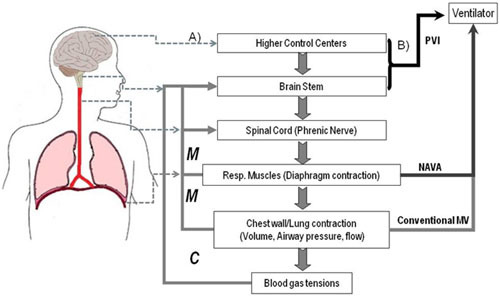
Patient Machine Interface for the Control of Mechanical Ventilation Devices
Abstract
:1. Introduction
2. Mechanical Ventilation and Neural Signals Associated to Voluntary and Involuntary Breathing
2.1. Current Needs for Mechanical Ventilation

2.2. Current Advances and State of the Art
- (1)
- Pressure trigger. The patient effort reduces the pressure till a cutoff value (sensitivity) that, when surpassed, activates the ventilator. The use of this technique is probably linked to the fact that pneumologists consider the mouth, the transdiaphragmatic and/or the esophageal pressures (Pes) as closely related to the neural respiratory drive [16,17,18]. Nevertheless, existing literature (see [3] and references there in) confirms that up to one third of patient efforts can be unrewarded with this method.
- (2)
- Flow trigger. The ventilator is activated when the flow, induced by the patient effort, surpass a cutoff value (sensitivity). Though considered more sensitive than (1), it might fail to avoid or eliminate patient-ventilator asynchrony [19].
- (3)
2.3. Neural Control Signals for a Patient Ventilator Interface (PVI)

2.3.1. Brainstem Signals Driving the Respiratory Rhythm
2.3.2. Cortical Premotor Signals for a Posteriori Assessment
2.3.3. Closing the PVI Loop in the Respiratory Cycle: Corollary/Afferent Responses as a Potential Source of Scalp EEG Feedback Related Potentials
2.3.4. Illustrating the Proposed Approach: Discriminating the Inspiratory and Expiratory Periods during the Voluntary Control of Breathing in a Healthy Control

2.4. Challenges and Workarounds in Developing a Patient Ventilator Interface (PVI)
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bolton, E.C.; Chen, R.; Wijdicks, E.F.M.; Zifko, U.A. Neurology of Breathing; Elsevier: Philadelphia, PA, USA, 2004. [Google Scholar]
- Petersen, E.S. The Control of Breathing Pattern. In The Control of Breathing in Man; Whipp, B.J., Ed.; Manchester University Press: Manchester, UK, 1987. [Google Scholar]
- Tobin, M.J. Advances in mechanical ventilation. N. Engl. J. Med. 2001, 344, 1986–1996. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Vaughan, T.M.; Heetderks, W.J.; Trejo, L.J.; Rymer, W.Z.; Weinrich, M.; Moore, M.M.; Kubler, A.; Dobkin, B.H.; Birbaumer, N.; Donchin, E.; et al. Brain-computer interface technology: A review of the Second International Meeting. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 94–109. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C.; Muller, G.R.; Obermaier, B.; Krausz, G.; Schlogl, A.; Scherer, R.; Graimann, B.; Keinrath, C.; Skliris, D.; et al. Graz-BCI: State of the art and clinical applications. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 177–180. [Google Scholar]
- Birbaumer, N.; Kubler, A.; Ghanayim, N.; Hinterberger, T.; Perelmouter, J.; Kaiser, J.; Iversen, I.; Kotchoubey, B.; Neumann, N.; Flor, H. The thought translation device (TTD) for completely paralyzed patients. IEEE Trans. Rehabil. Eng. 2000, 8, 190–193. [Google Scholar] [CrossRef]
- Kotur, P.F. Mechanical ventilation: Past, present and future. Indian J. Anaesth. 2004, 48, 430–432. [Google Scholar]
- Tobin, M.J. Mechanical ventilation. N. Engl. J. Med. 1994, 330, 1056–1061. [Google Scholar] [CrossRef]
- Sinderby, C.; Navalesi, P.; Beck, J.; Skrobik, Y.; Comtois, N.; Friberg, S.; Gottfried, S.B.; Lindström, L. Neural control of mechanical ventilation in respiratory failure. Nat. Med. 1999, 5, 1433–1436. [Google Scholar] [CrossRef]
- Calfee, C.S.; Matthay, M.A. Recent advances in mechanical ventilation. Am. J. Med. 2005, 118, 584–591. [Google Scholar] [CrossRef]
- MacIntyre, N. New Advances in Mechanical Ventilation. Available online: http://cme.medscape.com/viewarticle/568522 (accessed on 8 November 2013).
