Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Training and Maintenance on an Extended Access Schedule of Intravenous Methamphetamine Self-Administration
2.3. Progressive Ratio
2.4. Extinction
2.5. Reinstatement
2.6. Estrus Cycle Tracking
2.7. Slice Preparation and Electrophysiology
2.8. 5-bromo-2′-deoxyuridine (BrdU) Injections
2.9. Brain Tissue Collection for Immunohistochemistry and Western Blotting
2.10. Immunohistochemistry (IHC)
2.11. Analysis of Microglial Cells
2.12. Western Blotting
2.13. Statistical Analyses
3. Results
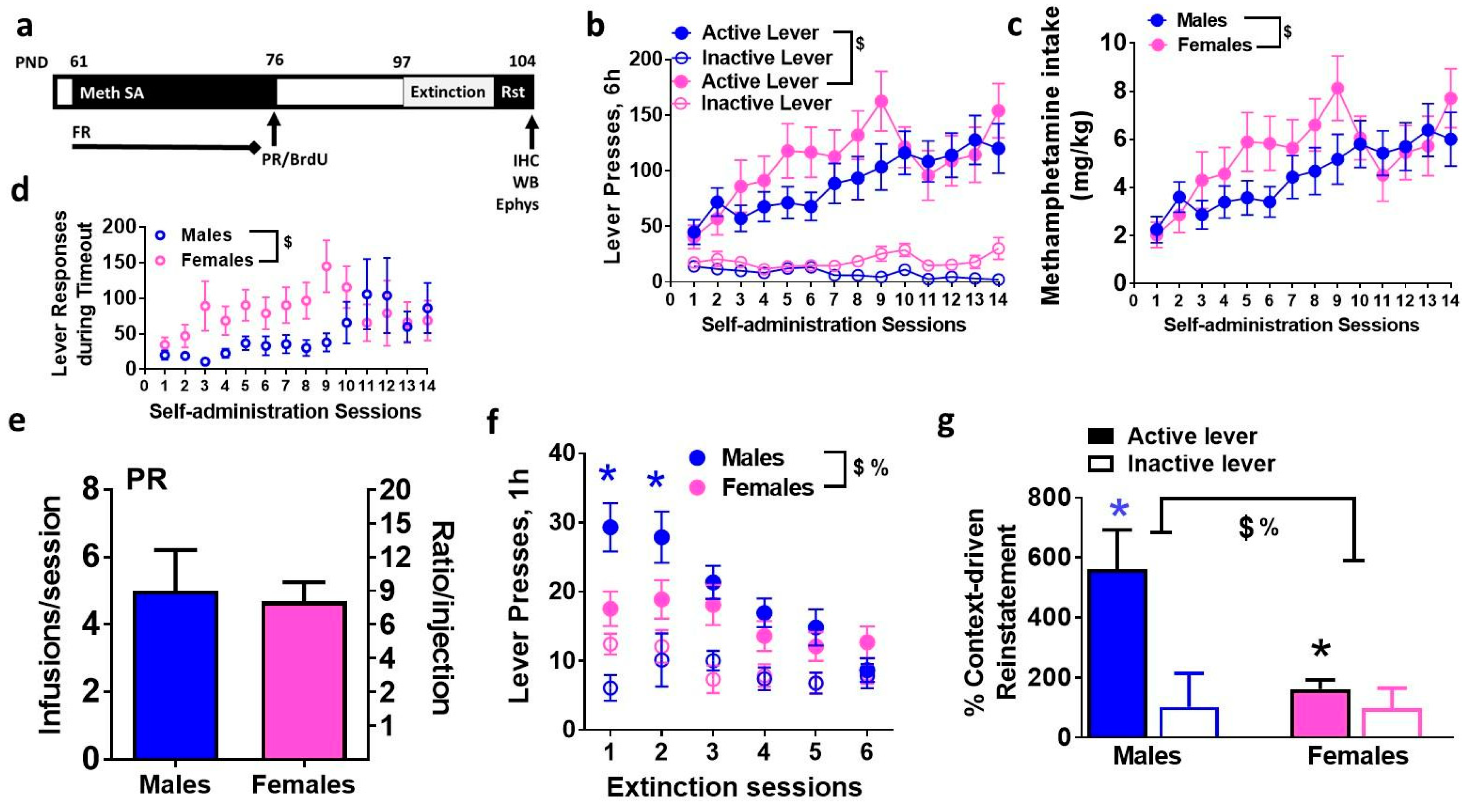
3.1. Extended Access to Meth Self-Administration Resulted in Escalation of Meth Intake in Male and Female Rats
3.2. Males and Females Distinctly Extinguish Operant Behavior and Demonstrate Varied Context-Driven Reinstatement of Methamphetamine Seeking
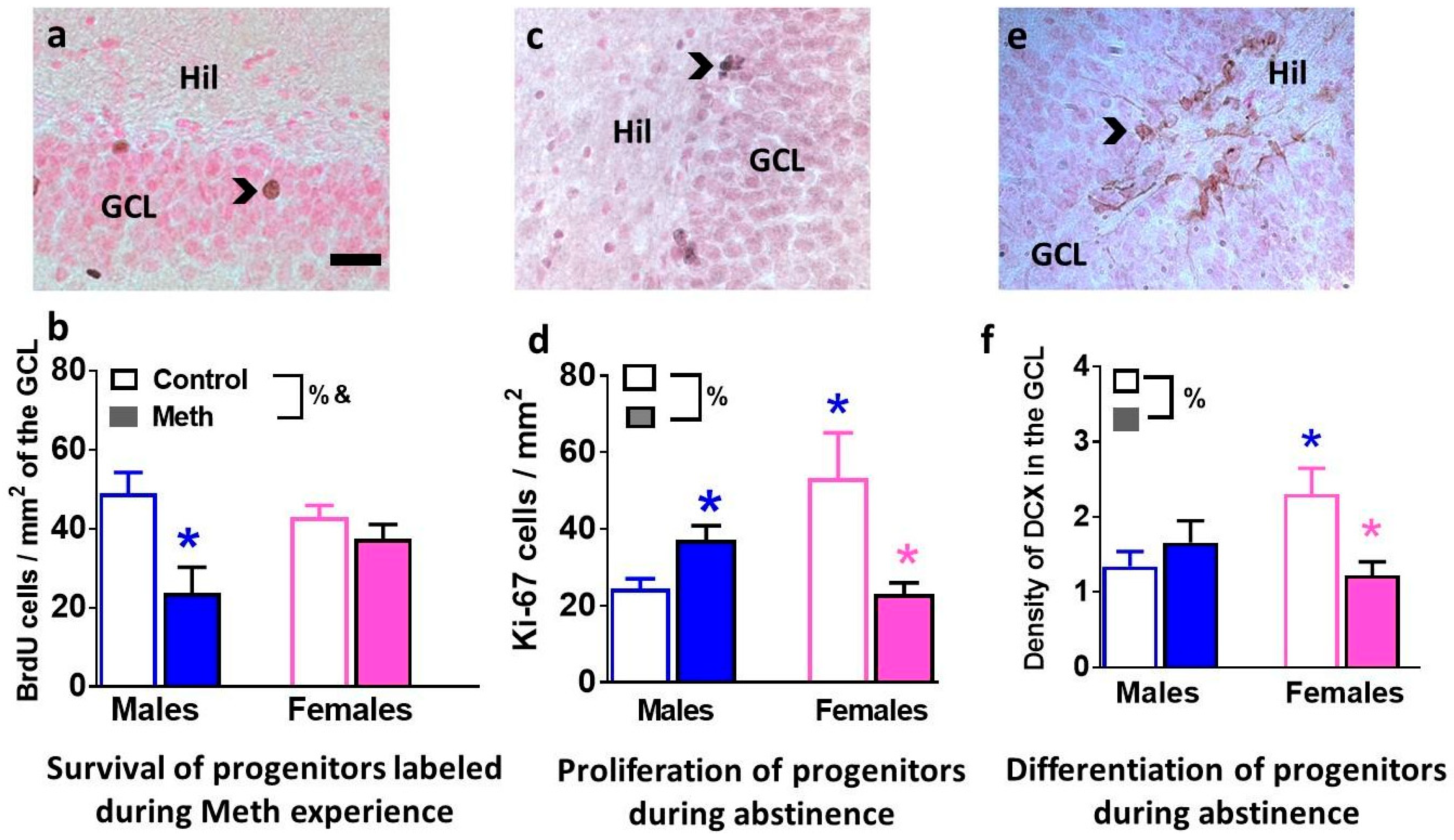
3.3. Immunohistochemical Analysis with Markers of Proliferation, Differentiation and Survival Reveal Distinct Effects of Methamphetamine and Abstinence on Progenitor Cells in Males and Females
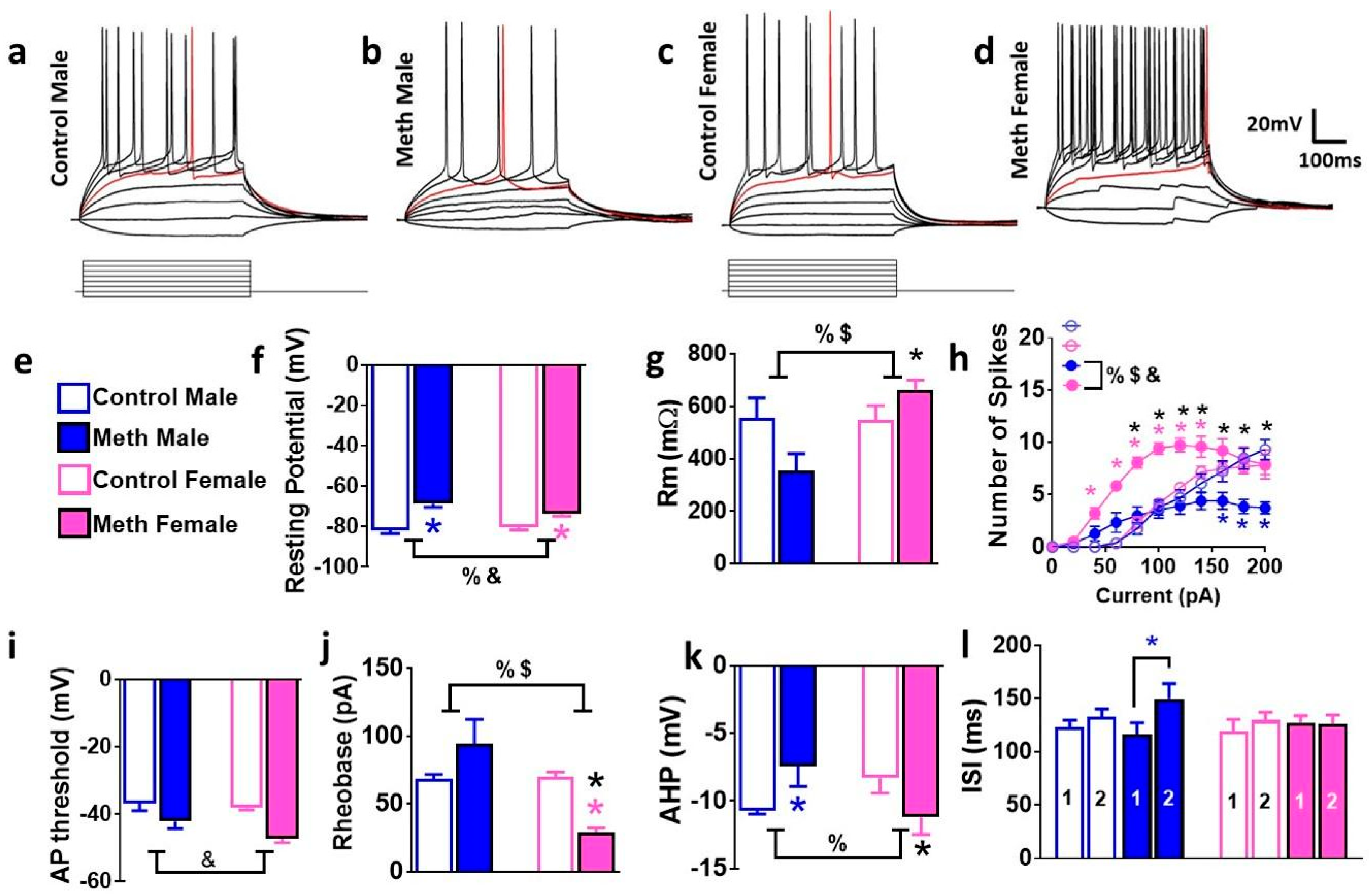
3.4. Intrinsic Firing Properties of GCNs are Differentially Altered in Male and Female Rats that Experienced Context-Driven Methamphetamine Seeking
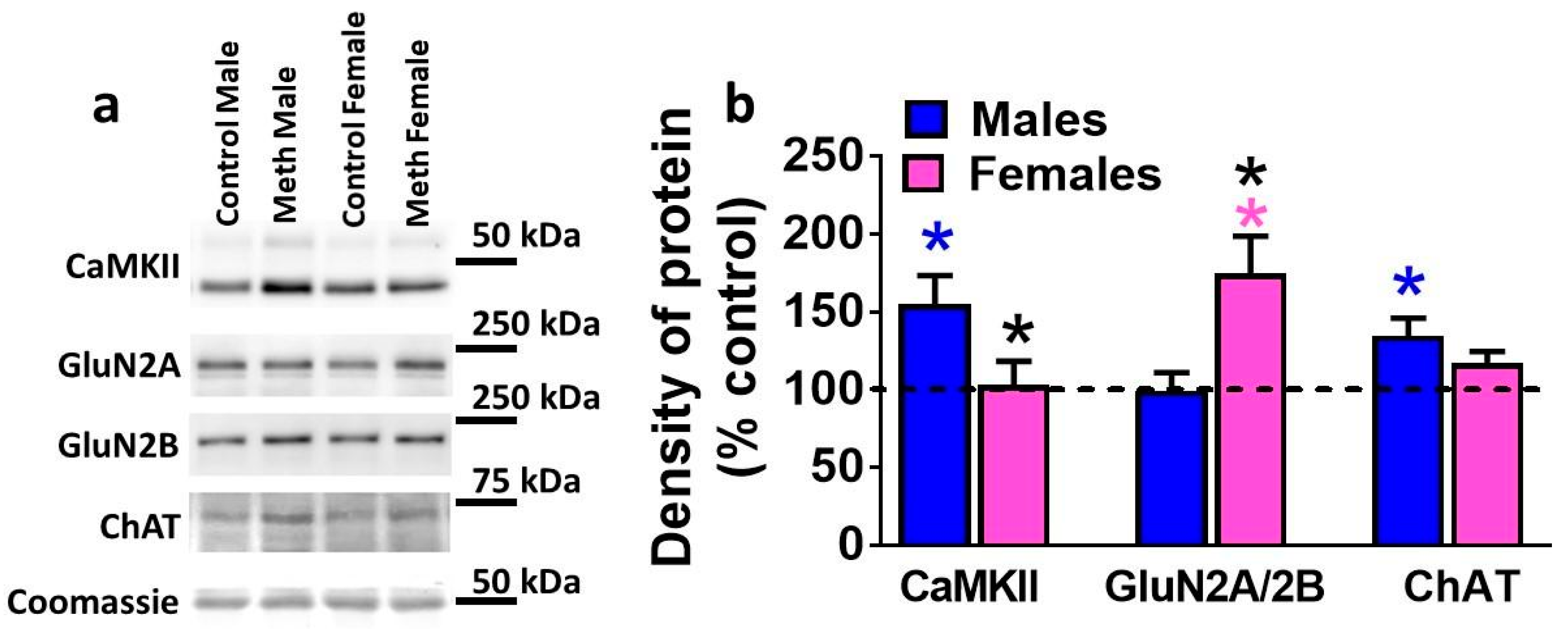
3.5. Expression of Plasticity-Related Proteins in the DG is Differentially Altered by Reinstatement of Methamphetamine Seeking in Males and Females
3.6. Density of Mossy Fiber Tracts in the Hilus and Pyramidal Projections of the Dentate Gyrus are Similarly Effected by Context-Driven Methamphetamine in Male and Female Rats
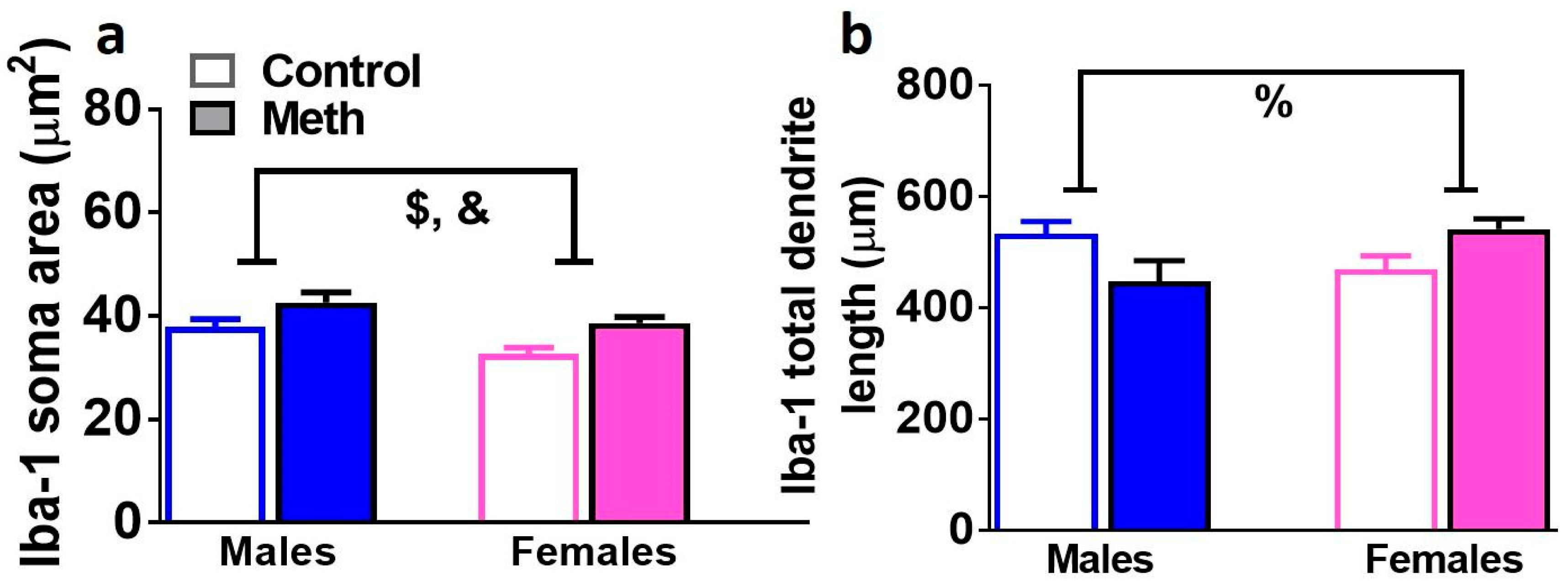
3.7. Analysis of 3D Structure of Iba-1 Cells Demonstrates Neuroimmune Response in Male and Female Methamphetamine Rats
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, M.E.; Anker, J.J.; Perry, J.L. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol. Depend. 2009, 104, S70–S78. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kandel, D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol. Depend. 2002, 68, 65–85. [Google Scholar] [CrossRef]
- Brady, K.T.; Randall, C.L. Gender differences in substance use disorders. Psychiatr. Clin. N. Am. 1999, 22, 241–252. [Google Scholar] [CrossRef]
