Reducing Fall Risk with Combined Motor and Cognitive Training in Elderly Fallers
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods
2.1.1. Study Design and Randomisation
2.1.2. Subjects
2.1.3. Trainings and Active Control Condition
2.2. Outcomes
2.3. Sample Size
2.4. Statistical Analysis
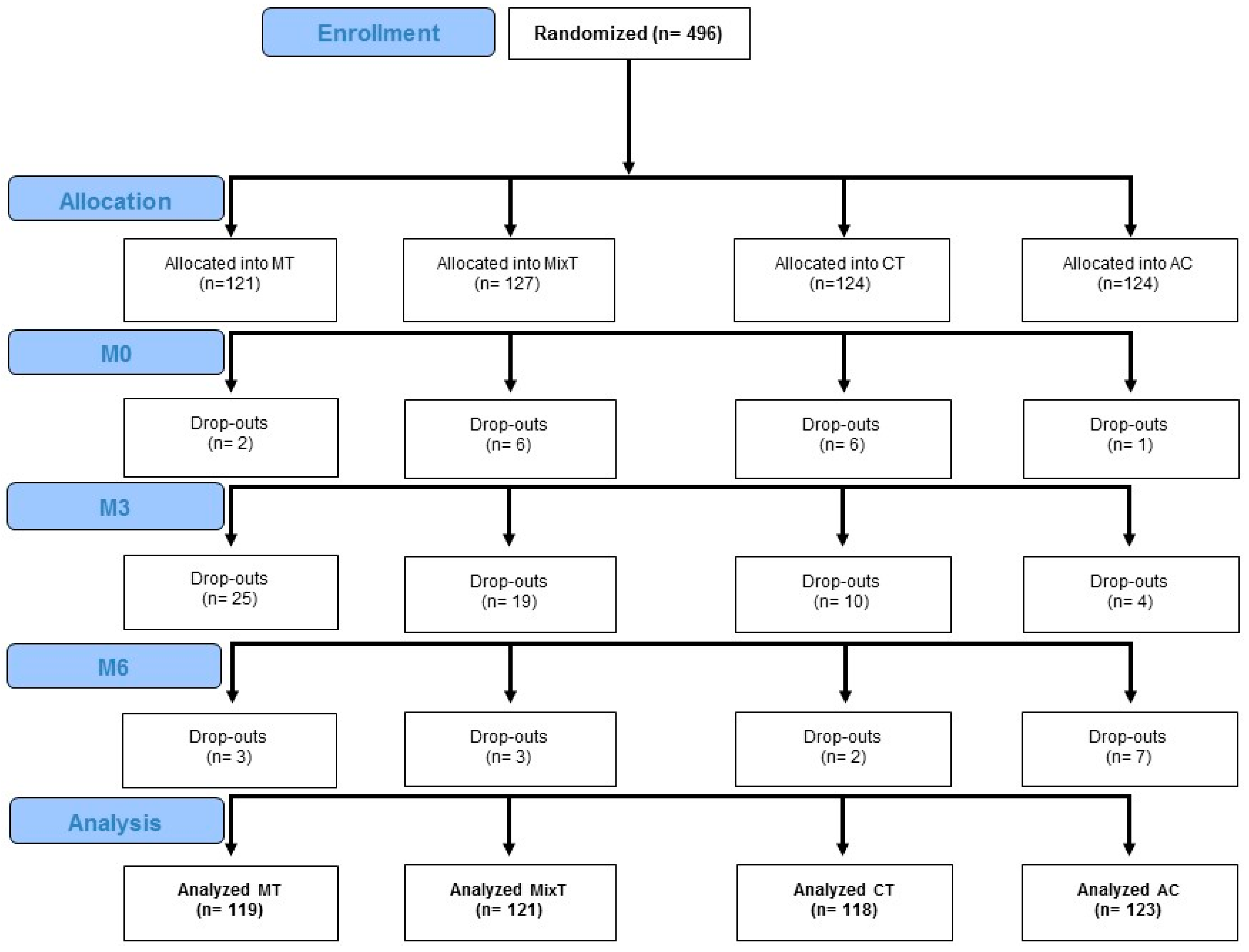
3. Results
3.1. Baseline
3.2. Treatments effects
3.3. Mobility
3.4. Cognition
3.5. Behavior and Functional Abilities
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Exercise | Contents | Objective of training |
|---|---|---|
| Memory | ||
| Remember the picture | The user is shown a picture, then this is immediately replaced with other pictures. The user is asked to find the previous picture among the subsequent ones. | Declarative episodic short-term visuo-spatial memory |
| Remember the melody | The user is presented with a piano playing some notes with corresponding key lighting up. The user is asked to reproduce the melody. | Declarative episodic short-term auditory memory |
| Hide and find | The user is shown one of three fully-furnished rooms (living room, bathroom, kitchen). During the encoding phase the user is asked to hide from 5 to 10 items in there (in the room). After 15 minutes, during the delayed recall phase, the user is asked to reposition the items in their initial location. | Declarative episodic long-term visuo-spatial memory |
| Do you remember your order | The user is shown two menus (his and his friend’s) and is asked to memorize them. Immediately after, the user is asked to recognize the ordered dishes among distracters. | Declarative episodic short-term verbal memory |
| Remember the design | The user is shown a design drawn on a 9-dot matrix and is asked to encode it. Immediately after the design disappears, the user is asked to reproduce it on the same 9-dot matrix. | Declarative episodic short-term visual memory |
| Find the pairs | The user is shown a grid of uncovered paired cards. After the cards are covered, the user is asked to find the pairs of identical pictures. | Declarative episodic short-term visual memory |
| Who belongs where | The user has to choose the correct profession of famous people among two alternatives. | Semantic memory |
| Executive functions and attention | ||
| Similarities | The user is asked to select the correct sentence among 3 describing the similarity between two concepts. | Abstraction |
| Differences | The user is asked to select the correct sentence among 3 describing the difference between two concepts. | Abstraction |
| Analogies | The user is given one pair of related concepts and another concept without its pair. The subject is asked to choose the correct concept out of four to form another pair. | Abstraction |
| Picture sort | The user is presented a series of pictures and is asked to move each one into one of two boxes following a rule. This must be inferred by the feedback given each time a picture is moved into the correct or incorrect box. | Abstraction |