- Beers, M.H.; Berkow, R.; Porter, R.S. The Merck Manual of Diagnosis and Therapy, 18th ed.; Porter, R.S., Ed.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Esteban, A.; Anzueto, A.; Alía, I.; Gordo, F.; Apezteguía, C.; Pálizas, F.; Cide, D.; Goldwaser, R.; Soto, L.; Bugedo, G.; et al. How is mechanical ventilation employed in the intensive care unit? An international review. Am. J. Respir. Crit. Care Med. 2000, 161, 1450–1458. [Google Scholar] [CrossRef]
- Tobin, M.J. Principles and Practice of Mechanical Ventilation; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Topeli, A.; Laghi, F.; Tobin, M.J. The voluntary drive to breathe is not decreased in hypercapnic patients with severe COPD. Eur. Respir. J. 2001, 18, 53–60. [Google Scholar] [CrossRef]
- Sumners, D.P.; Green, D.A.; Mileva, K.N.; Bowtell, J.L. Increases in inspiratory neural drive in response to rapid oscillating airflow braking forces (vibration). Respir. Physiol. Neurobiol. 2008, 160, 350–352. [Google Scholar]
- Luo, Y.M.; Qiu, Z.H.; Wu, H.D.; Steier, J.; Jolley, C.; Zhong, N.S.; Moxham, J.; Polkey, M.I. Neural drive during continuous positive airway pressure (CPAP) and pressure relief CPAP. Sleep Med. 2009, 10, 731–738. [Google Scholar] [CrossRef]
- Chao, D.C.; Scheinhorn, D.J.; Stearn-Hassenpflug, M. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 1997, 112, 1592–1599. [Google Scholar] [CrossRef]
- Stephenson, S.E.; Young, W.; Montgomery, L.H.; Batson, R. Physiologic auto-control of mechanical respirators. Dis. Chest 1961, 39, 363–371. [Google Scholar] [CrossRef]
- Schmidt, M.; Demoule, A.; Cracco, C.; Gharbi, A.; Fiamma, M.N.; Straus, C.; Duguet, A.; Gottfried, S.B.; Similowski, T. Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology 2010, 112, 670–681. [Google Scholar] [CrossRef]
- Chiappa, K.H. Evoked Potentials in Clinical Medicine, 3rd ed.; Chiappa, K.H., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1997. [Google Scholar]
- Nakazawa, K.; Granata, A.R.; Cohen, M.I. Synchronized fast rhythms in inspiratory and expiratory nerve discharges during fictive vocalization. J. Neurophysiol. 2000, 83, 1415–1425. [Google Scholar]
- Funk, G.D.; Parkis, M.A. High frequency oscillations in respiratory networks: Functionally significant or phenomenological? Respir. Physiol. Neurobiol. 2002, 131, 101–120. [Google Scholar] [CrossRef]
- Parkis, M.A.; Feldman, J.L.; Robinson, D.M.; Funk, G.D. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J. Neurosci. 2003, 23, 8152–8158. [Google Scholar]
- Gonzalez, S.L.; Grave de Peralta, R.; Thut, G.; Millán Jdel, R.; Morier, P.; Landis, T. Very high frequency oscillations (VHFO) as a predictor of movement intentions. Neuroimage 2006, 32, 170–179. [Google Scholar] [CrossRef]
- Raux, M.; Straus, C.; Redolfi, S.; Morelot-Panzini, C.; Couturier, A.; Hug, F.; Similowski, T. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J. Physiol. 2007, 578, 569–578. [Google Scholar]
- Raux, M.; Tremoureux, L.; Couturier, A.; Hug, F.; Similowski, T. Simplified recording technique for the identification of inspiratory premotor potentials in humans. Respir. Physiol. Neurobiol. 2010, 171, 67–70. [Google Scholar] [CrossRef]
- Tremoureux, L.; Raux, M.; Jutand, L.; Similowski, T. Sustained preinspiratory cortical potentials during prolonged inspiratory threshold loading in humans. J. Appl. Physiol. 2010, 108, 1127–1133. [Google Scholar] [CrossRef]
- Birbaumer, N.; Elbert, T.; Canavan, A.G.; Rockstroh, B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990, 70, 1–41. [Google Scholar]
- Raux, M.; Ray, P.; Prella, M.; Duguet, A.; Demoule, A.; Similowski, T. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology 2007, 107, 746–755. [Google Scholar] [CrossRef]
- Blankertz, B.; Dornhege, G.; Curio, G. Single Trial Detection of EEG Error Potentials: A Tool for Increasing BCI Transmission Rates. In Artificial Neural Networks—ICANN 2002; Springer: Berlin, Germany, 2002. [Google Scholar]
- Schalk, G.; Wolpaw, J.R.; McFarland, D.J.; Pfurtscheller, G. EEG-based communication: Presence of an error potential. Clin. Neurophysiol. 2000, 111, 2138–2144. [Google Scholar] [CrossRef]