- Hedden, S.L. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health; HHS Publication No. SMA 15-4927; Substance Abuse and Mental Health Services Administration, Department of Heath & Human Services: Rockville, MD, USA, 2015.
- Dluzen, D.E.; Liu, B. Gender differences in methamphetamine use and responses: A review. Gend. Med. 2008, 5, 24–35. [Google Scholar] [CrossRef]
- Rungnirundorn, T.; Verachai, V.; Gelernter, J.; Malison, R.T.; Kalayasiri, R. Sex Differences in Methamphetamine Use and Dependence in a Thai Treatment Center. J. Addict. Med. 2017, 11, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Holtz, N.A.; Lozama, A.; Prisinzano, T.E.; Carroll, M.E. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol. Depend. 2012, 120, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, J.; Vrskova, D.; Sulcova, A. Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non-estrogenized ovariectomized female rats. Neuro Endocrinol. Lett. 2009, 30, 663–670. [Google Scholar] [PubMed]
- Roth, M.E.; Carroll, M.E. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology 2004, 172, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Reichel, C.M.; Chan, C.H.; Ghee, S.M.; See, R.E. Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences in rats. Psychopharmacology 2012, 223, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Nelson, S.E.; Carroll, M.E. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp. Clin. Psychopharmacol. 2008, 16, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Milesi-Halle, A.; McMillan, D.E.; Laurenzana, E.M.; Byrnes-Blake, K.A.; Owens, S.M. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007, 86, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.W.; Bross, J.G.; Thorndike, E.B. Gender differences in the behavioral effects of methamphetamine. Eur. J. Pharmacol. 2002, 442, 231–235. [Google Scholar] [CrossRef]
- Kerstetter, K.A.; Aguilar, V.R.; Parrish, A.B.; Kippin, T.E. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology 2008, 198, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.A.; Evans, K.A.; Mehta, R.H.; Case, J.M.; See, R.E. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 2005, 179, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.J.; Perry, J.L.; Gliddon, L.A.; Carroll, M.E. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol. Biochem. Behav. 2009, 93, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Liu, B.; Dluzen, D.E.; Di Paolo, T. Sex differences in methamphetamine toxicity in mice: Effect on brain dopamine signaling pathways. Psychoneuroendocrinology 2011, 36, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Galinato, M.H.; Orio, L.; Mandyam, C.D. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience 2015, 286, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, J.C.; Kim, A.; Fannon, M.J.; Mandyam, C.D. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct. Funct. 2016, 221, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Shaham, Y.; Shalev, U.; Lu, L.; De Wit, H.; Stewart, J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology 2003, 168, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]
- Wee, S.; Wang, Z.; Woolverton, W.L.; Pulvirenti, L.; Koob, G.F. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology 2007, 32, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Galinato, M.H.; Lockner, J.W.; Fannon-Pavlich, M.J.; Sobieraj, J.C.; Staples, M.C.; Somkuwar, S.S.; Ghofranian, A.; Chaing, S.; Navarro, A.I.; Joea, A.; et al. A synthetic small-molecule Isoxazole-9 protects against methamphetamine relapse. Mol. Psychiatry 2018, 23, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Fannon, M.J.; Galinato, M.H.; Steiner, N.L.; An, M.; Zemljic-Harpf, A.E.; Somkuwar, S.S.; Head, B.P.; Mandyam, C.D. Neuroadaptations in the dentate gyrus following contextual cued reinstatement of methamphetamine seeking. Brain Struct. Funct. 2018, 223, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon-Pavlich, M.J.; Ghofranian, A.; Quigley, J.A.; Dutta, R.R.; Galinato, M.H.; Mandyam, C.D. Wheel running reduces ethanol seeking by increasing neuronal activation and reducing oligodendroglial/neuroinflammatory factors in the medial prefrontal cortex. Brain Behav. Immun. 2016, 58, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon, M.J.; Staples, M.C.; Zamora-Martinez, E.R.; Navarro, A.I.; Kim, A.; Quigley, J.A.; Edwards, S.; Mandyam, C.D. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct. Funct. 2016, 221, 4319–4335. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Staples, M.C.; Fannon, M.J.; Mysore, K.K.; Dutta, R.R.; Ongjoco, A.T.; Quach, L.W.; Kharidia, K.M.; Somkuwar, S.S.; Mandyam, C.D. Dietary restriction reduces hippocampal neurogenesis and granule cell neuron density without affecting the density of mossy fibers. Brain Res. 2017, 1663, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using Image. J. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Zamora-Martinez, E.R.; Edwards, S.; Mandyam, C.D. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct. Funct. 2015, 220, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Leong, K.C.; Berini, C.; Reichel, C.M. Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacol. Biochem. Behav. 2017, 161, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M.; Bentzley, B.S.; Regen-Tuero, H.; See, R.E.; Reichel, C.M.; Aston-Jones, G. Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand. Biol. Psychiatry 2017, 81, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, S.T.; Chou, S.; Barrett, S.T.; Catalano, I.; Lydiatt, M.; Bevins, R.A. Nicotine- and cocaine-triggered methamphetamine reinstatement in female and male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2017, 159, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ruda-Kucerova, J.; Amchova, P.; Babinska, Z.; Dusek, L.; Micale, V.; Sulcova, A. Sex Differences in the Reinstatement of Methamphetamine Seeking after Forced Abstinence in Sprague-Dawley Rats. Front. Psychiatry 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.A.; Logan, C.N.; Leong, K.C.; Peris, J.; Knackstedt, L.; Reichel, C.M. Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats. Int. J. Neuropsychopharmacol. 2018, 21, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Molenda, H.; Hummer, D.L. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann. N. Y. Acad. Sci. 2001, 937, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Ohia-Nwoko, O.; Haile, C.N.; Kosten, T.A. Sex differences in the acute locomotor response to methamphetamine in BALB/c mice. Behav. Brain Res. 2017, 327, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M.; Young, A.B.; See, R.E.; Reichel, C.M. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 2013, 38, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.B.; Wissman, A.M.; Loriaux, A.L.; Kourrich, S.; Self, D.W. Optogenetic stimulation of accumbens shell or shell projections to lateral hypothalamus produce differential effects on the motivation for cocaine. J. Neurosci. 2015, 35, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Hiranita, T.; Nawata, Y.; Sakimura, K.; Yamamoto, T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology 2008, 55, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Galinato, M.H.; Takashima, Y.; Fannon, M.J.; Quach, L.W.; Morales Silva, R.J.; Mysore, K.K.; Terranova, M.J.; Dutta, R.R.; Ostrom, R.W.; Somkuwar, S.S.; et al. Neurogenesis during Abstinence Is Necessary for Context-Driven Methamphetamine-Related Memory. J. Neurosci. 2018, 38, 2029–2042. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, C.D.; Wee, S.; Crawford, E.F.; Eisch, A.J.; Richardson, H.N.; Koob, G.F. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol. Psychiatry 2008, 64, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.J.; Quiocho, J.M.; Kim, A.; Wee, S.; Mandyam, C.D. Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol. Biochem. Behav. 2011, 100, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Recinto, P.; Samant, A.R.; Chavez, G.; Kim, A.; Yuan, C.J.; Soleiman, M.; Grant, Y.; Edwards, S.; Wee, S.; Koob, G.F.; et al. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 2012, 37, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [PubMed]
- Brown, J.P.; Couillard-Despres, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Romer, B.; Krebs, J.; Overall, R.W.; Fabel, K.; Babu, H.; Overstreet-Wadiche, L.; Brandt, M.D.; Williams, R.W.; Jessberger, S.; Kempermann, G. Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection: I. Co-regulation by activity. Front. Neurosci. 2011, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.J.; Shin, Y.J.; Lindquist, D.H. Mitigation of postnatal ethanol-induced neuroinflammation ameliorates trace fear memory deficits in juvenile rats. Behav. Brain Res. 2018, 338, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Klaus, F.; Paterna, J.C.; Marzorati, E.; Sigrist, H.; Gotze, L.; Schwendener, S.; Bergamini, G.; Jehli, E.; Azzinnari, D.; Fuertig, R.; et al. Differential effects of peripheral and brain tumor necrosis factor on inflammation, sickness, emotional behavior and memory in mice. Brain Behav. Immun. 