| Be a piano player | The user is presented with a piano playing some notes. When a note plays the corresponding key lights up and the user is asked to press the key and reproduce the note. Notes follow one another at growing speed. | Attention |
| Take away menu | The user must order a take away menu following some rules (e.g., maximum price, foods not to be ordered). | Inhibition, planning, problem-solving |
| Train guidance | The user is presented with a train rail and some parts of this are interrupted or missing. The user is asked to straighten or complete the rail in order to ensure the train passage. | Planning, problem-solving |
| Lost in the city | The user must pay attention to the direction given by the central person among five giving different directions. | Selective attention—interference inhibition |
| Guess Who | The user is asked to identify the mystery character by excluding those characters who do not meet the descriptions of the right one. | Visuo-spatial attention |
| N-back | The user is presented with a sequence of pictures and is asked to indicate when the current picture matches the one from n steps earlier in the sequence. | Working memory |
| Remember the sequence | The user is shown a sequence of pictures. These are positioned in the array each time in a different sequence. The user is asked to remember the pictures in their right sequence. At the same time, if a picture that follows the specified rule is placed in the sequence, the user must clap his hands. | Working memory |
| Constructional praxis | ||
| Puzzle | The user is asked to combine the pieces of a puzzle to complete the final figure | Constructional praxis |
| Copy of figures | The user is asked to copy the geometrical figures. | Constructional praxis |
| Language | ||
| Synonymous | The user must connect words of two different lists coupling synonymous words. | Lexicon access |
| Antonymous | The user must connect words of two different lists coupling antonymous words. | Lexicon access |
| Logical reasoning | ||
| Incomplete grids | The user is asked to complete the grid by inserting the right tile in a multiple choice. | Logical reasoning |
| Symbol addition | The user is asked to solve arithmetic operations using symbols instead of digits. | Logical reasoning |
| Domino | The user is asked to pair identical images by placing each tile next to the corresponding one. | Logical reasoning |
| Orientation | ||
| My home | The user must move a person into a house following a precise trail. | Spatial orientation |
| Travelling in Europe | The user is shown a map of Europe and must select different countries according a specified order during a trip. | Spatial orientation |
| Warm-up pool | Balance pool | Gait pool |
|---|---|---|
| Stretching | Lift up heels | Moving i-walker forward |
| Squat with spread legs | Lift up tiptoes | Moving i-walker forward oblique |
| Squat with spread legs in anteroposterior | Lift up heels/tiptoes | Moving i-walker forward flexing torso |
| Lateral load shift | Moving i-walker forward oblique flexing torso | |
| Lateral load shift with contralateral leg flexion | Load shift with arms | |
