Mechanisms Underlying Auditory Hallucinations—Understanding Perception without Stimulus
Abstract
:1. Introduction: An Overview of Auditory Verbal Hallucinations
1.1. Phenomenology and Epidemiology
1.2. AVH as a Continuum Phenomenon?
2. The Neurocognitive Models
2.1. The Feed-Forward Model
2.2. The Aberrant Memory Model
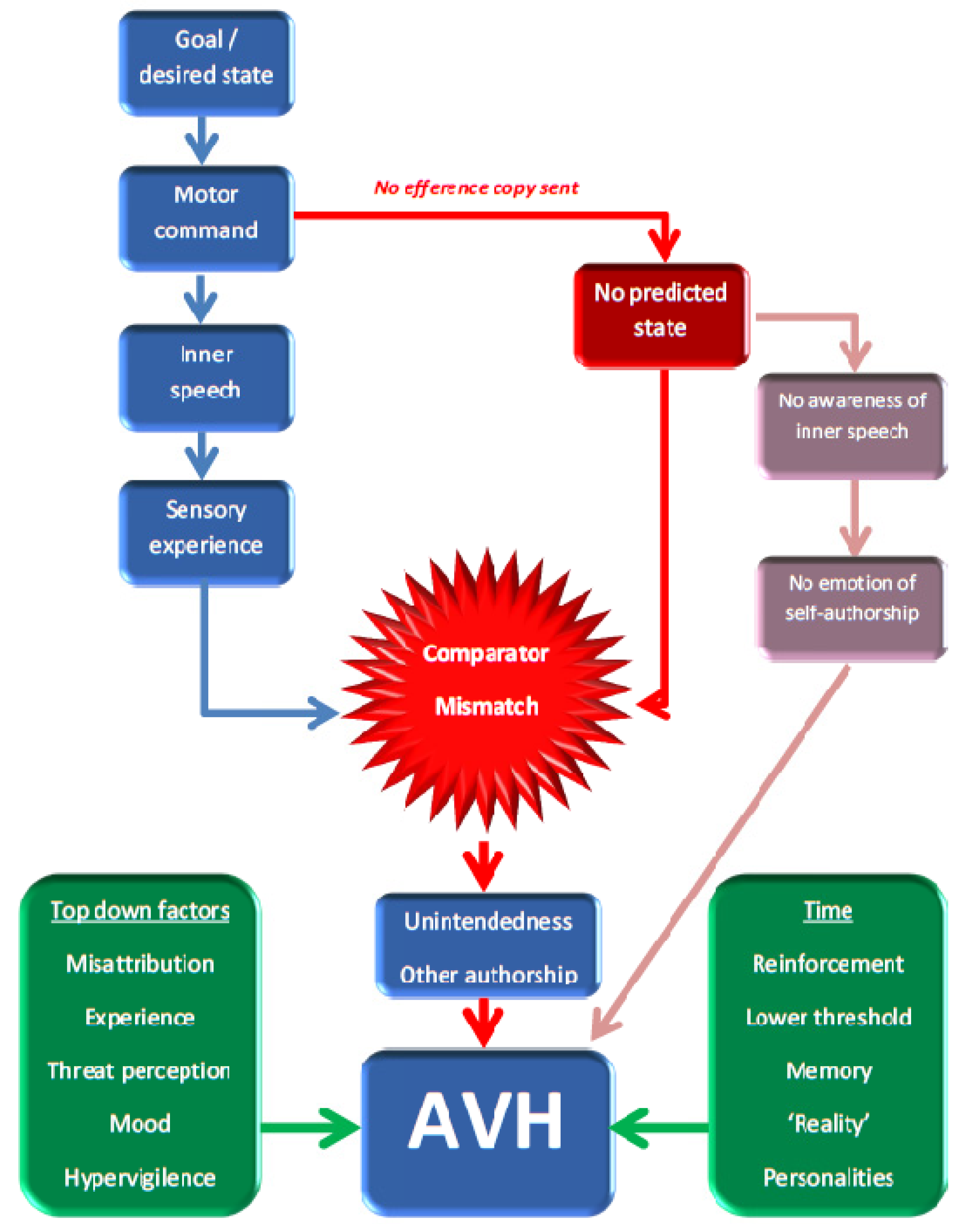