2016, 58, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Geil, C.R.; Nixon, K. Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration. Brain Sci. 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.B.; Sparkman, N.L.; Johnson, R.W. A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J. Neuroinflamm. 2010, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; DeSpenza, T., Jr.; Li, M.; Gulledge, A.T.; Luikart, B.W. Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J. Neurosci. 2015, 35, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hung, F.S.; Zhu, Y.; Xie, Z.; Wang, J.H. Calcium signal-dependent plasticity of neuronal excitability developed postnatally. J. Neurobiol. 2004, 61, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Crestani, A.P.; Krueger, J.N.; Barragan, E.V.; Nakazawa, Y.; Nemes, S.E.; Quillfeldt, J.A.; Gray, J.A.; Wiltgen, B.J. Metaplasticity contributes to memory formation in the hippocampus. Neuropsychopharmacology 2018. [Google Scholar] [CrossRef] [PubMed]
- Sametsky, E.A.; Disterhoft, J.F.; Ohno, M. Autophosphorylation of alphaCaMKII downregulates excitability of CA1 pyramidal neurons following synaptic stimulation. Neurobiol. Learn. Mem. 2009, 92, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Milshtein-Parush, H.; Frere, S.; Regev, L.; Lahav, C.; Benbenishty, A.; Ben-Eliyahu, S.; Goshen, I.; Slutsky, I. Sensory Deprivation Triggers Synaptic and Intrinsic Plasticity in the Hippocampus. Cereb. Cortex 2017, 27, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Klug, J.R.; Mathur, B.N.; Kash, T.L.; Wang, H.D.; Matthews, R.T.; Robison, A.J.; Anderson, M.E.; Deutch, A.Y.; Lovinger, D.M.; Colbran, R.J.; et al. Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability. PLoS ONE 2012, 7, e45323. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Gittis, A.H.; du Lac, S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 2005, 46, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Van Welie, I.; du Lac, S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J. Neurophysiol. 2011, 105, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Sametsky, E.A.; Silva, A.J.; Disterhoft, J.F. Differential effects of alphaCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur. J. Neurosci. 