| Lateral load shift with contralateral leg flexion | Load shift with arms and kick | |
| and torso rotation | Move i-walker forward / backward | |
| Forward load shift | Move i-walker forward / backward in line | |
| Hip lift up opposite the support leg | Move i-walker forward / backward marching | |
| Load holding for 10 seconds | Move i-walker on a wide curve | |
| Load holding with heel lift up | Move i-walker on a curve marching in place | |
| Leg flexion / alternate leg flexion | ||
| Leg flexion and extension / alternate leg | ||
| flexion and extension | ||
| Leg flexion and extension backwards | ||
| Foot sliding forth and back |
References
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.F. Falls in the elderly. Am. Fam. Phys. 2000, 61, 2159–2168. [Google Scholar]
- Friedman, S.M.; Munoz, B.; West, S.K.; Rubin, G.S.; Fried, L.P. Falls and fear of falling: Which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J. Am. Geriatr. Soc. 2002, 50, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Legters, K. Fear of falling. Phys. Ther. 2002, 82, 264–272. [Google Scholar] [PubMed]
- Jørstad, E.C.; Hauer, K.; Becker, C.; Lamb, S.E.; ProFaNE Group. Measuring the psychological outcomes of falling: A systematic review. J. Am. Geriatr. Soc. 2005, 53, 501–510. [Google Scholar]
- Boyd, R.; Stevens, J.A. Falls and fear of falling: Burden, beliefs and behaviours. Age Ageing 2009, 38, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Plotnik, M.; Bove, F.; Berardelli, A. The neurobiology of falls. Neurol. Sci. 2012, 33, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Lamb, S.E.; Jorstad-Stein, E.C.; Hauer, K.; Becker, C. Prevention of Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials: The Prevention of Falls Network Europe consensus. J. Am. Geriatr. Soc. 2005, 53, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Robertson, M.C. Implementation of multifactorial interventions for fall and fracture prevention. Age Ageing 2006, 35, ii60–ii64. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait dynamics, fractals and falls: Finding meaning in the stride-to-stride fluctuations of human walking. Hum. Mov. Sci. 2007, 26, 555–589. [Google Scholar] [CrossRef] [PubMed]
- Ganz, D.A.; Bao, Y.; Shekelle, P.G.; Rubenstein, L.Z. Will my patient fall? JAMA 2007, 297, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Carty, C.P.; Cronin, N.J.; Nicholson, D.; Lichtwark, G.A.; Mills, P.M.; Kerr, G.; Cresswell, A.G.; Barrett, R.S. Reactive stepping behaviour in response to forward loss of balance predicts future falls in community-dwelling older adults. Age Ageing 2015, 44, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, V.J.; van der Geest, J.N.; Hofman, A.; Ikram, M.A. Cognition and gait show a distinct pattern of association in the general population. Alzheimers Dement. 2014, 10, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Lundin-Olsson, L.; Nyberg, L.; Gustafson, Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet 1997, 349, 617. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.; Kerns, K.A.; Baldwin, M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M232–M240. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, G.L. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 2003, 18, 29–34. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M. Attentional demands and postural control: The effect of sensory context. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M10–M16. [Google Scholar] [PubMed]
