Characterization of TGF-β by Induced Oxidative Stress in Human Trabecular Meshwork Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Line and Cell Culture
2.3. H2O2 Sufficiency
2.4. shTGF-β1/2 Knockdown HTMC Establishment
2.5. siRNA Knockdown and Transfection
2.6. Cell Viability Assay
2.7. Detection of ROS Increase
2.8. Intracellular Calcium-Level Measurement
2.9. Apoptosis Assay
2.10. Immunoblotting Analysis
2.11. Immunofluorescence
2.12. Treatment of Cells with Recombinant Human TGF-β Protein1 (rhTGF-β1)
2.13. Statistical Analysis
3. Results
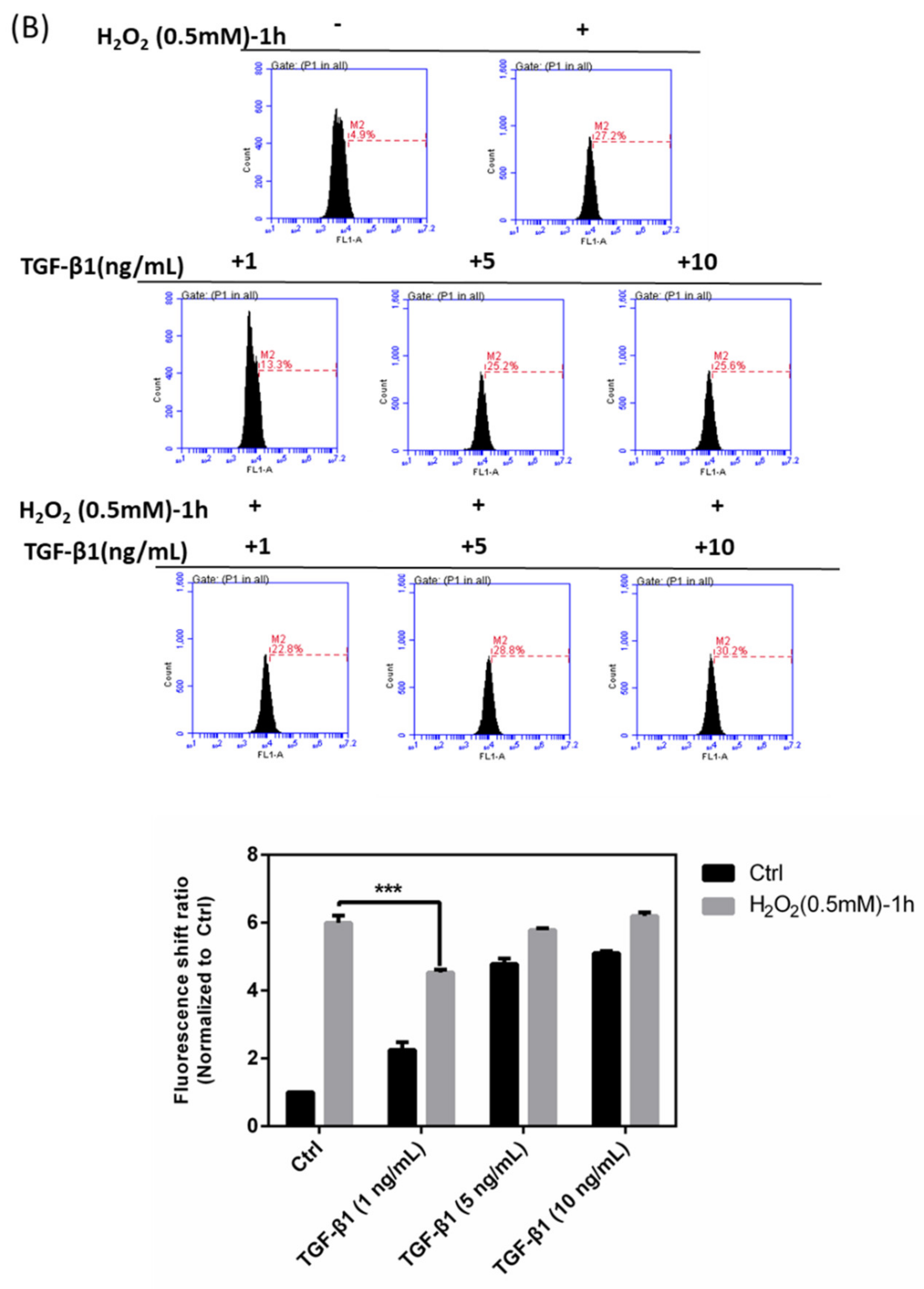
