In Silico Analysis of Microarray-Based Gene Expression Profiles Predicts Tumor Cell Response to Withanolides
Abstract
:1. Introduction
2. Results
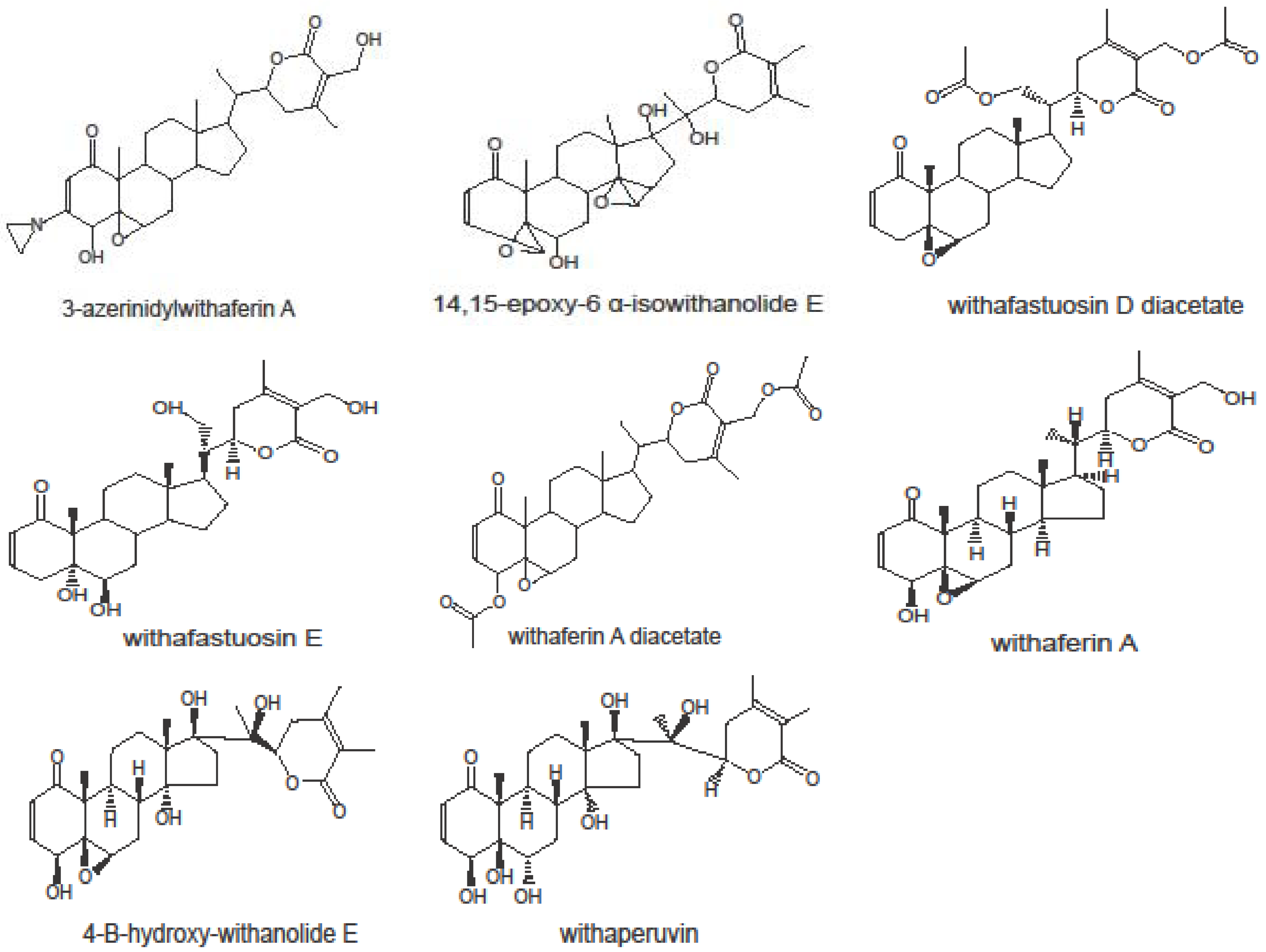
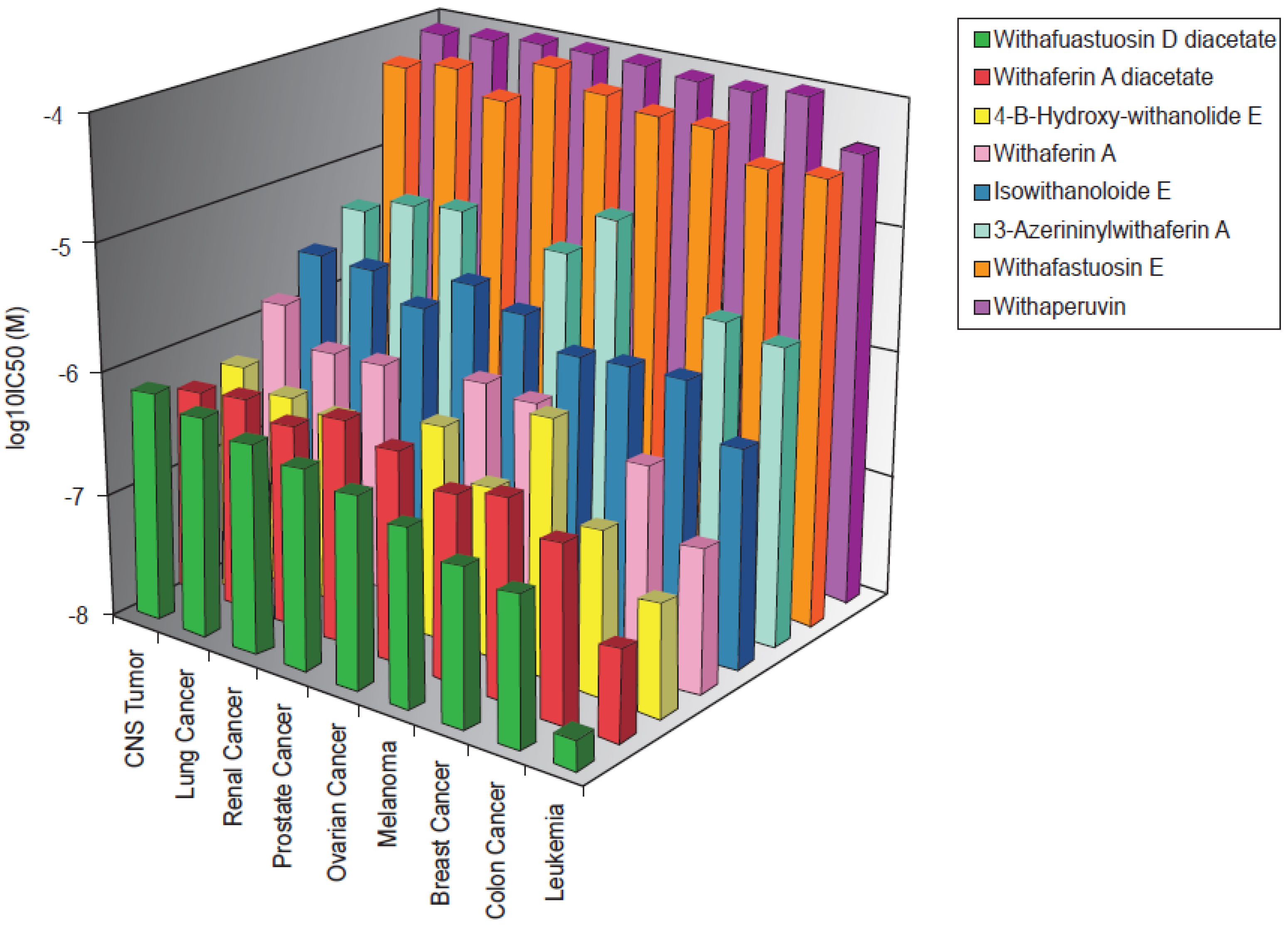
2.1. Cytotoxicity of Withanolides towards Cancer Cells
2.2. Cross-Resistance of the NCI Cell Line Panel Towards Withanolides
| Compounds | R-/P-Values | Withafastuosin D diacetate | 4-B-hydroxy-withanolide E | Withaferin A diacetate | Withaferin A | Isowithanololide E | 3-Azerininyl-withaferin A | Withafastuosin E | Withaperuvin |
|---|---|---|---|---|---|---|---|---|---|
| Withanolides: | |||||||||
| Withafastuosin D diacetate | R-value | 0.517 | 0.853 | 0.67 | 0.608 | 0.528 | 0.413 | 0.544 | |
| P-value | 1.00 × 10−4 | 9.18 × 10−18 | 4.37 × 10−7 | 2.08 × 10−7 | 8.22 × 10−5 | 8.64 × 10−4 | 5.11 × 10−6 | ||
| 4-B-hydroxy-withanolide E | R-value | 0.548 | 0.548 | 0.452 | 0.42 | 0.492 | 0.469 | ||
| P-value | 3.29 × 10−5 | 1.05 × 10−4 | 7.06 × 10−4 | 0.003 | 3.01 × 10−4 | 4.48 × 10−4 | |||
| Withaferin A diacetate | R-value | 0.701 | 0.7 | 0.475 | 0.426 | 0.623 | |||
| P-value | 5.95 × 10−8 | 3.47 × 10−10 | 3.69 × 10−4 | 5.98 × 10−4 | 6.99 × 10−8 | ||||
| Withaferin A | R-value | 0.609 | 0.549 | 0.358 | 0.466 | ||||
| P-value | 5.70 × 10−6 | 8.41 × 10−5 | 0.001 | 7.17 × 10−4 | |||||
| Isowithanololide E | R-value | 0.348 | 0.439 | 0.513 | |||||
| P-value | 0.008 | 3.94 × 10−4 | 1.63 × 10−5 | ||||||
| 3-Azerininyl-withaferin A | R-value | 0.28 | 0.349 | ||||||
| P-value | 0.033 | 0.008 | |||||||
| Withafastuosin E | R-value | 0.291 | |||||||
| P-value | 0.016 | ||||||||
| Alkylating agents: | |||||||||
| Carmustine (BCNU) | R-value | 0.607 | 0.466 | 0.747 | 0.508 | 0.59 | 0.393 | 0.411 | 0.721 |
| P-value | 1.74 × 10−7 | 4.21 × 10−4 | 5.64 × 10−12 | 2.16 × 10−4 | 4.47 × 10−7 | 0.003 | 8.27 × 10−4 | 5.79 × 10−11 | |
| Lomustine (CCNU) | R-value | 0.515 | 0.509 | 0.579 | 0.517 | 0.43 | 0.304 | 0.381 | 0.479 |
| P-value | 1.51 × 10−5 | 1.09 × 10−4 | 7.78 × 10−7 | 1.65 × 10−4 | 3.37 × 10−4 | 0.019 | 0.002 | 6.15 × 10−5 | |
| Semustine (me-CCNU) | R-value | 0.542 | 0.389 | 0.653 | 0.381 | 0.519 | 0.305 | 0.362 | 0.618 |
| P-value | 5.63 × 10−6 | 0.003 | 1.36 × 10−8 | n.s. | 1.48 × 10−5 | 0.019 | 0.003 | 1.2 × 10−7 | |
| Melphalan | R-value | 0.471 | 0.268 | 0.496 | 0.349 | 0.429 | 0.186 | 0.253 | 0.55 |
| P-value | 8.47 × 10−5 | 0.033 | 3.25 × 10−5 | 0.01 | 3.53 × 10−4 | n.s. | 0.03 | 3.24 × 10−6 | |
| Ifosfamide | R-value | 0.162 | 0.222 | 0.14 | 0.265 | 0.136 | 0.204 | 0.199 | 0.083 |