- Muir, S.W.; Gopaul, K.; Montero Odasso, M.M. The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis. Age Ageing 2012, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, K.; Kochan, N.A.; Close, J.C.; Menant, J.C.; Sturnieks, D.L.; Brodaty, H.; Sachdev, P.S.; Lord, S.R. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am. J. Geriatr. Psychiatry 2012, 20, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.K.; Vonschewelov, T.; Karlsson, C.; Cöster, M.; Rosengen, B.E. Prevention of falls in the elderly: A review. Scand. J. Public Health 2013, 41, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. N. S. W. Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Arcoverde, C.; Deslandes, A.; Rangel, A.; Rangel, A.; Pavao, R.; Nigri, F.; Engelhardt, E.; Laks, J. Role of physical activity on the maintenance of cognition and activities of daily living in elderly with Alzheimer’s disease. Arq. Neuro. Psiquiat. 2008, 66, 323–327. [Google Scholar] [CrossRef]
- Yaffe, K.; Barnes, D.; Nevitt, M.; Lui, L.Y.; Covinsky, K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch. Intern. Med. 2001, 161, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Smith, A.L.; Keele, S.W. The impact of aerobic activity on cognitive function in older adults: A new synthesis based on the concept of executive control. Eur. J. Cogn. Psychol. 2001, 13, 279–300. [Google Scholar] [CrossRef]
- Van het Reve, E.; de Bruin, E.D. Strength-balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: A multicenter randomized-controlled trial. BMC Geriatr. 2014, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ray, R.L.; Hughes, S.L.; Prohaska, T.R.; Little, D.M.; Jurivich, D.A.; Hedeker, D. Impact of cognitive training on balance and gait in older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2015, 70, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Pichierri, G.; Wolf, P.; Murer, K.; de Bruin, E.D. Cognitive and cognitive-motor interventions affecting physical functioning: A systematic review. BMC Geriatr. 2011, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, D.J.; Roberts, C. Understanding controlled trials. Randomisation methods: Concealment. BMJ 1999, 319, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 89–98. [Google Scholar]