3.1. Impairments by H2O2 of HTMCs
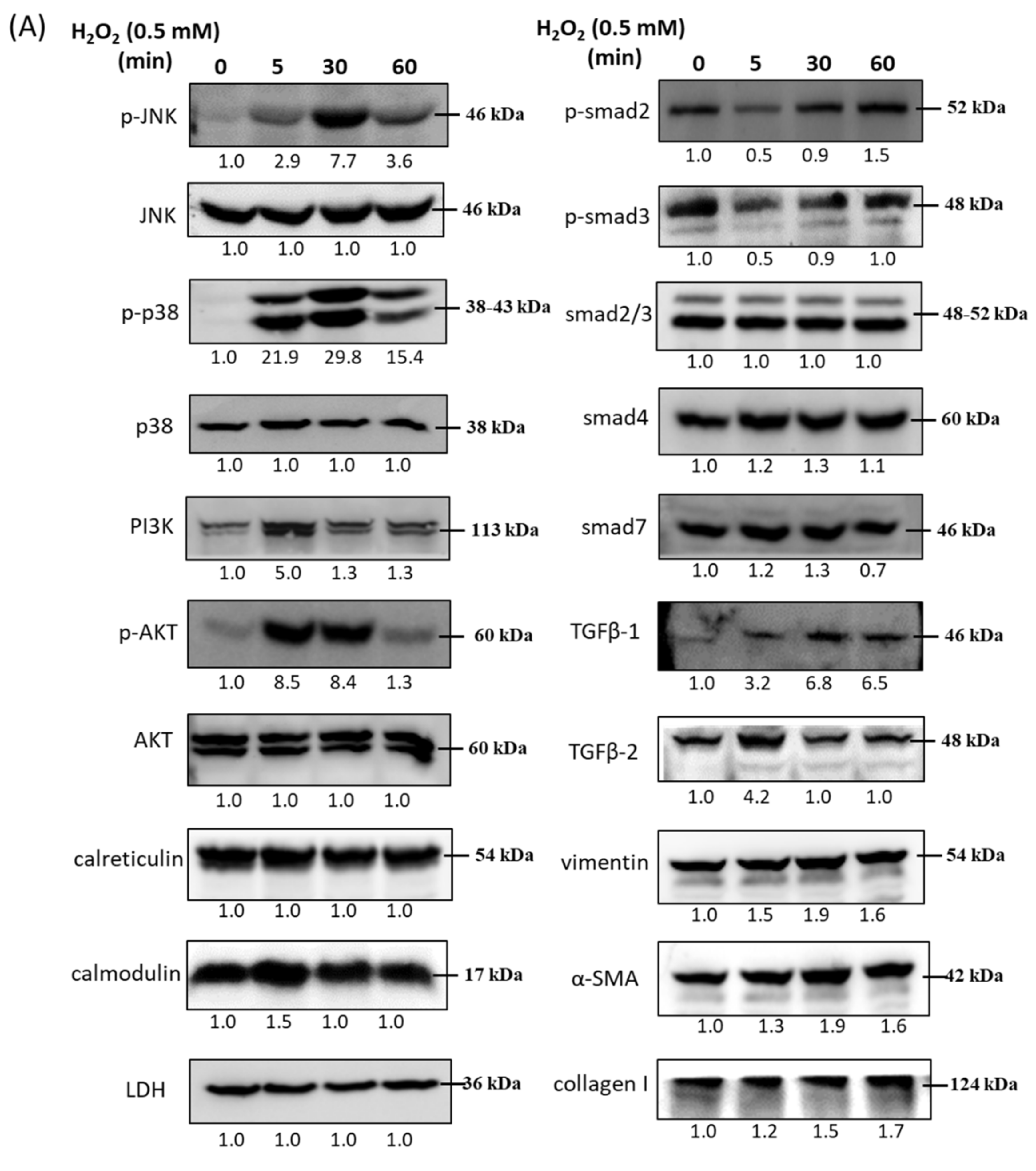
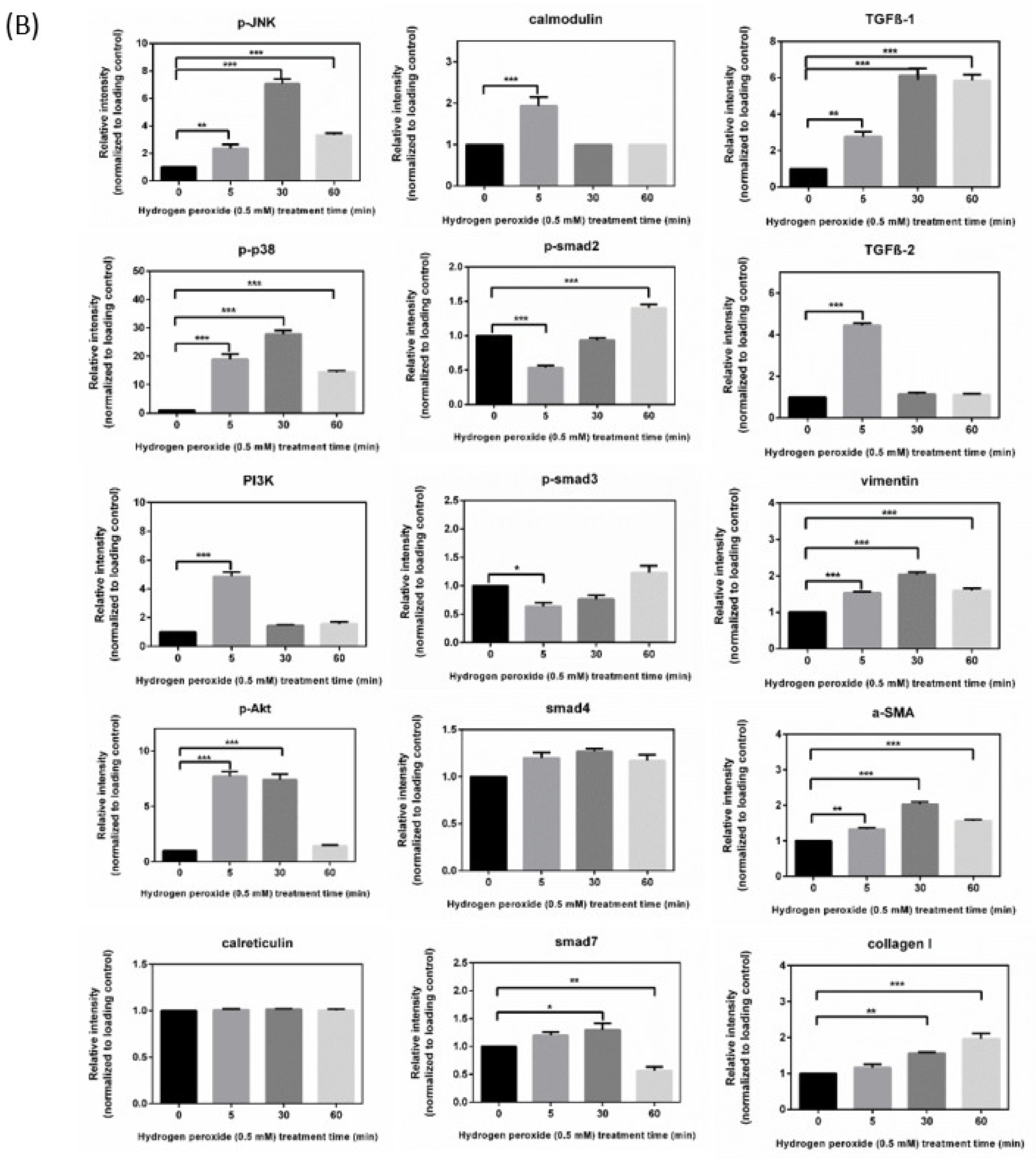
3.2. Immunoblot Analysis and Identification of H2O2-Induced Oxidative