2006, 23, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hieber, C.; Jonas, P.; Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 2004, 429, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Neubrandt, M.; Van-Weert, S.; Andrasi, T.; Kleine Borgmann, F.B.; Jessberger, S.; Szabadics, J. Adult-born granule cells mature through two functionally distinct states. eLife 2014, 3, e03104. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Yang, C.H.; Hsu, K.S.; Ming, G.L.; Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 2007, 54, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Marin-Burgin, A.; Mongiat, L.A.; Pardi, M.B.; Schinder, A.F. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 2012, 335, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Dieni, C.V.; Nietz, A.K.; Panichi, R.; Wadiche, J.I.; Overstreet-Wadiche, L. Distinct determinants of sparse activation during granule cell maturation. J. Neurosci. 2013, 33, 19131–19142. [Google Scholar] [CrossRef] [PubMed]
- Dieni, C.V.; Panichi, R.; Aimone, J.B.; Kuo, C.T.; Wadiche, J.I.; Overstreet-Wadiche, L. Low excitatory innervation balances high intrinsic excitability of immature dentate neurons. Nat. Commun. 2016, 7, 11313. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; Potter, M.C.; Choi, J.; Lee, J.Y.; Stringer, T.P.; Callaway, E.M.; Gage, F.H.; Suh, H.; van Praag, H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat. Commun. 2012, 3, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ide, Y.; Fujiyama, F.; Okamoto-Furuta, K.; Tamamaki, N.; Kaneko, T.; Hisatsune, T. Rapid integration of young newborn dentate gyrus granule cells in the adult hippocampal circuitry. Eur. J. Neurosci. 2008, 28, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Kuhn, C.M.; Winkler, J.; Kuhn, H.G. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J. Neurosci. Res. 2004, 77, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mohapel, P.; Leanza, G.; Kokaia, M.; Lindvall, O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol. Aging 2005, 26, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Okano, H.; Sawamoto, K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 2006, 11, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Vaughn, T.C.; Co, C. Acetylcholine turnover rates in rat brain regions during cocaine self-administration. J. Neurochem. 