- Tinetti, M.E.; Kumar, C. The patient who falls: “It is always a trade-off”. JAMA 2010, 303, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Stone, K.L.; Cauley, J.A.; Tracy, J.K.; Hochberg, M.C.; Rodondi, N.; Cawthon, P.M. Study of Osteoporotic Fractures Research Group. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Navarro, M.; Duaso, E.; Chivite, D.; Ruiz, D.; Perez-Castejon, J.M.; Lopez-Soto, A.; Pujol, R. Factors associated with hip fracture-related falls among patients with a history of recurrent falling. Bone 2008, 43, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Cortés, U.; Martínez-Velasco, A.; Barrué, C.; Benedico, T.; Campana, F.; Caltagirone, C.; Annicchiarico, R. A SHARE-it service to elders’ mobility using the i-Walker. Gerontechnology. 2008, 7, 95. [Google Scholar] [CrossRef]
- SOCIABLE. Available online: http://www.cognitivetraining.eu/ (accessed on 8 February 2017).
- Barban, F.; Annicchiarico, R.; Pantelopoulos, S.; Federici, A.; Perri, R.; Fadda, L.; Carlesimo, G.A.; Ricci, C.; Giuli, S.; Scalici, F.; et al. Protecting cognition from aging and Alzheimer’s disease: A computerized cognitive training combined with reminiscence therapy. Int. J. Geriatr. Psychiatry 2016, 31, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K.; Belfor, N.; Jagust, W.J.; DeCarli, C.; Reed, B.R.; Kramer, J.H. Computer-based cognitive training for mild cognitive impairment: Results from a pilot randomized, controlled trial. Alzheimer Dis Assoc. Disord. 2009, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Reitan, R.M. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955, 19, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Borkowisky, J.G.; Benton, A.L.; Spreen, O. Word fluency and brain damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- Rey, A. Memorisation D’une Serie de 15 Mots en 5 Repetitions; L’examen Clinique en Psycologie; Presses Universitaires de France: Paris, France, 1958. [Google Scholar]
- Rey, A. L’examen psychologique dans les cas d’encéphalopatie traumatique. Arch. Psychol. (Geneve) 1941, 28, 286–340. [Google Scholar]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982–1983, 17, 37–49. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait. Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Halvarsson, A.; Oddsson, L.; Olsson, E.; Farén, E.; Pettersson, A.; Ståhle, A. Effects of new, individually adjusted, progressive balance group training for elderly people with fear of falling and tend to fall: A randomized controlled trial. Clin. Rehabil. 2011, 25, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Tripepi, G.; Dekker, F.W.; Zoccali, C.; Tanck, M.W.; Jager, K.J. Sample size calculations: Basic principles and common pitfalls. Nephrol. Dial. Transplant. 2010, 25, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxford, UK, 1998. [Google Scholar]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 9, CD007146. [Google Scholar]
- Austin, N.; Devine, A.; Dick, I.; Prince, R.; Bruce, D. Fear of falling in older women: A longitudinal study of incidence, persistence, and predictors. J. Am. Geriatr. Soc. 2007, 55, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kearney, F.C.; Harwood, R.H.; Gladman, J.R.; Lincoln, N.; Masud, T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013, 36, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.A.; van Haastregt, J.C.; van Rossum, E.; van Eijk, J.T.; Yardley, L.; Kempen, G.I. Interventions