Stress Response Signaling Pathways
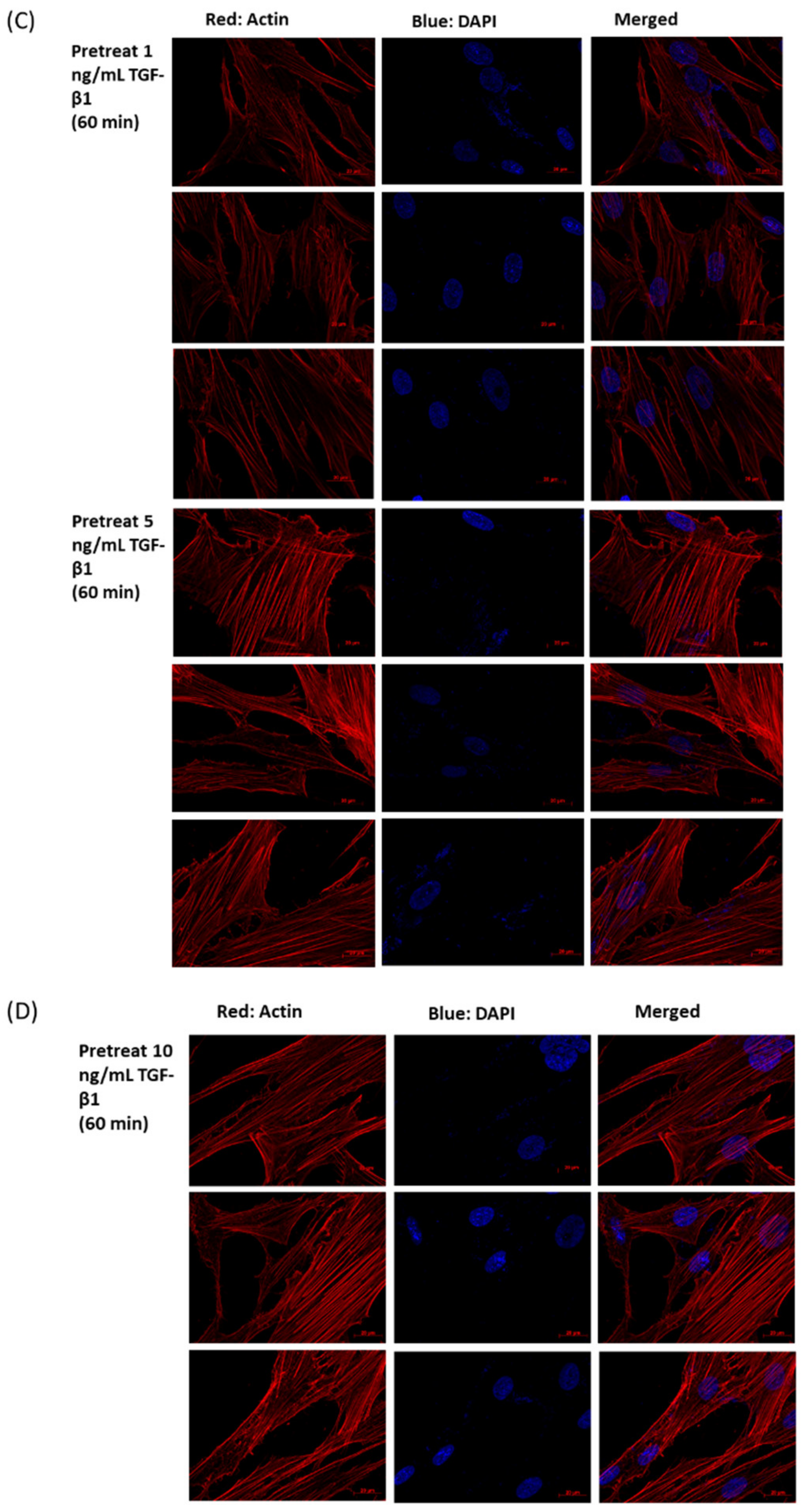
3.3. Immunofluorescence Analysis of H2O2 (0.5 mM)-Treated HTMCs
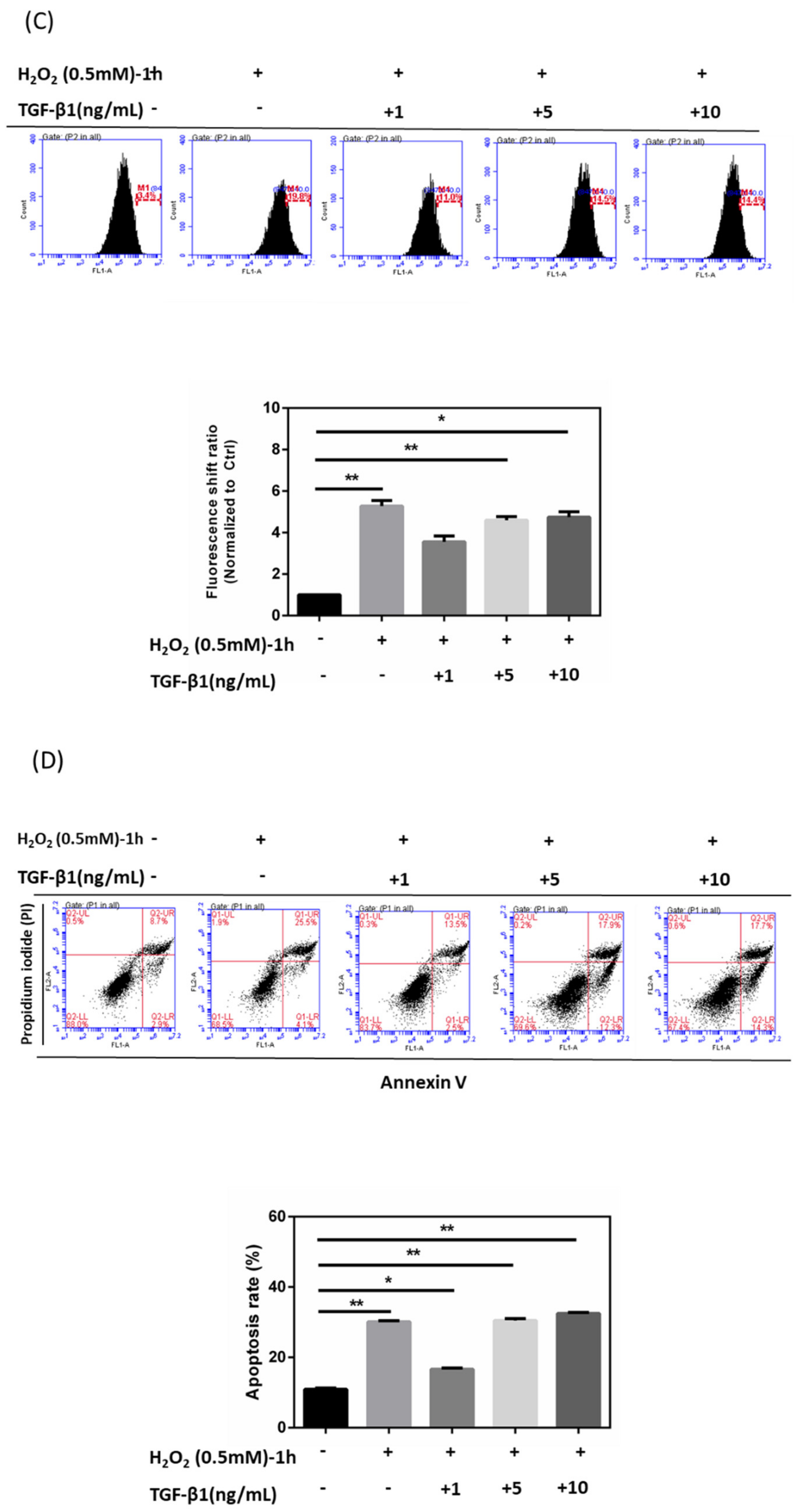