2.3. Critiquing the Neurocognitive Models
3. Imaging Data
3.1. Initial Imaging Data and Structural Scans
3.2. fMRI: Building up a Network of Involved Regions
3.3. State and Trait Analysis
3.4. Non-Schizophrenia Populations
3.5. Challenges in Functional Imaging Studies
4. Dysconnectivity as the Common Mechanism? Joining the Cognitive Model and the Imaging
4.1. Normal Connectivity: Intrinsic and Extrinsic Networks
4.2. Connectivity Analysis in Schizophrenia
4.3. Dysconnectivity and AVH
4.4. Transcranial Magnetic Stimulation: Altering Connectivity?
4.5. Challenges for Connectivity Studies
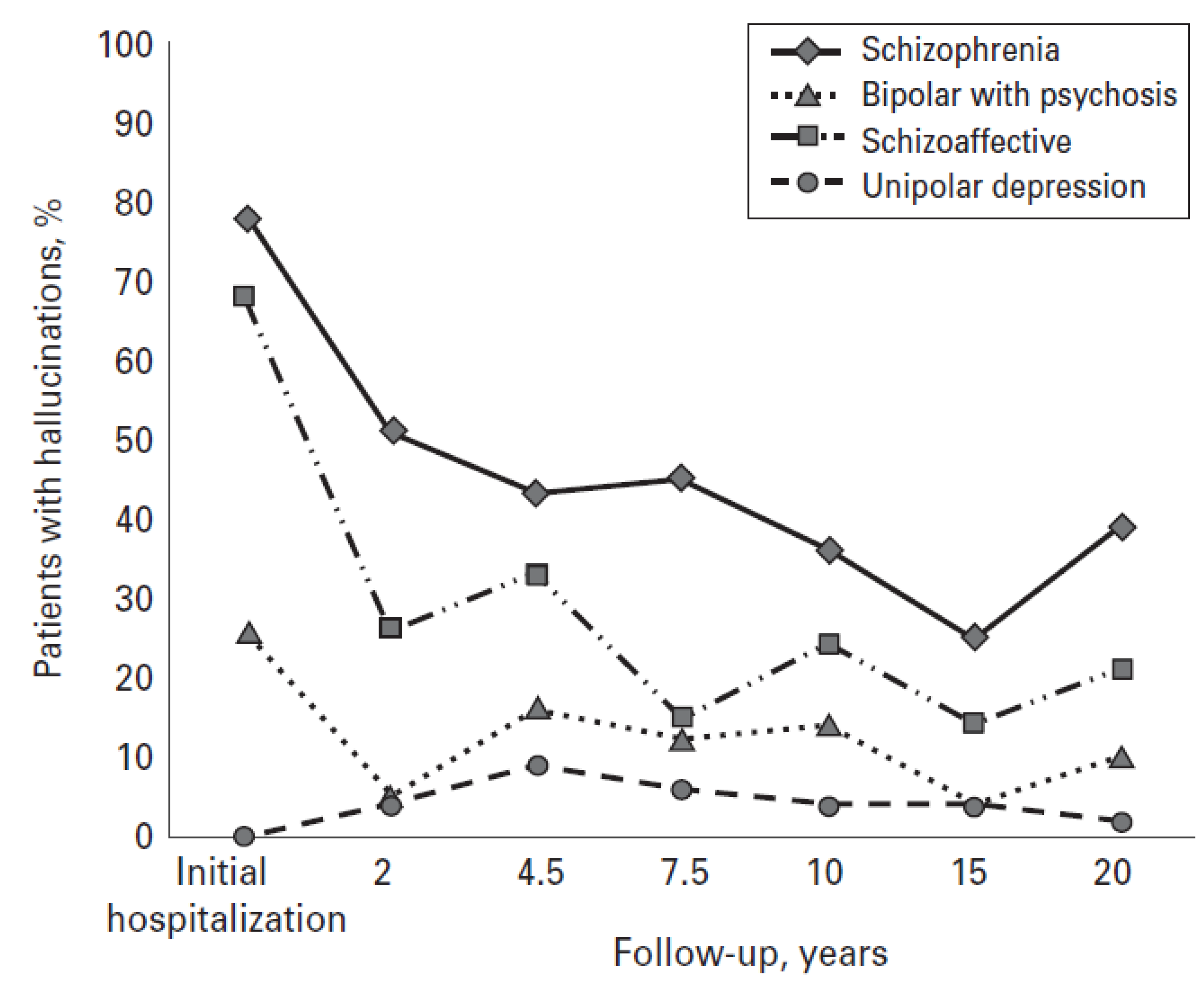
5. Outcomes in Clinical AVH
6. Conclusions
Conflict of Interest
Acknowledgements
References
- Sartorius, N.; Jablensky, A.; Korten, A.; Ernberg, G.; Anker, M.; Cooper, J.E.; Day, R. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the who collaborative study on determinants of outcome of severe mental disorders. Psychol. Med. 1986, 16, 909–928. [Google Scholar] [CrossRef]
- Shergill, S.S.; Murray, R.M.; McGuire, P.K. Auditory hallucinations: A review of psychological treatments. Schizophr. Res. 1998, 32, 137–150. [Google Scholar] [CrossRef]
- Slotema, C.W.; Daalman, K.; Blom, J.D.; Diederen, K.M.; Hoek, H.W.; Sommer, I.E. Auditory verbal hallucinations in patients with borderline personality disorder are similar to those in schizophrenia. Psychol. Med. 2012, 42, 1873–1878. [Google Scholar] [CrossRef]
- Hauf, M.; Wiest, R.; Schindler, K.; Jann, K.; Dierks, T.; Strik, W.; Schroth, G.; Hubl, D. Common mechanisms of auditory hallucinations-perfusion studies in epilepsy. Psychiatry Res. 2012, 211, 268–270. [Google Scholar]
- Andreasen, N.C.; Flaum, M. Schizophrenia: The characteristic symptoms. Schizophr. Bull. 1991, 17, 27–49. [Google Scholar] [CrossRef]
- Laroi, F.; Sommer, I.E.; Blom, J.D.; Fernyhough, C.; Ffytche, D.H.; Hugdahl, K.; Johns, L.C.; McCarthy-Jones, S.; Preti, A.; Raballo, A.; et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: State-of-the-art overview and future directions. Schizophr. Bull. 2012, 38, 724–733. [Google Scholar] [CrossRef]
- Nayani, T.H.; David, A.S. The auditory hallucination: A phenomenological survey. Psychol. Med. 1996, 26, 177–189. [Google Scholar] [CrossRef]
- Badcock, J.C.; Hugdahl, K. Cognitive mechanisms of auditory verbal hallucinations in psychotic and non-psychotic groups. Neurosci. Biobehav. Rev. 2012, 36, 431–438. [Google Scholar] [CrossRef]
- Beavan, V.; Read, J.; Cartwright, C. The prevalence of voice-hearers in the general population: A literature review. J. Ment. Health 2011, 20, 281–292. [Google Scholar]
- Smeets, F.; Lataster, T.; van Winkel, R.; de Graaf, R.; Ten Have, M.; van Os, J. Testing the hypothesis that psychotic illness begins when subthreshold hallucinations combine with delusional ideation. Acta Psychiatr. Scand. 2013, 127, 34–47. [Google Scholar] [CrossRef]
- Sommer, I.E.; Daalman, K.; Rietkerk, T.; Diederen, K.M.; Bakker, S.; Wijkstra, J.; Boks, M.P. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr. Bull. 2010, 36, 633–641. [Google Scholar] [CrossRef]
- Birchwood, M.; Meaden, A.; Trower, P.; Gilbert, P.; Plaistow, J. The power and omnipotence of voices: Subordination and entrapment by voices and significant others. Psychol. Med. 2000, 30, 337–344. [Google Scholar] [CrossRef]
- Johns, L.C.; Hemsley, D.; Kuipers, E. A comparison of auditory hallucinations in a psychiatric and non-psychiatric group. Br. J. Clin. Psychol. 2002, 41, 81–86. [Google Scholar] [CrossRef]
- Falkenburg, J.; Tracy, D.K. Sex and schizophrenia: A review of gender differences. Psychos. Psychol. Soc. Integr. Approaches 2012. [Google Scholar] [CrossRef]
- Johns, L.C.; Nazroo, J.Y.; Bebbington, P.; Kuipers, E. Occurrence of hallucinatory experiences in a community sample and ethnic variations. Br. J. Psychiatry 2002, 180, 174–178. [Google Scholar] [CrossRef]
- Murphy, J.; Shevlin, M.; Houston, J.; Adamson, G. A population based analysis of subclinical psychosis and help-seeking behavior. Schizophr. Bull. 2012, 38, 360–367. [Google Scholar] [CrossRef]