to reduce fear of falling in community-living older people: A systematic review. J. Am. Geriatr. Soc. 2007, 55, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Lustig, C.; Shah, P.; Seidler, R.; Reuter-Lorenz, P.A. Aging, training, and the brain: A review and future directions. Neuropsychol. Rev. 2009, 19, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Duff, K.; Schoenberg, M.R.; Scott, J.G.; Adams, R.L. The relationship between executive functioning and verbal and visual learning and memory. Arch. Clin. Neuropsychol. 2005, 20, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.W.; Bamford, C.; Deary, V.; Finch, T.L.; Gray, J.; MacDonald, C.; McMeekin, P.; Sabin, N.J.; Steen, I.N.; Whitney, S.L.; et al. Cognitive-behavioural therapy-based intervention to reduce fear of falling in older people: Therapy development and randomised controlled trial—The Strategies for Increasing Independence, Confidence and Energy (STRIDE) study. Health Technol. Assess. 2016, 20, 1–206. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, D.; Kumar, A.; Carpenter, H.; Zijlstra, G.A.; Skelton, D.A.; Cook, J.R.; Stevens, Z.; Belcher, C.M.; Haworth, D.; Gawler, S.J.; et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst. Rev. 2014, 11, CD009848. [Google Scholar]
- Kumar, A.; Delbaere, K.; Zijlstra, G.A.; Carpenter, H.; Iliffe, S.; Masud, T.; Skelton, D.; Morris, R.; Kendrick, D. Exercise for reducing fear of falling in older people living in the community: Cochrane systematic review and meta-analysis. Age Ageing 2016, 45, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Ishikawa-Takata, K.; Yamazaki, H.; Morita, T.; Ohta, T. The effects of Tai Chi Chuan on physiological function and fear of falling in the less robust elderly: An intervention study for preventing falls. Arch. Gerontol. Geriatr. 2006, 42, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Logghe, I.H.; Zeeuwe, P.E.; Verhagen, A.P.; Wijnen-Sponselee, R.M.; Willemsen, S.P.; Bierma-Zeinstra, S.M.; van Rossum, E.; Faber, M.J.; Koes, B.W. Lack of effect of Tai Chi Chuan in preventing falls in elderly people living at home: A randomized clinical trial. J. Am. Geriatr. Soc. 2009, 57, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, G.; Williams, H.G.; Hussey, J.; Durstine, J.L.; McClenaghan, B.A. Effects of Feldenkrais exercises on balance, mobility, balance confidence, and gait performance in community-dwelling adults age 65 and older. J. Altern. Complement. Med. 2010, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Kruse, A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin. Interv. Aging 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, F.; Cassou, B.; Latouche, A.; Aegerter, P.; Charles, M.A.; Dargent-Molina, P. Effectiveness of two year balance training programme on prevention of fall induced injuries in at risk women aged 75–85 living in community: Ossébo randomised controlled trial. BMJ 2015, 351, h3830. [Google Scholar] [CrossRef] [PubMed]
- Durlak, J.A. How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 2009, 34, 917–928. [Google Scholar] [CrossRef] [PubMed]