| P-value | n.s. | n.s. | n.s. | 0.041 | n.s. | n.s. | n.s. | n.s. | |
| Platin compounds: | |||||||||
| Cisplatin | R-value | 0.199 | 0.11 | 0.296 | 0.191 | 0.282 | 0.05 | 0.131 | 0.379 |
| P-value | n.s. | n.s. | 0.011 | n.s. | 0.015 | n.s. | n.s. | 0.002 | |
| Carboplatin | R-value | 0.124 | 0.22 | 0.279 | 0.195 | 0.308 | 0.11 | 0.127 | 0.391 |
| P-value | n.s. | n.s. | 0.016 | n.s. | 0.009 | n.s. | n.s. | 0.001 | |
| DNA topoisomerase I inhibitors: | |||||||||
| Camptothcin | R-value | 0.205 | −0.018 | 0.131 | 0.076 | 0.05 | 0.125 | 0.069 | 0.307 |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Topotecan | R-value | 0.242 | 0.119 | 0.178 | 0.108 | 0.102 | 0.104 | 0.198 | 0.308 |
| P-value | 0.032 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.009 | |
| DNA topoisomerase II inhibitors: | |||||||||
| Adriamycin | R-value | 0.327 | 0.198 | 0.362 | 0.203 | 0.196 | 0.267 | 0.207 | 0.413 |
| P-value | 0.006 | n.s. | 0.002 | n.s. | n.s. | 0.035 | n.s. | 5.70 × 10−4 | |
| Daunorubicin | R-value | 0.44 | 0.213 | 0.447 | 0.305 | 0.21 | 0.333 | 0.247 | 0.46 |
| P-value | 2.44 × 10−4 | n.s. | 1.95 × 10−4 | 0.022 | n.s. | 0.011 | 0.033 | 1.25 × 10−4 | |
| Etoposide | R-value | 0.237 | 0.063 | 0.244 | 0.256 | 0.199 | 0.15 | 0.109 | 0.246 |
| P-value | 0.036 | n.s. | 0.031 | 0.047 | n.s. | n.s. | n.s. | 0.03 | |
| Teniposide | R-value | 0.343 | 0.215 | 0.358 | 0.217 | 0.203 | 0.264 | 0.185 | 0.437 |
| P-value | 0.004 | n.s. | 0.003 | n.s. | n.s. | 0.037 | n.s. | 2.67 × 10−4 | |
| Antimetabolites: | |||||||||
| 5-Fluorouracil | R-value | 0.357 | 0.16 | 0.365 | 0.054 | 0.3 | 0.25 | 0.272 | 0.129 |
| P-value | 0.003 | n.s. | 0.002 | n.s. | 0.011 | 0.046 | 0.021 | n.s. | |
| Methotrexate | R-value | 0.451 | 0.114 | 0.418 | 0.154 | 0.27 | 0.299 | 0.218 | 0.21 |
| P-value | 1.66 × 10−4 | n.s. | 4.94 × 10−4 | n.s. | 0.019 | 0.021 | n.s. | n.s. | |
| Mitotic spindle poisons: | |||||||||
| Vincristine | R-value | 0.283 | 0.266 | 0.307 | 0.177 | 0.102 | 0.446 | 0.258 | 0.156 |
| P-value | 0.015 | 0.034 | 0.009 | n.s. | n.s. | 8.32 × 10−4 | 0.028 | n.s. | |
| Vinblastine | R-value | 0.033 | −0.222 | 0.041 | −0.040 | 0.017 | −0.007 | 0.085 | 0.058 |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Paclitaxel | R-value | 0.283 | 0.193 | 0.308 | 0.143 | 0.048 | 0.399 | 0.304 | 0.315 |
| P-value | 0.015 | n.s. | 0.009 | n.s. | n.s. | 0.003 | 0.011 | 0.008 | |
| Taxotere | R-value | 0.202 | −0.041 | 0.158 | 0.146 | 0.098 | 0.337 | 0.177 | 0.215 |