- Van Os, J.; Linscott, R.J.; Myin-Germeys, I.; Delespaul, P.; Krabbendam, L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 2009, 39, 179–195. [Google Scholar] [CrossRef]
- Daalman, K.; Boks, M.P.; Diederen, K.M.; de Weijer, A.D.; Blom, J.D.; Kahn, R.S.; Sommer, I.E. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J. Clin. Psychiatry 2011, 72, 320–325. [Google Scholar] [CrossRef]
- van der Meer, L.; van’t Wout, M.; Aleman, A. Emotion regulation strategies in patients with schizophrenia. Psychiatry Res. 2009, 170, 108–113. [Google Scholar] [CrossRef]
- Fett, A.K.; Shergill, S.S.; Joyce, D.W.; Riedl, A.; Strobel, M.; Gromann, P.M.; Krabbendam, L. To trust or not to trust: The dynamics of social interaction in psychosis. Brain 2012, 135, 976–984. [Google Scholar]
- Weiser, M.; van Os, J.; Davidson, M. Time for a shift in focus in schizophrenia: From narrow phenotypes to broad endophenotypes. Br. J. Psychiatry 2005, 187, 203–205. [Google Scholar] [CrossRef]
- Cardno, A.G.; Rijsdijk, F.V.; Sham, P.C.; Murray, R.M.; McGuffin, P. A twin study of genetic relationships between psychotic symptoms. Am. J. Psychiatry 2002, 159, 539–545. [Google Scholar] [CrossRef]
- Lewis, C.M.; Levinson, D.F.; Wise, L.H.; DeLisi, L.E.; Straub, R.E.; Hovatta, I.; Williams, N.M.; Schwab, S.G.; Pulver, A.E.; Faraone, S.V.; et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am. J. Hum. Genet. 2003, 73, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef]
- Maher, B.A. The relationship between delusions and hallucinations. Curr. Psychiatry Rep. 2006, 8, 179–183. [Google Scholar] [CrossRef]
- Bentall, R.P.; Fernyhough, C. Social predictors of psychotic experiences: Specificity and psychological mechanisms. Schizophr. Bull. 2008, 34, 1012–1020. [Google Scholar] [CrossRef]
- Freeman, D. Suspicious minds: The psychology of persecutory delusions. Clin. Psychol. Rev. 2007, 27, 425–457. [Google Scholar] [CrossRef]
- Varese, F.; Bentall, R.P. The metacognitive beliefs account of hallucinatory experiences: A literature review and meta-analysis. Clin. Psychol. Rev 2011, 31, 850–864. [Google Scholar] [CrossRef]
- Joyce, D.W.; Averbeck, B.B.; Frith, C.D.; Shergill, S.S. Examining belief and confidence in schizophrenia. Psychol. Med. 2013. [Google Scholar] [CrossRef]
- Boks, M.P.; Leask, S.; Vermunt, J.K.; Kahn, R.S. The structure of psychosis revisited: The role of mood symptoms. Schizophr. Res. 2007, 93, 178–185. [Google Scholar] [CrossRef]
- van Rossum, I.; Dominguez, M.D.; Lieb, R.; Wittchen, H.U.; van Os, J. Affective dysregulation and reality distortion: A 10-year prospective study of their association and clinical relevance. Schizophr. Bull. 2011, 37, 561–571. [Google Scholar] [CrossRef]
- Varghese, D.; Scott, J.; Welham, J.; Bor, W.; Najman, J.; O’Callaghan, M.; Williams, G.; McGrath, J. Psychotic-like experiences in major depression and anxiety disorders: A population-based survey in young adults. Schizophr. Bull. 2011, 37, 389–393. [Google Scholar] [CrossRef]
- Freeman, D.; Garety, P. Connecting neurosis and psychosis: The direct influence of emotion on delusions and hallucinations. Behav. Res. Ther. 2002, 41, 923–927. [Google Scholar] [CrossRef]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef]
- Fowler, D.; Hodgekins, J.; Garety, P.; Freeman, D.; Kuipers, E.; Dunn, G.; Smith, B.; Bebbington, P.E. Negative cognition, depressed mood, and paranoia: A longitudinal pathway analysis using structural equation modeling. Schizophr. Bull. 2012, 38, 1063–1073. [Google Scholar] [CrossRef]
- Wigman, J.T.; van Nierop, M.; Vollebergh, W.A.; Lieb, R.; Beesdo-Baum, K.; Wittchen, H.U.; van Os, J. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity—implications for diagnosis and ultra-high risk research. Schizophr. Bull. 2012, 38, 247–257. [Google Scholar] [CrossRef]
- Olfson, M.; Lewis-Fernandez, R.; Weissman, M.M.; Feder, A.; Gameroff, M.J.; Pilowsky, D.; Fuentes, M. Psychotic symptoms in an urban general medicine practice. Am. J. Psychiatry 2002, 159, 1412–1419. [Google Scholar] [CrossRef]
