Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. EAE Induction and Clinical Evaluation
2.3. IHC and HC Analysis
2.4. Statistical Analysis
3. Results
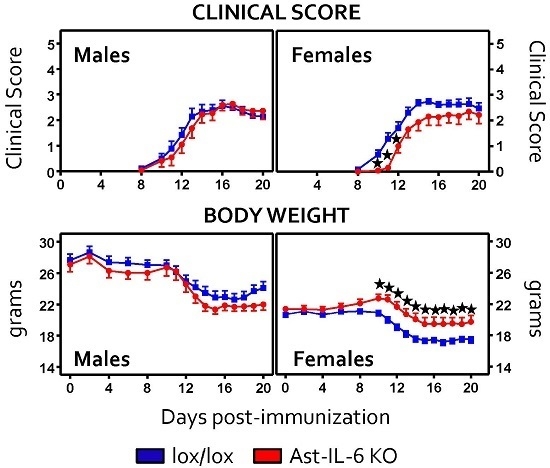
3.1. Lack of Astrocytic IL-6 Alters the Clinical Course of EAE in Ast-IL-6 KO Mice and Ameliorates EAE Symptomatology in a Sex-Dependent Manner
3.2. Reduced Cellular Infiltrates and Demyelination in the Spinal Cord of Ast-IL-6 KO Female Mice
3.3. Reduced Gliosis and Vasogenesis in Spinal Cord and Brain of EAE-Induced Ast-IL-6 KO Females
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hirano, T.; Taga, T.; Nakano, N.; Yasukawa, K.; Kashiwamura, S.; Shimizu, K.; Nakajima, K.; Pyun, K.H.; Kishimoto, T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc. Natl. Acad. Sci. USA 1985, 82, 5490–5494. [Google Scholar] [CrossRef] [PubMed]
- Gruol, D.L.; Nelson, T.E. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 1997, 15, 307–339. [Google Scholar] [CrossRef] [PubMed]
- Schöbitz, B.; de Kloet, E.R.; Sutanto, W.; Holsboer, F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mrna in rat brain. Eur. J. Neurosci. 1993, 5, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, N.J.; Benveniste, E.N. Interleukin-6 expression and regulation in astrocytes. J. Neuroimmunol. 1999, 100, 124–139. [Google Scholar] [CrossRef]
- Milo, R.; Kahana, E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun. Rev. 2010, 9, A387–A394. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.G. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 2007, 7, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.E.; Benveniste, E.N. Cytokines in inflammatory brain lesions: Helpful and harmful. Trends Neurosci. 1996, 19, 331–338. [Google Scholar] [CrossRef]
- Maimone, D.; Guazzi, G.C.; Annunziata, P. IL-6 detection in multiple sclerosis brain. J. Neurol. Sci. 1997, 146, 59–65. [Google Scholar] [CrossRef]
- Eugster, H.-P.; Frei, K.; Kopf, M.; Lassmann, H.; Fontana, A. IL-6 deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 1998, 28, 2178–2187. [Google Scholar] [CrossRef]
- Mendel, I.; Katz, A.; Kozak, N.; Ben-Nun, A.; Revel, M. Interleukin-6 functions in autoimmune encephalomyelitis: A study in gene-targeted mice. Eur. J. Immunol. 1998, 28, 1727–1737. [Google Scholar] [CrossRef]
- Okuda, Y.; Sakoda, S.; Bernard, C.C.; Fujimura, H.; Saeki, Y.; Kishimoto, T.; Yanagihara, T. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 1998, 10, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, E.B.; Horton, J.L.; Hilliard, B.; Liu, T.S.; Chen, Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: Roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 1998, 161, 6480–6486. [Google Scholar] [PubMed]
- Giralt, M.; Ramos, R.; Quintana, A.; Ferrer, B.; Erta, M.; Castro-Freire, M.; Comes, G.; Sanz, E.; Unzeta, M.; Pifarré, P.; et al. Induction of atypical EAE mediated by transgenic production of IL-6 in astrocytes in the absence of systemic IL-6. Glia 2013, 61, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Serada, S.; Fujimoto, M.; Mihara, M.; Koike, N.; Ohsugi, Y.; Nomura, S.; Yoshida, H.; Nishikawa, T.; Terabe, F.; Ohkawara, T.; et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9041–9046. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Erta, M.; Ferrer, B.; Comes, G.; Giralt, M.; Hidalgo, J. Astrocyte-specific deficiency of interleukin-6 and its receptor reveal specific roles in survival, body weight and behavior. Brain Behav. Immun. 2013, 27, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Giralt, M.; Esposito, F.L.; Fernandez-Gayol, O.; Hidalgo, J. Astrocytic IL-6 mediates locomotor activity, exploration, anxiety, learning and social behavior. Horm. Behav. 2015, 73, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Hu, H.; Lee, Y.; d’Azzo, A.; Messing, A.; Brenner, M. Expression specificity of GFAP transgenes. Neurochem. Res. 2004, 29, 2075–2093. [Google Scholar] [CrossRef] [PubMed]
- Mycko, M.P.; Papoian, R.; Boschert, U.; Raine, C.S.; Selmaj, K.W. Cdna microarray analysis in multiple sclerosis lesions: Detection of genes associated with disease activity. Brain 2003, 126, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Gijbels, K.; Brocke, S.; Abrams, J.S.; Steinman, L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1995, 1, 795–805. [Google Scholar] [PubMed]