| P-value | n.s. | n.s. | n.s. | n.s. | n.s. | 0.01 | n.s. | n.s. | |
2.3. COMPARE and Cluster Analyses of Microarray-Based mRNA Hybridization:
| Symbol | COMPARE Coefficient | ID No. | Genebank | Name | Function |
|---|---|---|---|---|---|
| Cluster 1: | |||||
| CORO1A | 0.65 | GC9728 | AA047478 | Coronin, actin binding protein, 1A | Cytoskeleton component |
| LSM2 | 0.646 | GC31813 | AJ245416 | LSM2 homolog, U6 small nuclear RNA associated (S. cerevisiae) | Pre-mRNA splicing |
| HCLS1 | 0.676 | GC34797 | X16663 | Hematopoietic cell-specific Lyn substrate 1 | Transcription factor |
| Cluster 2: | |||||
| unknown | 0.643 | GC34785 | X79234 | unknown | Unknown |
| PLS3 | −0.606 | GC37799 | M22299 | Plastin 3 | Actin-bundling protein |
| RAD54L | 0.654 | GC32858 | X97795 | RAD54-like (S. cerevisiae) | DNA repair and mitotic recombination |
| RPL5 | 0.648 | GC36655 | U14966 | Ribosomal protein L5 | Structural constituent of ribosome |
| IKZF1 | 0.648 | GC61547 | AI247840 | IKAROS family zinc finger 1 (Ikaros) | Transcriptional regulator |
| DLG2 | −0.612 | GC10718 | R41930 | Discs, large homolog 2 (Drosophila) | Signal transducer, required for perception of chronic pain through NMDA receptor signaling |
| RPS23 | 0.669 | GC37806 | D14530 | Ribosomal protein S23 | Structural constituent of ribosome |
| unknown | 0.674 | GC33814 | D11327 | unknown | Unknown |
| RNF138 | 0.688 | GC67595 | AI608790 | Ring finger protein 138 | ubiquitin-protein ligase, ubiquitinylation |
| unknown | 0.663 | GC31615 | X79234 | unknown | Unknown |
| LCP1 | 0.649 | GC27422 | J02923 | Lymphocyte cytosolic protein 1 (L-plastin) | Actin-binding protein, T-cell activation |
| LAMB1 | −0.610 | GC18026 | AA004918 | Laminin, beta-1 | Extracellular matrix structural constituent |
| SH3BP4 | −0.608 | GC16071 | W72796 | SH3-domain binding protein | Signal transducer, functions in transferrin receptor internalization at the plasma membrane |
| UACA | −0.624 | GC14684 | N66980 | Uveal autoantigen with coiled-coil domains and ankyrin repeats | Regulation of stress-induced apoptosis |
| BCAR3 | −0.636 | GC14433 | N48319 | Breast cancer anti-estrogen resistance 3 | Adapter protein for activated growth factor receptors to signaling pathways that regulate cell proliferation |
| ZNF112 | −0.640 | GC15668 | W15410 | Zinc finger protein 112 homolog (mouse) | DNA binding, transcriptional regulator |
| LOC440055 | 0.721 | GC36107 | AA977163 | Similar to ribosomal protein S12 | Unknown |
| Unknown | −0.601 | GC14769 | N92652 | unknown | Unknown |