- Cassano, P.; Chang, T.; Trinh, N.H.; Baer, L.; Fava, M.; Mischoulon, D. Differential impact of isolated psychotic symptoms on treatment outcome of major depressive disorder in the STAR*D cohort of Whites, Blacks and Latinos. J. Affect. Disord. 2013. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar]
- Frith, C.D.; Blakemore, S.; Wolpert, D.M. Explaining the symptoms of schizophrenia: Abnormalities in the awareness of action. Brain Res. Brain Res. Rev. 2000, 31, 357–363. [Google Scholar] [CrossRef]
- Blakemore, S.J. Deluding the motor system. Conscious. Cogn. 2003, 12, 647–655. [Google Scholar] [CrossRef]
- Shergill, S.S.; White, T.; Joyce, D.; Bays, P.M.; Wolpert, D.M.; Frith, C. Modulation of somatosensory processing by action. NeuroImage 2013, 70, 356–362. [Google Scholar] [CrossRef]
- Laroi, F. How do auditory verbal hallucinations in patients differ from those in non-patients? Front. Hum. Neurosci. 2012, 6, 25. [Google Scholar] [CrossRef]
- Shergill, S.S.; Samson, G.; Bays, P.M.; Frith, C.D.; Wolpert, D.M. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry 2005, 162, 2384–2386. [Google Scholar] [CrossRef]
- Waters, F.; Woodward, T.; Allen, P.; Aleman, A.; Sommer, I. Self-recognition deficits in schizophrenia patients with auditory hallucinations: A meta-analysis of the literature. Schizophr. Bull. 2012, 38, 741–750. [Google Scholar] [CrossRef]
- Rains, L.S.; Fallica, G.; O’Daly, O.; Gilleen, J.; Giampetro, V.; Morley, L.; Shergill, S. Exploring psychotic symptoms: A comparison of motor related neuronal activation during and after acute psychosis. BMC Psychiatry 2012, 12, 102. [Google Scholar] [CrossRef]
- Frith, C. The self in action: Lessons from delusions of control. Conscious. Cogn. 2005, 14, 752–770. [Google Scholar] [CrossRef]
- Seal, M.L.; Aleman, A.; McGuire, P.K. Compelling imagery, unanticipated speech and deceptive memory: Neurocognitive models of auditory verbal hallucinations in schizophrenia. Cogn. Neuropsychiatry 2004, 9, 43–72. [Google Scholar] [CrossRef]
- Shergill, S.S.; Brammer, M.J.; Williams, S.C.; Murray, R.M.; McGuire, P.K. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch. Gen. Psychiatry 2000, 57, 1033–1038. [Google Scholar] [CrossRef]
- Shergill, S.S.; Bullmore, E.T.; Brammer, M.J.; Williams, S.C.; Murray, R.M.; McGuire, P.K. A functional study of auditory verbal imagery. Psychol. Med. 2001, 31, 241–253. [Google Scholar]
- Aleman, A.; Bocker, K.B.; Hijman, R.; de Haan, E.H.; Kahn, R.S. Cognitive basis of hallucinations in schizophrenia: Role of top-down information processing. Schizophr. Res. 2003, 64, 175–185. [Google Scholar] [CrossRef]
- Jones, S.R.; Fernyhough, C. Thought as action: Inner speech, self-monitoring, and auditory verbal hallucinations. Conscious. Cogn. 2007, 16, 391–399. [Google Scholar] [CrossRef]
- Dodgson, G.; Gordon, S. Avoiding false negatives: Are some auditory hallucinations an evolved design flaw? Behav. Cogn. Psychother. 2009, 37, 325–334. [Google Scholar] [CrossRef]
- Fett, A.K.; Gromann, P.M.; Giampietro, V.; Shergill, S.S.; Krabbendam, L. Default distrust? An fMRI investigation of the neural development of trust and cooperation. Soc. Cogn. Affect. Neurosci. 2012. [Google Scholar] [CrossRef]
- Waters, F.; Allen, P.; Aleman, A.; Fernyhough, C.; Woodward, T.S.; Badcock, J.C.; Barkus, E.; Johns, L.; Varese, F.; Menon, M.; et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: A review and integrated model of cognitive mechanisms. Schizophr. Bull. 2012, 38, 683–693. [Google Scholar] [CrossRef]
- Lataster, T.; van Os, J.; Drukker, M.; Henquet, C.; Feron, F.; Gunther, N.; Myin-Germeys, I. Childhood victimisation and developmental expression of non-clinical delusional ideation and hallucinatory experiences: Victimisation and non-clinical psychotic experiences. Soc. Psychiatry Psychiatr. Epidemiol. 2006, 41, 423–428. [Google Scholar] [CrossRef]
- Read, J.; van Os, J.; Morrison, A.P.; Ross, C.A. Childhood trauma, psychosis and schizophrenia: A literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005, 112, 330–350. [Google Scholar] [CrossRef]