| Motor | Non-Motor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Cognitive | Cognitive | Cognitive | Non-Cognitive | ||||||
| Kind of Treatment | MT | MixT | CT | AC | M/noM | C/noC | |||
| N = 119 | N = 121 | N = 118 | N = 123 | χ2 F | p | χ2 F | p | ||
| Sex f(m) | 82(37) | 87(34) | 65(53) | 80(43) | 5.575 | 0.018 | 0.593 | 0.441 | |
| Domain | Variable | m(sd) | m(sd) | m(sd) | m(sd) | ||||
| Demographic | Age (years) | 75.5(8.5) | 74.5(7.9) | 74.1(7.2) | 76(8.8) | 0.002 | 0.969 | 0.428 | 0.513 |
| Education (years) | 9.7(4.3) | 10.2(4.8) | 9.9(4.2) | 10(4.2) | 0.000 | 0.991 | 0.653 | 0.419 | |
| Mobility | FES-I | 30(10.2) | 32(9.3) | 29.9(9.7) | 31.3(11) | 0.145 | 0.703 | 0.102 | 0.749 |
| POMA B | 11.9(3.4) | 11.1(3.5) | 11.5(3.3) | 11.3(3.5) | 0.116 | 0.734 | 1.076 | 0.300 | |
| POMA G | 8.7(2.6) | 8.1(2.8) | 8.2(2.6) | 8.2(2.9) | 0.390 | 0.533 | 1.479 | 0.224 | |
| Cognitive | TMT B-A | 117.7(72.2) | 135.4(76.1) | 119.5(68.3) | 117(65.5) | 1.699 | 0.193 | 2.502 | 0.114 |
| PF | 25.7(12.4) | 24.8(12.3) | 24.4(12.6) | 25.2(10.8) | 0.185 | 0.667 | 0.560 | 0.445 | |
| RAVLT d | 6.3(3.8) | 5.7(3.5) | 5.8(3.7) | 5.9(3.5) | 0.206 | 0.650 | 1.106 | 0.293 | |
| ROCF d | 10.1(7.4) | 8.2(6.7) | 9.9(7.2) | 7.9(6.5) | 0.195 | 0.659 | 0.002 | 0.963 | |
| Behavioral | GDS | 5.2(3) | 5.6(3.4) | 5(3) | 5.8(3.2) | 0.005 | 0.946 | 0.344 | 0.558 |
| STAI-Y s | 36.4(10.1) | 37.1(10.7) | 36.4(10.2) | 36.4(10.1) | 0.146 | 0.702 | 0.176 | 0.675 | |
| STAI-Y t | 39.6(9.9) | 39.7(9.9) | 38.8(9.9) | 39(9.6) | 0.678 | 0.411 | 0.002 | 0.964 | |
| Functional | BI | 86(19.9) | 84.6(20.7) | 86.6(19.7) | 86.1(18) | 0.328 | 0.567 | 0.061 | 0.804 |
| IADL | 6.3(2.3) | 6.2(2.4) | 6.2(2.2) | 5.9(2.5) | 0.755 | 0.385 | 0.197 | 0.657 | |
| Motor/Non-Motor * | Cognitive/Non-Cognitive | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Group | Int | FU-Int | Time | Group | Int | FU-Int | ||||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | ||
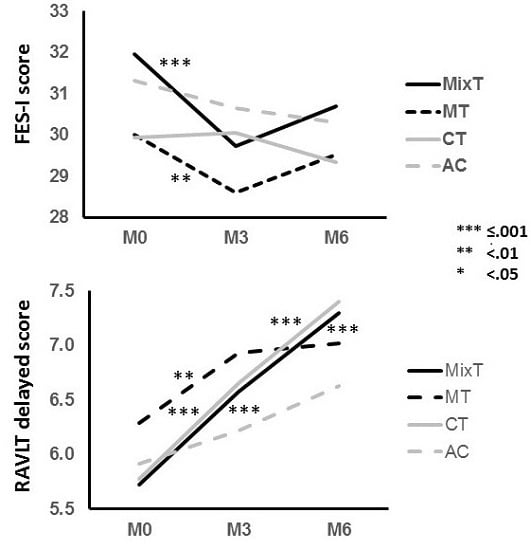
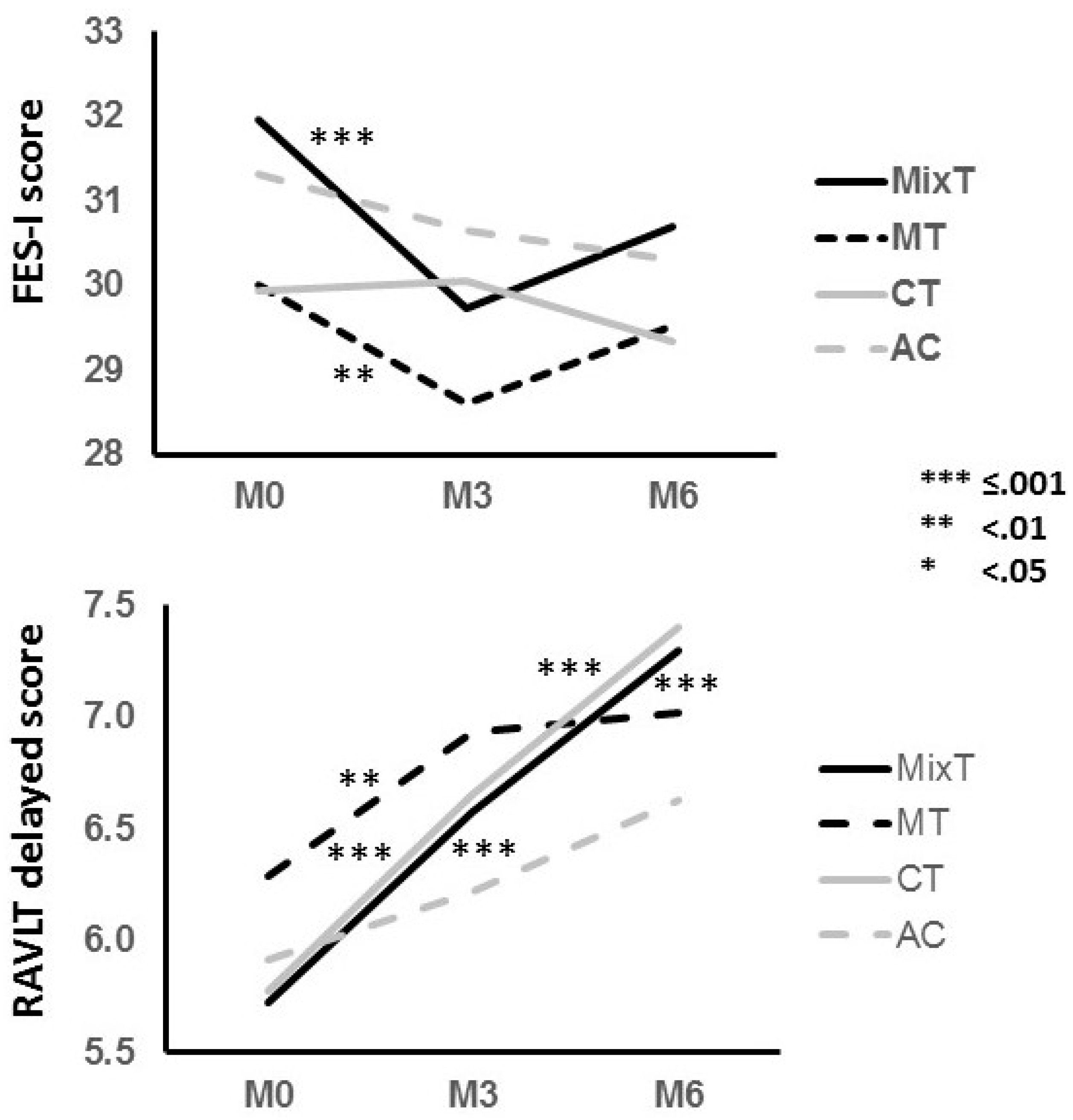
| Mobility | FES-I | 2.553 | 0.111 | 1.030 | 0.311 | 6.786 | 0.009 | 4.900 | 0.009 | 13.564 | <0.001 | 0.108 | 0.743 | 0.004 | 0.952 | 0.059 | 0.934 |
| POMA B | 37.422 | <0.001 | 1.166 | 0.281 | 3.442 | 0.064 | 2.016 | 0.146 | 61.258 | <0.001 | 1.219 | 0.270 | 0.000 | 0.999 | 0.155 | 0.797 | |
| POMA G | 5.626 | 0.018 | 0.599 | 0.439 | 0.005 | 0.943 | 0.318 | 0.707 | 24.402 | <0.001 | 1.016 | 0.314 | 0.901 | 0.343 | 0.590 | 0.539 | |
| Cognition | TMT B-A | 3.564 | 0.060 | 1.149 | 0.284 | 0.276 | 0.599 | 0.225 | 0.788 | 15.247 | <0.001 | 1.833 | 0.176 | 1.216 | 0.271 | 1.292 | 0.275 |
| PF | 7.450 | 0.007 | 0.302 | 0.583 | 0.185 | 0.667 | 1.403 | 0.247 | 7.634 | 0.006 | 0.367 | 0.545 | 0.445 | 0.505 | 0.277 | 0.744 | |
| RAVLT d | 20.425 | <0.001 | 0.379 | 0.538 | 0.845 | 0.358 | 0.517 | 0.583 | 53.692 | <0.001 | 0.238 | 0.626 | 4.699 | 0.031 | 9.040 | <0.001 | |
| ROCF d | 5.148 | 0.024 | 0.813 | 0.368 | 0.369 | 0.544 | 0.171 | 0.829 | 28.979 | <0.001 | 0.374 | 0.541 | 5.048 | 0.025 | 2.411 | 0.094 | |