| ALDH7A1 | −0.642 | GC16889 | AA024918 | Aldehyde dehydrogenase 7 family, member A1 | Aldehyde dehydrogenase (NAD), oxidoreductase |
| ADAM9 | −0.611 | GC15762 | W47533 | ADAM metallopeptidase domain 9 | Mediates cell-cell or cell-matrix interactions |
| TRIM3 | −0.616 | GC14991 | N71362 | Tripartite motif-containing 3 | Transcriptional repressor, control of cell proliferation |
| ITGB1 | −0.638 | GC19072 | AA044261 | Integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12) | integrin binding |
| RGS12 | −0.639 | GC15931 | W67134 | Regulator of G-protein signaling 12 | Signal transducer, regulator of G proteins |
| TJP1 | −0.612 | GC12455 | R79560 | Tight junction protein 1 (zona occludens 1) | Signal transduction for tight junction assembly and stabilizing junctions |
| ASAP2 | −0.638 | GC15131 | N70773 | ArfGAP with SH3 domain, ankyrin repeat and PH domain 2 | GTPase activator, Regulates the formation of post-Golgi vesicles, modulates cell migration |
| STMN4 | −0.650 | GC11515 | H29581 | Stathmin-like 4 | Unknown |
| WAS | 0.654 | GC69113 | AI655719 | Wiskott-Aldrich syndrome (eczema-thrombocytopenia) | effector for Rho-type GTPases, regulates structure and dynamics of the actin cytoskeleton |
| NACA | 0.652 | GC30164 | AF054187 | Nascent polypeptide-associated complex alpha subunit | Prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum (ER) |
| NASP | 0.657 | GC83792 | AW003362 | Nuclear autoantigenic sperm protein (histone-binding) | Involved in DNA replication, normal cell cycle progression and cell proliferation |
| LOC729362 | 0.679 | GC31589 | T89651 | Similar to ribosomal protein L36a | Unknown |
| GNA11 | −0.639 | GC31915 | N36926 | Guanine nucleotide binding protein (G protein), alpha 11 (Gq class) | Signal transducer, activator of phospholipase C, GTPase |
| unknown | −0.658 | GC32458 | M69013 | unknown | Unknown |
| Cluster 3: | |||||
| CD53 | 0.666 | GC89937 | M37033 | cell differentiation antigen 53,32-40kDa | Growth regulation in hematopoietic cells |
| PPIH | 0.662 | GC28763 | AF016371 | Peptidylprolyl isomerase H (cyclophilin H) | Chaperone |
| PTPN7 | 0.707 | GC90165 | M64322 | Protein tyrosine phosphatase, non-receptor type 7 | Regulation of T and B-lymphocyte development and signal transduction |
| ANXA2P3 | −0.652 | GC90123 | M62895 | Annexin A2 pseudogene 3 | Unknown |
| ANXA2 | −0.659 | GC85483 | D00017 | Annexin A2 | Phospholipase inhibitor, involved in heat-stress response |
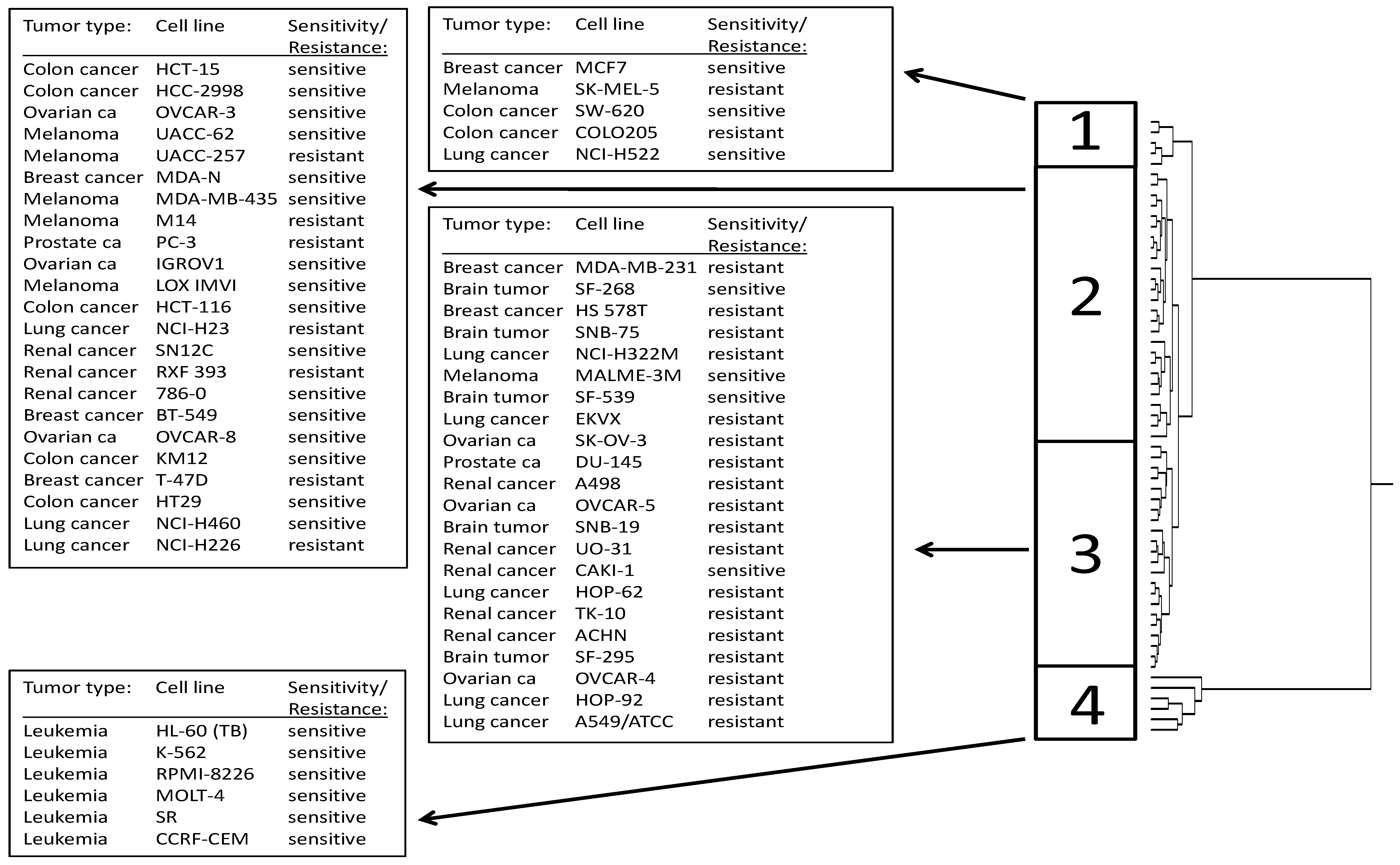