- Waters, F.A.; Badcock, J.C.; Michie, P.T.; Maybery, M.T. Auditory hallucinations in schizophrenia: Intrusive thoughts and forgotten memories. Cogn. Neuropsychiatry 2006, 11, 65–83. [Google Scholar] [CrossRef]
- Wible, C.G.; Lee, K.; Molina, I.; Hashimoto, R.; Preus, A.P.; Roach, B.J.; Ford, J.M.; Mathalon, D.H.; McCarthey, G.; Turner, J.A.; et al. fMRI activity correlated with auditory hallucinations during performance of a working memory task: Data from the FBIRN consortium study. Schizophr. Bull. 2009, 35, 47–57. [Google Scholar] [CrossRef]
- Brebion, G.; David, A.S.; Jones, H.M.; Ohlsen, R.; Pilowsky, L.S. Temporal context discrimination in patients with schizophrenia: Associations with auditory hallucinations and negative symptoms. Neuropsychologia 2007, 45, 817–823. [Google Scholar] [CrossRef]
- Brebion, G.; Gorman, J.M.; Amador, X.; Malaspina, D.; Sharif, Z. Source monitoring impairments in schizophrenia: Characterisation and associations with positive and negative symptomatology. Psychiatry Res. 2002, 112, 27–39. [Google Scholar] [CrossRef]
- Krishnan, R.R.; Keefe, R.; Kraus, M. Schizophrenia is a disorder of higher order hierarchical processing. Med. Hypotheses 2009, 72, 740–744. [Google Scholar] [CrossRef]
- Laruelle, M.; Abi-Dargham, A. Dopamine as the wind of the psychotic fire: New evidence from brain imaging studies. J. Psychopharmacol. 1999, 13, 358–371. [Google Scholar] [CrossRef]
- Levey, A.I.; Hersch, S.M.; Rye, D.B.; Sunahara, R.K.; Niznik, H.B.; Kitt, C.A.; Price, D.L.; Maggio, R.; Brann, M.R.; Ciliax, B.J. Localization of d1 and d2 dopamine receptors in brain with subtype-specific antibodies. Proc. Natl. Acad. Sci. USA 1993, 90, 8861–8865. [Google Scholar] [CrossRef]
- Sendt, K.V.; Giaroli, G.; Tracy, D.K. Beyond dopamine: Glutamate as a target for future antipsychotics. ISRN Pharmacol. 2012, 2012, 427267. [Google Scholar]
- Laroi, F.; Woodward, T.S. Hallucinations from a cognitive perspective. Harv. Rev. Psychiatry 2007, 15, 109–117. [Google Scholar] [CrossRef]
- O’Daly, O.G.; Joyce, D.; Stephan, K.E.; Murray, R.M.; Shergill, S.S. Functional magnetic resonance imaging investigation of the amphetamine sensitization model of schizophrenia in healthy male volunteers. Arch. Gen. Psychiatry 2011, 68, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, S. Neurocognitive models of schizophrenia: A neurophenomenological critique. Psychopathology 2004, 37, 8–19. [Google Scholar] [CrossRef]
- Tracy, D.K.; Ho, D.K.; O’Daly, O.; Michalopoulou, P.; Lloyd, L.C.; Dimond, E.; Matsumoto, K.; Shergill, S.S. It’s not what you say but the way that you say it: An fMRI study of differential lexical and non-lexical prosodic pitch processing. BMC Neurosci. 2011, 12, 128. [Google Scholar] [CrossRef]
- Matsumoto, K.; Samson, G.T.; O’Daly, O.D.; Tracy, D.K.; Patel, A.D.; Shergill, S.S. Prosodic discrimination in patients with schizophrenia. Br. J. Psychiatry 2006, 189, 180–181. [Google Scholar] [CrossRef]
- Hugdahl, K. “Hearing voices”: Auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand. J. Psychol. 2009, 50, 553–560. [Google Scholar] [CrossRef]
- Allen, P.P.; Johns, L.C.; Fu, C.H.; Broome, M.R.; Vythelingum, G.N.; McGuire, P.K. Misattribution of external speech in patients with hallucinations and delusions. Schizophr. Res. 2004, 69, 277–287. [Google Scholar] [CrossRef]
- Matsuda, H.; Gyobu, T.; Masayasu, I.; Hisada, K. Increased accumulation of n-isopropyl-(i-123) p-iodoamphetamine in the left auditory area in a schizophrenic patient with auditory hallucinations. Clin. Nuclear Med. 1988, 13, 53–55. [Google Scholar] [CrossRef]
- Silbersweig, D.A.; Stern, E.; Frith, C.; Cahill, C.; Holmes, A.; Grootoonk, S.; Seaward, J.; McKenna, P.; Chua, S.E.; Schnorr, L.; et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995, 378, 176–179. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wolf, A.P.; Van Gelder, P.; Brodie, J.D.; Overall, J.E.; Cancro, R.; Gomez-Mont, F. Phenomenological correlates of metabolic activity in 18 patients with chronic schizophrenia. Am. J. Psychiatry 1987, 144, 151–158. [Google Scholar]