2004, 88, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.R.; Druhan, J.P. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur. J. Neurosci. 2000, 12, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Neill, D.B.; Justice, J.B., Jr. Increased sensitivity to cocaine place-preference conditioning by septal lesions in rats. Brain Res. 1995, 683, 221–227. [Google Scholar] [CrossRef]
- Imperato, A.; Obinu, M.C.; Mascia, M.S.; Casu, M.A.; Zocchi, A.; Cabib, S.; Puglisi-Allegra, S. Strain-dependent effects of dopamine agonists on acetylcholine release in the hippocampus: An in vivo study in mice. Neuroscience 1996, 70, 653–660. [Google Scholar] [CrossRef]
- Martin, K.J.; Vyas, S. Increase in acetylcholine concentrations in the brain of ‘old’ rats following treatment with pyrithioxin (Encephabol). Br. J. Pharmacol. 1987, 90, 561–565. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takashima, Y.; Tseng, J.; Fannon, M.J.; Purohit, D.C.; Quach, L.W.; Terranova, M.J.; Kharidia, K.M.; Oliver, R.J.; Mandyam, C.D. Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus. Brain Sci. 2018, 8, 208. https://doi.org/10.3390/brainsci8120208
Takashima Y, Tseng J, Fannon MJ, Purohit DC, Quach LW, Terranova MJ, Kharidia KM, Oliver RJ, Mandyam CD. Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus. Brain Sciences. 2018; 8(12):208. https://doi.org/10.3390/brainsci8120208
Chicago/Turabian StyleTakashima, Yoshio, Joyee Tseng, McKenzie J. Fannon, Dvijen C. Purohit, Leon W. Quach, Michael J. Terranova, Khush M. Kharidia, Robert J. Oliver, and Chitra D. Mandyam. 2018. "Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus" Brain Sciences 8, no. 12: 208. https://doi.org/10.3390/brainsci8120208
APA StyleTakashima, Y., Tseng, J., Fannon, M. J., Purohit, D. C., Quach, L. W., Terranova, M. J., Kharidia, K. M., Oliver, R. J., & Mandyam, C. D. (2018). Sex Differences in Context-Driven Reinstatement of Methamphetamine Seeking is Associated with Distinct Neuroadaptations in the Dentate Gyrus. Brain Sciences, 8(12), 208. https://doi.org/10.3390/brainsci8120208