| Partition | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|---|
| sensitive | <−6.5 | 3 | 9 | 4 | 6 |
| resistant | >−6.5 | 2 | 17 | 18 | 0 |
| χ2-test | P = 0.00208 |
4. Experimental Section
5. Conclusions
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Efferth, T.; Li, P.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Ribnicky, D.M.; Lipsky, P.E.; Raskin, I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 2007, 3, 360–366. [Google Scholar] [CrossRef]
- Efferth, T.; Kahl, S.; Paulus, K.; Adams, M.; Rauh, R.; Boechzelt, H.; Hao, X.; Kaina, B.; Bauer, R. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese Materia Medica with activity against tumour cells. Mol. Cancer Therap. 2008, 7, 152–161. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar]
- Mahringer, A.; Karamustafa, S.; Klotz, D.; Kahl, S.; Konkimalla, V.B.; Wang, Y.F.; Wang, J.S.; Liu, H.Y.; Boechzelt, H.; Hao, X.J.; Bauer, R.; Fricker, G.; Efferth, T. Inhibition of P-glycoprotein at the blood brain barrier by phytochemicals derived from traditional Chinese medicine. Cancer Genomics Proteomics 2010, 7, 191–205. [Google Scholar]
- Efferth, T. Personalized cancer medicine: From molecular diagnostics to targeted therapy with natural products. Planta Med. 2010, 76, 1–12. [Google Scholar]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targ. 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Konkimalla, V.B.; Efferth, T. Molecular mechanisms and interactions responsible for radio- and chemoresistance of tumors and their modulation by natural products from Ayurveda. In Herbal Medicine. A Cancer Chemopreventive and Therapeutic Perspective; Arora, R., Ed.; Jaypee Brothers Medical Publishers Pvt. Ltd.: New Delhi, India; St. Louis, MI, USA, 2010; pp. 513–530. [Google Scholar]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–336. [Google Scholar]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Paul, A.; Devasagayam, T. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef]
- Lu, L.; Liu, Y.; Zhu, W.; Shi, J.; Liu, Y.; Ling, W.; Kosten, T.R. Traditional medicine in the treatment of drug addiction. Am. J. Drug Alcohol. Abuse 2009, 35, 1–11. [Google Scholar] [CrossRef]
- Maurya, R. Chemistry and pharmacology of Withania coagulans: An Ayurvedic remedy. J. Pharm. Pharmacol. 2010, 62, 153–160. [Google Scholar] [CrossRef]