- O’Daly, O.G.; Frangou, S.; Chitnis, X.; Shergill, S.S. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. 2007, 156, 15–21. [Google Scholar] [CrossRef]
- Modinos, G.; Costafreda, S.G.; van Tol, M.J.; McGuire, P.K.; Aleman, A.; Allen, P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex 2012, 49, 1046–1055. [Google Scholar]
- Shergill, S.S.; Kanaan, R.A.; Chitnis, X.A.; O’Daly, O.; Jones, D.K.; Frangou, S.; Williams, S.C.; Howard, R.J.; Barker, G.J.; Murray, R.M.; et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry 2007, 164, 467–473. [Google Scholar] [CrossRef]
- Dierks, T.; Linden, D.E.; Jandl, M.; Formisano, E.; Goebel, R.; Lanfermann, H.; Singer, W. Activation of Heschl’s gyrus during auditory hallucinations. Neuron 1999, 22, 615–621. [Google Scholar] [CrossRef]
- Lennox, B.R.; Park, S.B.; Medley, I.; Morris, P.G.; Jones, P.B. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000, 100, 13–20. [Google Scholar] [CrossRef]
- McGuire, P.K.; Shah, G.M.; Murray, R.M. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet 1993, 342, 703–706. [Google Scholar] [CrossRef]
- Raij, T.T.; Valkonen-Korhonen, M.; Holi, M.; Therman, S.; Lehtonen, J.; Hari, R. Reality of auditory verbal hallucinations. Brain 2009, 132, 2994–3001. [Google Scholar] [CrossRef]
- Sommer, I.E.; Diederen, K.M.; Blom, J.D.; Willems, A.; Kushan, L.; Slotema, K.; Boks, M.P.; Daalman, K.; Hoek, H.W.; Neggers, S.F.; et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain 2008, 131, 3169–3177. [Google Scholar] [CrossRef]
- Copolov, D.L.; Seal, M.L.; Maruff, P.; Ulusoy, R.; Wong, M.T.; Tochon-Danguy, H.J.; Egan, G.F. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: A pet correlation study. Psychiatry Res. 2003, 122, 139–152. [Google Scholar] [CrossRef]
- Woodruff, P.; Brammer, M.; Mellers, J.; Wright, I.; Bullmore, E.; Williams, S. Auditory hallucinations and perception of external speech. Lancet 1995, 346, 1035. [Google Scholar] [CrossRef]
- Jardri, R.; Pouchet, A.; Pins, D.; Thomas, P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. Am. J. Psychiatry 2011, 168, 73–81. [Google Scholar] [CrossRef]
- Kuhn, S.; Gallinat, J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2012, 38, 779–786. [Google Scholar] [CrossRef]
- Allen, P.; Modinos, G.; Hubl, D.; Shields, G.; Cachia, A.; Jardri, R.; Thomas, P.; Woodward, T.; Shotbolt, P.; Plaze, M.; et al. Neuroimaging auditory hallucinations in schizophrenia: From neuroanatomy to neurochemistry and beyond. Schizophr. Bull. 2012, 38, 695–703. [Google Scholar] [CrossRef]
- Linden, D.E.; Thornton, K.; Kuswanto, C.N.; Johnston, S.J.; van de Ven, V.; Jackson, M.C. The brain’s voices: Comparing nonclinical auditory hallucinations and imagery. Cereb Cortex 2011, 21, 330–337. [Google Scholar] [CrossRef]
- Diederen, K.M.; Daalman, K.; de Weijer, A.D.; Neggers, S.F.; van Gastel, W.; Blom, J.D.; Kahn, R.S.; Sommer, I.E. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr. Bull. 2012, 38, 1074–1082. [Google Scholar] [CrossRef]
- Diederen, K.M.; Neggers, S.F.; de Weijer, A.D.; van Lutterveld, R.; Daalman, K.; Eickhoff, S.B.; Clos, M.; Kahn, R.S.; Sommer, I.E. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 2012. [Google Scholar] [CrossRef]
- Fornito, A.; Harrison, B.J. Brain connectivity and mental illness. Front. Psychiatry 2012, 3, 72. [Google Scholar]
- Vul, E.; Pashler, H. Voodoo and circularity errors. NeuroImage 2012, 62, 945–948. [Google Scholar] [CrossRef]
- Kapur, S.; Phillips, A.G.; Insel, T.R. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol. Psychiatry 2012, 17, 1174–1179. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Vertes, P.E.; Alexander-Bloch, A.F.; Gogtay, N.; Giedd, J.N.; Rapoport, J.L.; Bullmore, E.T. Simple models of human brain functional networks. Proc. Natl. Acad. Sci. USA 2012, 109, 5868–5873. [Google Scholar]
- Behrens, T.E.; Sporns, O. Human connectomics. Curr. Opin. Neurobiol. 2012, 22, 144–153. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Doucet, G.; Naveau, M.; Petit, L.; Zago, L.; Crivello, F.; Jobard, G.; Delcroix, N.; Mellet, E.; Tzourio-Mazoyer, N.; Mazoyer, B.; et al. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage 2012, 59, 3194–3200. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar]
