, 361–373. [Google Scholar]
- Tendolkar, I.; Enajat, M.; Zwiers, M.P.; van Wingen, G.; de Leeuw, F.E.; van Kuilenburg, J.; Bouwels, L.; Pop, G.; Pop-Purceleanu, M. One-year cholesterol lowering treatment reduces medial temporal lobe atrophy and memory decline in stroke-free elderly with atrial fibrillation: Evidence from a parallel group randomized trial. Int. J. Geriatr. Psychiatry 2012, 27, 49–58. [Google Scholar] [CrossRef]
- Carlsson, C.M.; Xu, G.; Wen, Z.; Barnet, J.H.; Blazel, H.M.; Chappell, R.J.; Stein, J.H.; Asthana, S.; Sager, M.A.; Alsop, D.C.; et al. Effects of atorvastatin on cerebral blood flow in middle-aged adults at risk for Alzheimer’s disease: A pilot study. Curr. Alzheimer Res. 2011, 9, 990–997. [Google Scholar]
- Kurata, T.; Miyazaki, K.; Kozuki, M.; Morimoto, N.; Ohta, Y.; Ikeda, Y.; Abe, K. Progressive neurovascular disturbances in the cerebral cortex of Alzheimer’s disease-model mice: Protection by atorvastatin and pitavastatin. Neuroscience 2011, 197, 358–368. [Google Scholar] [CrossRef]
- Tong, X.K.; Lecrux, C.; Hamel, E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J. Neurosci. 2012, 32, 4705–4715. [Google Scholar] [CrossRef]
- Longenberger, J.; Shah, Z.A. Simvastatin and other hmg-coareductase inhibitors on brain cholesterol levels in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 434–442. [Google Scholar]
- Pac-Soo, C.; Lloyd, D.G.; Vizcaychipi, M.P.; Ma, D. Statins: The role in the treatment and prevention of Alzheimer’s neurodegeneration. J. Alzheimers Dis. 2011, 27, 1–10. [Google Scholar]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch. Neurol. 2011, 68, 1385–1392. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gregory, S.M.; Parker, B.; Thompson, P.D. Physical Activity, Cognitive Function, and Brain Health: What Is the Role of Exercise Training in the Prevention of Dementia? Brain Sci. 2012, 2, 684-708. https://doi.org/10.3390/brainsci2040684
Gregory SM, Parker B, Thompson PD. Physical Activity, Cognitive Function, and Brain Health: What Is the Role of Exercise Training in the Prevention of Dementia? Brain Sciences. 2012; 2(4):684-708. https://doi.org/10.3390/brainsci2040684
Chicago/Turabian StyleGregory, Sara M., Beth Parker, and Paul D. Thompson. 2012. "Physical Activity, Cognitive Function, and Brain Health: What Is the Role of Exercise Training in the Prevention of Dementia?" Brain Sciences 2, no. 4: 684-708. https://doi.org/10.3390/brainsci2040684