- Ven Murthy, M.R.; Ranjekar, P.K.; Ramassamy, C.; Deshpande, M. Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: Ashwagandha. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 238–236. [Google Scholar]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Mehrotra, B.N. Compendium of Indian Medicinal Plants; Central Drug Research Institute: New Delhi, India; St. Louis, MI, USA, 1998. [Google Scholar]
- Davis, L.; Kuttan, G. Effect of Withania somnifera on 20-methylcholanthrene induced fibrosarcoma. J. Exp. Clin. Cancer Res. 2000, 19, 165–167. [Google Scholar]
- Davis, L.; Kuttan, G. Effect of Withania somnifera on DMBA induced carcinogenesis. J. Ethnopharmacol. 2001, 75, 165–168. [Google Scholar] [CrossRef]
- Prakash, J.; Gupta, S.K.; Kochupillai, V.; Singh, N.; Gupta, Y.K.; Joshi, S. Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytother. Res. 2001, 15, 240–244. [Google Scholar] [CrossRef]
- Muralikrishnan, G.; Dinda, A.K.; Shakeel, F. Immunomodulatory effects of Withania somnifera on azoxymethane induced experimental colon cancer in mice. Immunol. Invest. 2010, 39, 688–698. [Google Scholar] [CrossRef]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U.; Udupa, N.; Srinivasan, K.K. Antitumor and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma in vivo. Acta Oncol. 1996, 35, 95–100. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ranga, R.S.; Burikhanov, R.; Han, S.S.; Chendil, D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007, 67, 246–243. [Google Scholar]
- Stan, S.D.; Zeng, Y.; Singh, S.V. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer. 60, 51–60.
- Malik, F.; Kumar, A.; Bhushan, S.; Mondhe, D.M.; Pal, H.C.; Sharma, R.; Khajuria, A.; Singh, S.; Singh, G.; Saxena, A.K.; Suri, K.A.; Qazi, G.N.; Singh, J. Immune modulation and apoptosis induction: Two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera. Eur. J. Cancer. 2009, 45, 1494–1509. [Google Scholar]
- Yu, Y.; Hamza, A.; Zhang, T.; Gu, M.; Zou, P.; Newman, B.; Li, Y.; Gunatilaka, A.A.; Zhan, C.G.; Sun, D. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010, 79, 542–551. [Google Scholar] [CrossRef]
- Hahm, E.R.; Lee, J.; Huang, Y.; Singh, S.V. Withaferin a suppresses estrogen receptor-α expression in human breast cancer cells. Mol. Carcinog. 2011, 50, 614–624. [Google Scholar] [CrossRef]
- Devi, P.U.; Akagi, K.; Ostapenko, V.; Tanaka, Y.; Sugahara, T. Withaferin A: A new radiosensitizer from the Indian medicinal plant Withania somnifera. Int. J. Radiat. Biol. 1996, 69, 193–197. [Google Scholar] [CrossRef]
- Stan, S.D.; Hahm, E.R.; Warin, R.; Singh, S.V. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 68, 7661–7669.