- Kane, M.J.; Brown, L.H.; McVay, J.C.; Silvia, P.J.; Myin-Germeys, I.; Kwapil, T.R. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychol. Sci. 2007, 18, 614–621. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 2011, 53, 303–317. [Google Scholar]
- Sepulcre, J.; Sabuncu, M.R.; Johnson, K.A. Network assemblies in the functional brain. Curr. Opin. Neurol. 2012, 25, 384–391. [Google Scholar]
- Doucet, G.; Naveau, M.; Petit, L.; Delcroix, N.; Zago, L.; Crivello, F.; Jobard, G.; Tzourio-Mazoyer, N.; Mazoyer, B.; Mellet, E.; et al. Brain activity at rest: A multiscale hierarchical functional organization. J. Neurophysiol. 2011, 105, 2753–2763. [Google Scholar] [CrossRef]
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Pantelis, C.; Bullmore, E.T. Schizophrenia, neuroimaging and connectomics. NeuroImage 2012, 62, 2296–2314. [Google Scholar] [CrossRef]
- Hoffman, R.E.; Hampson, M. Functional connectivity studies of patients with auditory verbal hallucinations. Front. Hum. Neurosci. 2012, 6, 6. [Google Scholar]
- Pettersson-Yeo, W.; Allen, P.; Benetti, S.; McGuire, P.; Mechelli, A. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011, 35, 1110–1124. [Google Scholar] [CrossRef]
- Uhlhaas, P.J. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013, 23, 283–290. [Google Scholar] [CrossRef]
- Williamson, P.C.; Allman, J.M. A framework for interpreting functional networks in schizophrenia. Front. Hum. Neurosci. 2012, 6, 184. [Google Scholar]
- Palaniyappan, L.; Liddle, P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012, 37, 17–27. [Google Scholar]
- Michalopoulou, P.G.; Giampietro, V.P.; Morley, L.A.; Azim, A.; Kapur, S.; Lykouras, L.; Shergill, S.S. The effects of reality distortion syndrome on salient stimuli processing in patients with schizophrenia: An fMRI study. Psychiatry Res. 2010, 183, 93–98. [Google Scholar] [CrossRef]
- Shergill, S.S.; Brammer, M.J.; Amaro, E.; Williams, S.C.; Murray, R.M.; McGuire, P.K. Temporal course of auditory hallucinations. Br. J. Psychiatry 2004, 185, 516–517. [Google Scholar] [CrossRef]
- Diederen, K.M.; Neggers, S.F.; Daalman, K.; Blom, J.D.; Goekoop, R.; Kahn, R.S.; Sommer, I.E. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry 2010, 167, 427–435. [Google Scholar] [CrossRef]
- Hoffman, R.E.; Pittman, B.; Constable, R.T.; Bhagwagar, Z.; Hampson, M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry 2011, 198, 277–283. [Google Scholar] [CrossRef]
- Vercammen, A.; Knegtering, H.; den Boer, J.A.; Liemburg, E.J.; Aleman, A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry 2010, 67, 912–918. [Google Scholar] [CrossRef]
- Simons, C.J.; Tracy, D.K.; Sanghera, K.K.; O’Daly, O.; Gilleen, J.; Dominguez, M.D.; Krabbendam, L.; Shergill, S.S. Functional magnetic resonance imaging of inner speech in schizophrenia. Biol. Psychiatry 2010, 67, 232–237. [Google Scholar] [CrossRef]
- Giraud, A.L.; Kell, C.; Thierfelder, C.; Sterzer, P.; Russ, M.O.; Preibisch, C.; Kleinschmidt, A. Contributions of sensory input, auditory search and verbal comprehension to cortical activity during speech processing. Cereb. Cortex 2004, 14, 247–255. [Google Scholar]
- Hoffman, R.E.; Fernandez, T.; Pittman, B.; Hampson, M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol. Psychiatry 2011, 69, 407–414. [Google Scholar] [CrossRef]
- Hoffman, R.E.; Boutros, N.N.; Berman, R.M.; Roessler, E.; Belger, A.; Krystal, J.H.; Charney, D.S. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol. Psychiatry 1999, 46, 130–132. [Google Scholar] [CrossRef]
- Tracy, D.K.; O’Daly, O.; Joyce, D.W.; Michalopoulou, P.G.; Basit, B.B.; Dhillon, G.; McLoughlin, D.M.; Shergill, S.S. An evoked auditory response fMRI study of the effects of rTMS on putative avh pathways in healthy volunteers. Neuropsychologia 2010, 48, 270–277. [Google Scholar] [CrossRef]
- Gromann, P.M.; Tracy, D.K.; Giampietro, V.; Brammer, M.J.; Krabbendam, L.; Shergill, S.S. Examining frontotemporal connectivity and rTMS in healthy controls: Implications for auditory hallucinations in schizophrenia. Neuropsychology 2012, 26, 127–132. [Google Scholar] [CrossRef]