| Behavior | GDS | 8.374 | 0.004 | 0.145 | 0.704 | 0.176 | 0.675 | 0.427 | 0.632 | 13.878 | <0.001 | 0.245 | 0.621 | 0.170 | 0.680 | 0.575 | 0.546 |
| STAI-Y s | 2.234 | 0.136 | 0.077 | 0.782 | 0.705 | 0.402 | 0.551 | 0.564 | 1.561 | 0.212 | 0.031 | 0.861 | 0.824 | 0.364 | 1.241 | 0.288 | |
| STAI-Y t | 0.020 | 0.888 | 0.066 | 0.798 | 5.649 | 0.018 | 2.738 | 0.069 | 0.008 | 0.928 | 0.000 | 0.998 | .023 | 0.881 | 0.506 | 0.592 | |
| Function | BI | 2.497 | 0.115 | 0.011 | 0.916 | 1.578 | 0.210 | 1.219 | 0.279 | 11.288 | 0.001 | 0.004 | 0.948 | 0.868 | 0.352 | 1.483 | 0.227 |
| IADL | 5.630 | 0.018 | 0.656 | 0.419 | 0.051 | 0.821 | 0.111 | 0.858 | 9.713 | 0.002 | 0.162 | 0.687 | 0.080 | 0.777 | 0.518 | 0.561 | |
| Treatment | Test | Pre | Post | FU | Pre-Post | ES | Post-FU | ES | ||
|---|---|---|---|---|---|---|---|---|---|---|
| t | p | t | p | |||||||
| MT | FES-I | 30(10.2) | 28.6(9.2) | 29.5(9.5) | 2.777 | 0.006 | −0.14 | −1.773 | 0.079 | 0.10 |
| MixT | 32(9.3) | 29.7(8.5) | 30.7(8.7) | 3.889 | <0.001 | −0.25 | −2.181 | 0.031 | 0.11 | |
| CT | 29.9(9.7) | 30.1(9.4) | 29.3(9.9) | −0.187 | 0.852 | 0.01 | 1.293 | 0.199 | −0.07 | |
| AC | 31.3(11) | 30.6(10.5) | 30.3(10.3) | 1.245 | 0.215 | −0.06 | 0.716 | 0.475 | −0.03 | |
| MT | RAVLT d | 6.3(3.8) | 6.9(3.5) | 7(3.7) | −2.780 | 0.006 | 0.17 | −0.443 | 0.659 | 0.03 |
| MixT | 5.7(3.5) | 6.6(3.3) | 7.3(3.2) | −5.986 | <0.001 | 0.25 | −3.555 | 0.001 | 0.22 | |
| CT | 5.8(3.7) | 6.7(3.7) | 7.4(3.8) | −5.782 | <0.001 | 0.24 | −3.405 | 0.001 | 0.20 | |
| AC | 5.9(3.5) | 6.2(3.3) | 6.6(3.3) | −1.593 | 0.114 | 0.09 | −2.076 | 0.040 | 0.12 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barban, F.; Annicchiarico, R.; Melideo, M.; Federici, A.; Lombardi, M.G.; Giuli, S.; Ricci, C.; Adriano, F.; Griffini, I.; Silvestri, M.; et al. Reducing Fall Risk with Combined Motor and Cognitive Training in Elderly Fallers. Brain Sci. 2017, 7, 19. https://doi.org/10.3390/brainsci7020019
Barban F, Annicchiarico R, Melideo M, Federici A, Lombardi MG, Giuli S, Ricci C, Adriano F, Griffini I, Silvestri M, et al. Reducing Fall Risk with Combined Motor and Cognitive Training in Elderly Fallers. Brain Sciences. 2017; 7(2):19. https://doi.org/10.3390/brainsci7020019
Chicago/Turabian StyleBarban, Francesco, Roberta Annicchiarico, Matteo Melideo, Alessia Federici, Maria Giovanna Lombardi, Simone Giuli, Claudia Ricci, Fulvia Adriano, Ivo Griffini, Manuel Silvestri, and et al. 2017. "Reducing Fall Risk with Combined Motor and Cognitive Training in Elderly Fallers" Brain Sciences 7, no. 2: 19. https://doi.org/10.3390/brainsci7020019
APA StyleBarban, F., Annicchiarico, R., Melideo, M., Federici, A., Lombardi, M. G., Giuli, S., Ricci, C., Adriano, F., Griffini, I., Silvestri, M., Chiusso, M., Neglia, S., Ariño-Blasco, S., Cuevas Perez, R., Dionyssiotis, Y., Koumanakos, G., Kovačeić, M., Montero-Fernández, N., Pino, O., ... Caltagirone, C. (2017). Reducing Fall Risk with Combined Motor and Cognitive Training in Elderly Fallers. Brain Sciences, 7(2), 19. https://doi.org/10.3390/brainsci7020019