- Mandal, C.; Dutta, A.; Mallick, A.; Chandra, S.; Misra, L.; Sangwan, R.S.; Mandal, C. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis 2008, 13, 1450–1464. [Google Scholar] [CrossRef]
- Choi, M.J.; Park, E.J.; Min, K.J.; Park, J.W.; Kwon, T.K. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol. In Vitro 2011, 25, 692–698. [Google Scholar] [CrossRef]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; Pribluda, V.S. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Mathur, R.; Gupta, S.K.; Singh, N.; Mathur, S.; Kochupillai, V.; Velpandian, T. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J. Ethnopharmacol. 2006, 105, 336–341. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Hussain, S.; Yousuf, S.; Dar, A.; Mudassar, A.-R. Chlorinated and diepoxy withanolides from Withania somnifera and their cytotoxic effects against human lung cancer cell line. Phytochemistry 2010, 71, 2205–2209. [Google Scholar]
- Mondal, S.; Mandal, C.; Sangwan, R.; Chandra, S.; Mandal, C. Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. Mol. Cancer. 2010, 9, 239. [Google Scholar]
- Abdeljebbar, L.H.; Benjouad, A.; Morjani, H.; Merghoub, N.; El Haddar, S.; Humam, M.; Christen, P.; Hostettmann, K.; Bekkouche, K.; Amzazi, S. Antiproliferative effects of withanolides from Withania adpressa. Therapie 2009, 64, 121–127. [Google Scholar]
- Xu, Y.M.; Marron, M.T.; Seddon, E.; McLaughlin, S.P.; Ray, D.T.; Whitesell, L.; Gunatilaka, A.A. 2,3-Dihydrowithaferin A-3beta-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown Withania somnifera. Bioorg. Med. Chem. 2009, 17, 2210–2214. [Google Scholar]
- He, Q.P.; Ma, L.; Luo, J.Y.; He, F.Y.; Lou, L.G.; Hu, L.H. Cytotoxic withanolides from Physalis angulata L. Chem. Biodivers. 2007, 4, 443–449. [Google Scholar] [CrossRef]
- Subbaraju, G.V.; Vanisree, M.; Rao, C.V.; Sivaramakrishna, C.; Sridhar, P.; Jayaprakasam, B.; Nair, M.G. Ashwagandhanolide, a bioactive dimeric thiowithanolide isolated from the roots of Withania somnifera. J. Nat. Prod. 2006, 69, 1790–1792. [Google Scholar] [CrossRef]
- Mathur, S.; Kaur, P.; Sharma, M.; Katyal, A.; Singh, B.; Tiwari, M.; Chandra, R. The treatment of skin carcinoma, induced by UV B radiation, using 1-oxo-5beta, 6beta-epoxy-witha-2-enolide, isolated from the roots of Withania somnifera, in a rat model. Phytomedicine 2004, 11, 452–460. [Google Scholar] [CrossRef]
- Developmental Therapeutics Program NCI/NIH Hompage. Available online: http://dtp.nci.nih.gov (accessed on 21 May 2012).
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Monk, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–756. [Google Scholar] [CrossRef]
- Staunton, J.E.; Slonim, D.K.; Coller, H.A.; Tamayo, P.; Angelo, M.J.; Park, J.; Scherf, U.; Lee, J.K.; Reinhold, W.O.; Weinstein, J.N.; Mesirov, J.P.; Lander, E.S.; Golub, T.R. Chemosensitivity prediction by transcriptional profiling. Proc. Natl. Acad. Sci. USA 2001, 98, 10787–10792. [Google Scholar]
- OMIM®—nline Mendelian Inheritance in Man® Hompage. Available online: http://www.ncbi.nlm.nih.gov/Omim (accessed on 21 May 2012).
- GeneCard database. Weizman Institute of Science: Rehovot, Israel. 2012. Available online: http://bioinfo.weizmann.ac.il/cards/index.html (accessed on 21 May 2012).
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The antimalarial artesunate is also a potent anticancer drug. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar]
- Efferth, T.; Kahl, S.; Paulus, K.; Adams, M.; Rauh, R.; Boechzelt, H.; Hao, X.; Kaina, B.; Bauer, R. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and chinese materia medica with activity against tumor cells. Mol. Cancer Ther. 2008, 7, 152–161. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Bauer, J.; Hock, K.; Plinkert, P.K.; Efferth, T. Pharmacogenomic determination of genes associated with sensitivity or resistance of tumor cells to curcumin and curcumin derivatives. J. Nutr. Biochem. 2011, in press. [Google Scholar]
- Koduru, S.; Kumar, R.; Srinivasan, S.; Evers, M.B.; Damodaran, C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010, 9, 202–210. [Google Scholar]
- Senthilnathan, P.; Padmavathi, R.; Magesh, V.; Sakthisekaran, D. Chemotherapeutic efficacy of paclitaxel in combination with Withania somnifera on benzo(a)pyrene-induced experimental lung cancer. Cancer Sci. 2006, 97, 658–664. [Google Scholar] [CrossRef]
- Kalthur, G.; Pathirissery, U.D. Enhancement of the response of B16F1 melanoma to fractionated radiotherapy and prolongation of survival by withaferin A and/or hyperthermia. Integr. Cancer Ther. 2010, 9, 370–377. [Google Scholar] [CrossRef]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Withaferin A enhances radiation-induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl-2 downregulation and Akt inhibition. Chem. Biol. Interact. 2011, 190, 9–15. [Google Scholar] [CrossRef]
- Leteurtre, F.; Kohlhagen, G.; Paull, K.D.; Pommier, Y. Topoisomerase II inhibition and cytotoxicity of the anthrapyrazoles DuP 937 and DuP 941 (Losoxantrone) in the National Cancer Institute preclinical antitumor drug discovery screen. J. Natl. Cancer Inst. 1994, 86, 1239–1244. [Google Scholar] [CrossRef]