- Slotema, C.W.; Aleman, A.; Daskalakis, Z.J.; Sommer, I.E. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: Update and effects after one month. Schizophr. Res. 2012, 142, 40–45. [Google Scholar] [CrossRef]
- Aleman, A.; Sommer, I.E.; Kahn, R.S. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: A meta-analysis. J. Clin. Psychiatry 2007, 68, 416–421. [Google Scholar] [CrossRef]
- Freitas, C.; Fregni, F.; Pascual-Leone, A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr. Res. 2009, 108, 11–24. [Google Scholar] [CrossRef]
- Slotema, C.W.; Blom, J.D.; Hoek, H.W.; Sommer, I.E. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry 2010, 71, 873–884. [Google Scholar]
- Tranulis, C.; Sepehry, A.A.; Galinowski, A.; Stip, E. Should we treat auditory hallucinations with repetitive transcranial magnetic stimulation? A metaanalysis. Can. J. Psychiatry 2008, 53, 577–586. [Google Scholar]
- Bassett, D.S.; Bullmore, E.; Verchinski, B.A.; Mattay, V.S.; Weinberger, D.R.; Meyer-Lindenberg, A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008, 28, 9239–9248. [Google Scholar] [CrossRef]
- Lang, F.U.; Kosters, M.; Lang, S.; Becker, T.; Jager, M. Psychopathological long-term outcome of schizophrenia—a review. Acta Psychiatr. Scand. 2013, 127, 173–182. [Google Scholar] [CrossRef]
- Bromet, E.J.; Naz, B.; Fochtmann, L.J.; Carlson, G.A.; Tanenberg-Karant, M. Long-term diagnostic stability and outcome in recent first-episode cohort studies of schizophrenia. Schizophr. Bull. 2005, 31, 639–649. [Google Scholar] [CrossRef]
- Harrison, G.; Hopper, K.; Craig, T.; Laska, E.; Siegel, C.; Wanderling, J.; Dube, K.C.; Ganev, K.; Giel, R.; an der Heiden, W.; et al. Recovery from psychotic illness: A 15- and 25-year international follow-up study. Br. J. Psychiatry 2001, 178, 506–517. [Google Scholar] [CrossRef]
- Averbeck, B.B.; Bobin, T.; Evans, S.; Shergill, S.S. Emotion recognition and oxytocin in patients with schizophrenia. Psychol. Med. 2012, 42, 259–266. [Google Scholar] [CrossRef]
- White, C.; Stirling, J.; Hopkins, R.; Morris, J.; Montague, L.; Tantam, D.; Lewis, S. Predictors of 10-year outcome of first-episode psychosis. Psychol. Med. 2009, 39, 1447–1456. [Google Scholar] [CrossRef]
- Jaaskelainen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2012. [Google Scholar] [CrossRef]
- Evensen, J.; Rossberg, J.I.; Barder, H.; Haahr, U.; Hegelstad, W.T.; Joa, I.; Johannessen, J.O.; Larsen, T.K.; Melle, I.; Opjordsmoen, S.; et al. Flat affect and social functioning: A 10 year follow-up study of first episode psychosis patients. Schizophr. Res. 2012, 139, 99–104. [Google Scholar] [CrossRef]
- Ten Velden Hegelstad, W.; Haahr, U.; Larsen, T.K.; Auestad, B.; Barder, H.; Evensen, J.; Joa, I.; Johannessen, J.O.; Langeveld, J.; Melle, I.; et al. Early detection, early symptom progression and symptomatic remission after ten years in a first episode of psychosis study. Schizophr. Res. 2013, 143, 337–343. [Google Scholar] [CrossRef]
- Goghari, V.M.; Harrow, M.; Grossman, L.S.; Rosen, C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol. Med. 2012. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tracy, D.K.; Shergill, S.S. Mechanisms Underlying Auditory Hallucinations—Understanding Perception without Stimulus. Brain Sci. 2013, 3, 642-669. https://doi.org/10.3390/brainsci3020642
Tracy DK, Shergill SS. Mechanisms Underlying Auditory Hallucinations—Understanding Perception without Stimulus. Brain Sciences. 2013; 3(2):642-669. https://doi.org/10.3390/brainsci3020642
Chicago/Turabian StyleTracy, Derek K., and Sukhwinder S. Shergill. 2013. "Mechanisms Underlying Auditory Hallucinations—Understanding Perception without Stimulus" Brain Sciences 3, no. 2: 642-669. https://doi.org/10.3390/brainsci3020642
APA StyleTracy, D. K., & Shergill, S. S. (2013). Mechanisms Underlying Auditory Hallucinations—Understanding Perception without Stimulus. Brain Sciences, 3(2), 642-669. https://doi.org/10.3390/brainsci3020642