- Willenborg, D.O.; Fordham, S.A.; Cowden, W.B.; Ramshaw, I.A. Cytokines and murine autoimmune encephalomyelitis: Inhibition or enhancement of disease with antibodies to select cytokines, or by delivery of exogenous cytokines using a recombinant vaccinia virus system. Scand. J. Immunol. 1995, 41, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Pavelko, K.D.; McKinne, C.W.; Leibowitz, J.L. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J. Immunol. 1994, 153, 3811–3821. [Google Scholar] [PubMed]
- Campbell, I.L.; Abraham, C.R.; Masliah, E.; Kemper, P.; Inglis, J.D.; Oldstone, M.B.A.; Mucke, L. Neurologic disease in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 10061–10065. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.L.; Erta, M.; Lim, S.L.; Frausto, R.; May, U.; Rose-John, S.; Scheller, J.; Hidalgo, J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J. Neurosci. 2014, 34, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.M.; Baecher-Allan, C.; Hafler, D.A. Multiple sclerosis and regulatory T cells. J. Clin. Immunol. 2008, 28, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kishimoto, T. Il-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.C.; Lalor, S.J.; Lynch, M.A.; Mills, K.H. Infiltration of th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010, 24, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Mitsdoerffer, M.; Croxford, A.L.; Awasthi, A.; Dardalhon, V.A.; Galileos, G.; Vollmar, P.; Stritesky, G.L.; Kaplan, M.H.; Waisman, A.; et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional t cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18460–18465. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Reynolds, S.L.; Baker, B.J.; Li, X.; Benveniste, E.N.; Qin, H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J. Immunol. 2010, 184, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Ogura, H.; Murakami, M.; Okuyama, Y.; Tsuruoka, M.; Kitabayashi, C.; Kanamoto, M.; Nishihara, M.; Iwakura, Y.; Hirano, T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008, 29, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Swartz, K.R.; Liu, F.; Sewell, D.; Schochet, T.; Campbell, I.; Sandor, M.; Fabry, Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001, 896, 86–95. [Google Scholar] [CrossRef]
- Fee, D.; Grzybicki, D.; Dobbs., M.; Ihyer, S.; Clotfelter, J.; Macvilay, S.; Hart, M.N.; Sandor, M.; Fabry, Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine 2000, 12, 655–665. [Google Scholar] [CrossRef] [PubMed]



| Genotype | Experiment 1 (0–22 dpi) | Experiment 2 (0–20 dpi) | Experiment 3 (0–46 dpi) | ||
|---|---|---|---|---|---|
| Males | Females | Males | Females | Females | |
| Floxed | 7 | 3 | 11 | 17 | 8 |
| Ast-IL-6 KO | 3 | 7 | 8 | 8 | 8 |
| EAE | Females | Males | ||
|---|---|---|---|---|
| Ast-IL-6 KO | Floxed | Ast-IL-6 KO | Floxed | |
| Incidence | 21/23 | 28/28 | 11/11 | 17/18 |
| Mortality | 3/23 | 0/28 | 0/11 | 0/18 |
| Day of onset | 13.38 ± 0.59 * | 11.57 ± 0.29 | 12.27 ± 0.52 * | 11.65 ± 0.49 |
| Clinical Course | Females (0–20 dpi) | Females (46 dpi) | Males (0–20 dpi) | |||
|---|---|---|---|---|---|---|
| Ast-IL-6 KO (n = 15) | Floxed (n = 20) | Ast-IL-6 KO (n = 8) | Floxed (n = 8) | Ast-IL-6 KO (n = 11) | Floxed (n = 18) | |
| Time of peak score | 14.30 ± 0.53 | 14.80 ± 0.49 | 22.5 ± 2.46 * | 15.5 ± 1.92 | 15.18 ± 0.80 | 13.76 ± 0.6 |
| Peak score | 2.80 ± 0.23 | 3.45 ± 0.16 | 3.68 ± 0.50 | 3.18 ± 0.23 | 3.18 ± 0.18 | 3.14 ± 0.12 |
| Cumulative score | 19.40 ± 2.55 | 24.16 ± 1.32 | 88.87 ± 21.84 | 72.18 ± 10.35 | 20.32 ± 1.64 | 22.61 ± 1.34 |
| Grade of Remission | 0.64 ± 0.13 | 0.91 ± 0.11 | 1.00 ± 0.34 | 1.43 ± 0.27 | 0.82 ± 0.26 | 20.32 ± 1.64 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erta, M.; Giralt, M.; Jiménez, S.; Molinero, A.; Comes, G.; Hidalgo, J. Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice. Brain Sci. 2016, 6, 15. https://doi.org/10.3390/brainsci6020015
Erta M, Giralt M, Jiménez S, Molinero A, Comes G, Hidalgo J. Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice. Brain Sciences. 2016; 6(2):15. https://doi.org/10.3390/brainsci6020015
Chicago/Turabian StyleErta, Maria, Mercedes Giralt, Silvia Jiménez, Amalia Molinero, Gemma Comes, and Juan Hidalgo. 2016. "Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice" Brain Sciences 6, no. 2: 15. https://doi.org/10.3390/brainsci6020015