- Efferth, T.; Gebhart, E.; Ross, D.D.; Sauerbrey, A. Identification of gene expression profiles predicting tumor cell response to l-alanosine. Biochem. Pharmacol. 2003, 66, 613–621. [Google Scholar] [CrossRef]
- Efferth, T.; Olbrich, A.; Bauer, R. mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem. Pharmacol. 2002, 64, 617–623. [Google Scholar] [CrossRef]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.; Marcus, A.I. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer. 2011, 129, 2744–2755. [Google Scholar]
- Conner, B.P.; Rougas, J.; Pribluda, V.S. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Grover, A.; Shandilya, A.; Punetha, A.; Bisaria, V.S.; Sundar, D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genomics. 2010, 11, 25. [Google Scholar]
- Grover, A.; Shandilya, A.; Agrawal, V.; Pratik, P.; Bhasme, D.; Bisaria, V.S.; Sundar, D. Hsp90/Cdc37 chaperone/co-chaperone complex, a novel junction anticancer target elucidated by the mode of action of herbal drug Withaferin A. BMC Bioinformatics 2011, 12, 30. [Google Scholar]
- Swagemakers, S.M.; Essers, J.; de Wit, J.; Hoeijmakers, J.H.; Kanaar, R. The human RAD54 recombinational DNA repair protein is a double-stranded DNA-dependent ATPase. J. Biol. Chem. 1998, 273, 28292–28297. [Google Scholar]
- Fuqua, S.A.; Oesterreich, S.; Hilsenbeck, S.G.; Von Hoff, D.D.; Eckardt, J.; Osborne, C.K. Heat shock proteins and drug resistance. Breast Cancer Res. Treat. 1994, 32, 67–71. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Zhang, B.; Wei, X.; Yang, Y.; Qi, R.Z.; Ying, G.; Zhang, N.; Niu, R. Anxa2 plays a critical role in enhanced invasiveness of the multidrug resistant human breast cancer cells. J. Proteome Res. 2009, 8, 5041–5047. [Google Scholar] [CrossRef]
- Gong, X.G.; Lv, Y.F.; Li, X. Q.; Xu, F.G.; Ma, Q.Y. Gemcitabine resistance induced by interaction between alternatively spliced segment of tenascin-C and annexin A2 in pancreatic cancer cells. Biol. Pharm. Bull. 2010, 33, 1261–1267. [Google Scholar] [CrossRef]
- Chen, H.; Xie, Z.X.; Jiang, H.; Zhang, Z.W.; Wang, G.P. Effect of ribosomal protein L6 on drug resistance and apoptosis in K562/A02 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2007, 15, 292–295. [Google Scholar]
- Harris, E.H.; Burkhart, B.D.; Gillham, N.W.; Boynton, J.E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: Correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989, 123, 281–292. [Google Scholar]
- Stolovich, M.; Tang, H.; Hornstein, E.; Levy, G.; Cohen, R.; Bae, S.S.; Birnbaum, M.J.; Meyuhas, O. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinasemediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol. Cell Biol. 2002, 22, 8101–8113. [Google Scholar]
- Du, J.; Shi, Y.; Pan, Y.; Jin, X.; Liu, C.; Liu, N.; Han, Q.; Lu, Y.; Qiao, T.; Fan, D. Regulation of multidrug resistance by ribosomal protein l6 in gastric cancer cells. Cancer Biol. Ther. 2005, 4, 242–247. [Google Scholar] [CrossRef]
- Efferth, T.; Miyachi, H.; Bartsch, H. Pharmacogenomics of a traditional Japanese herbal medicine (Kampo) for cancer therapy. Cancer Genomics Proteomics 2007, 4, 81–92. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Sieber, S.; Sauer, A.; Weiss, J.; Plinkert, P.K.; Efferth, T. Efferth, T. Factors determining sensitivity or resistance of tumor cell lines towards artesunate. Chem-Biol. Interact. 2010, 185, 42–52. [Google Scholar] [CrossRef]
- Scherf, U.; Ross, D.T.; Waltham, M.; Smith, L.H.; Lee, J.K.; Tanabe, L.; Kohn, K.W.; Reinhold, W.C.; Myers, T.G.; Andrews, D.T.; et al. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. [Google Scholar] [CrossRef]
- Amundson, S.A.; Do, K.T.; Vinikoor, L.C.; Lee, R.A.; Koch-Paiz, C.A.; Ahn, J.; Reimers, M.; Chen, Y.; Scudiero, D.A.; Weinstein, J.N.; et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008, 68, 415–424. [Google Scholar]
- Efferth, T.; Fabry, U.; Osieka, R. Apoptosis and resistance to daunorubicin in human leukemic cells. Leukemia 1997, 11, 1180–1186. [Google Scholar]
- Wosikowski, K.; Schuurhuis, D.; Johnso, K.; Paull, K.D.; Myers, T.G.; Weinstein, J.N.; Bates, S.E. Identification of epidermal growth factor receptor and erbB2 pathway inhibitors by correlation with gene expression patterns. J. Natl. Cancer Inst. 1997, 89, 1505–1515. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Efferth, T.; Greten, H.J. In Silico Analysis of Microarray-Based Gene Expression Profiles Predicts Tumor Cell Response to Withanolides. Microarrays 2012, 1, 44-63. https://doi.org/10.3390/microarrays1010044
Efferth T, Greten HJ. In Silico Analysis of Microarray-Based Gene Expression Profiles Predicts Tumor Cell Response to Withanolides. Microarrays. 2012; 1(1):44-63. https://doi.org/10.3390/microarrays1010044
Chicago/Turabian StyleEfferth, Thomas, and Henry Johannes Greten. 2012. "In Silico Analysis of Microarray-Based Gene Expression Profiles Predicts Tumor Cell Response to Withanolides" Microarrays 1, no. 1: 44-63. https://doi.org/10.3390/microarrays1010044