- Murray, J.F.; Nadels, J.A. Murray and Nadel’s Textbook of Respiratory Medicine, 4th ed.; Robert, J.F.M., Mason, J., Nadel, J.A., Eds.; Elsevier: New York, NY, USA, 2005. [Google Scholar]
- Holroyd, C.B.; Hajcak, G.; Larsen, J.T. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006, 1105, 93–101. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Yeung, N.; Holroyd, C.B.; Schurger, A.; Cohen, J.D. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb. Cortex 2004, 14, 741–747. [Google Scholar] [CrossRef]
- Yeung, N.; Holroyd, C.B.; Cohen, J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb. Cortex 2005, 15, 535–544. [Google Scholar] [CrossRef]
- Mars, R.B.; Coles, M.G.; Grol, M.J.; Holroyd, C.B.; Nieuwenhuis, S.; Hulstijn, W.; Toni, I. Neural dynamics of error processing in medial frontal cortex. Neuroimage 2005, 28, 1007–1013. [Google Scholar] [CrossRef]
- Hajcak, G.; Moser, J.S.; Holroyd, C.B.; Simons, R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006, 71, 148–154. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Nieuwenhuis, S.; Yeung, N.; Cohen, J.D. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport 2003, 14, 2481–2484. [Google Scholar] [CrossRef]
- Booth, S.; Bausewein, C.; Higginson, I.; Moosavi, S.H. Pharmacological treatment of refractory breathlessness. Exp. Rev. Respir. Med. 2009, 3, 21–36. [Google Scholar] [CrossRef]
- Dyspnea, A.T.S. Mechanisms, assessment, and management: A consensus statement. Am. J. Respir. Crit. Care Med. 1999, 159, 1666–1682. [Google Scholar]
- Grave de Peralta Menendez, R.; Noirhomme, Q.; Cincotti, F.; Mattia, D.; Aloise, F.; González Andino, S. Modern electrophysiological methods for brain-computer interfaces. Comput. Intell. Neurosci. 2007, 2007. [Google Scholar] [CrossRef]
- Gonzalez Andino, S.L.; Grave de Peralta, R.; Khateb, A.; Pegna, A.J.; Thut, G.; Landis, T. A glimpse into your vision. Hum. Brain Mapp. 2007, 28, 614–624. [Google Scholar] [CrossRef]
- Deecke, L.G.B.; Kornhuber, H.H. Voluntary finger movement in man: Cerebral potentials and theory. Biol. Cybern. 1976, 23, 99–119. [Google Scholar] [CrossRef]
- Dornhege, G.; Blankertz, B.; Curio, G. Speeding up Classication of Multi-Channel Brain-Computer Interfaces: Common Spatial Patterns for Slow Cortical Potentials. In Proceedings of the 1st International IEEE EMBS Conference on Neural Engineering, Capri, Italy, 20–22 March 2003; IEEE: New York, NY, USA, 2003. [Google Scholar]
- Birbaumer, N.; Ghanayim, N.; Hinterberger, T.; Iversen, I.; Kotchoubey, B.; Kübler, A.; Perelmouter, J.; Taub, E.; Flor, H. A spelling device for the completely paralyzed. Nature 1999, 398, 297–298. [Google Scholar] [CrossRef]
- Bragin, A.; Engel, J., Jr.; Wilson, C.L.; Fried, I.; Mathern, G.W. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 1999, 40, 127–137. [Google Scholar] [CrossRef]
- Brovelli, A.; Lachaux, J.P.; Kahane, P.; Boussaoud, D. High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage 2005, 28, 154–164. [Google Scholar] [CrossRef]
- Barth, D.S. Submillisecond synchronization of fast electrical oscillations in neocortex. J. Neurosci. 2003, 23, 2502–2510. [Google Scholar]
- Vaisanen, O.; Malmivuo, J. Improving the SNR of EEG generated by deep sources with weighted multielectrode leads. J. Physiol. Paris 2009, 103, 306–314. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grave de Peralta, R.; Gonzalez Andino, S.; Perrig, S. Patient Machine Interface for the Control of Mechanical Ventilation Devices. Brain Sci. 2013, 3, 1554-1568. https://doi.org/10.3390/brainsci3041554
Grave de Peralta R, Gonzalez Andino S, Perrig S. Patient Machine Interface for the Control of Mechanical Ventilation Devices. Brain Sciences. 2013; 3(4):1554-1568. https://doi.org/10.3390/brainsci3041554
Chicago/Turabian StyleGrave de Peralta, Rolando, Sara Gonzalez Andino, and Stephen Perrig. 2013. "Patient Machine Interface for the Control of Mechanical Ventilation Devices" Brain Sciences 3, no. 4: 1554-1568. https://doi.org/10.3390/brainsci3041554