3.4. Effects of rhTGF-β1 Protein on Oxidative Stress Response in HTMCs
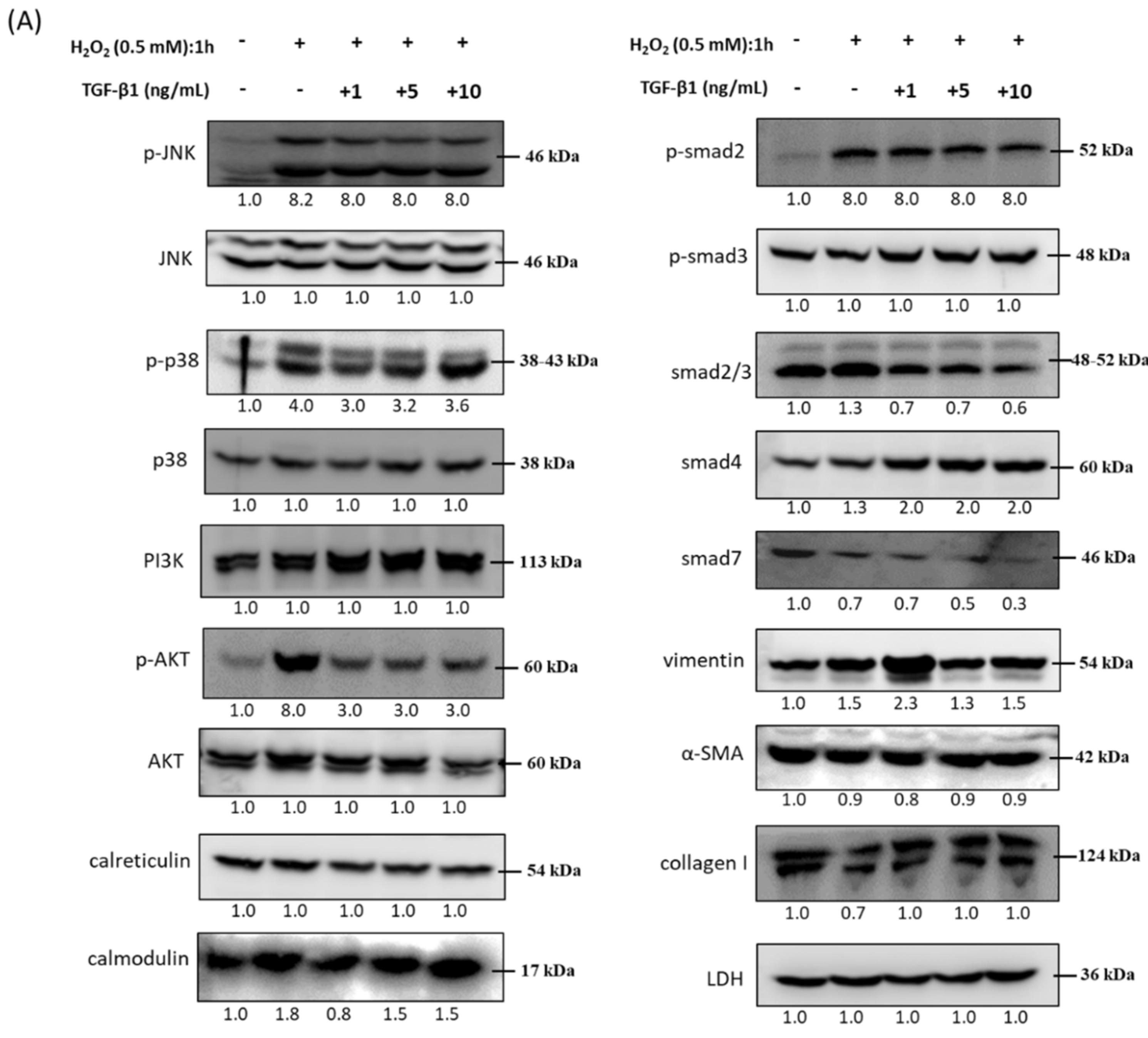
3.5. Immunoblot Analysis and Identification of rhTGF-β1 Protein in Oxidative Stress Pathways
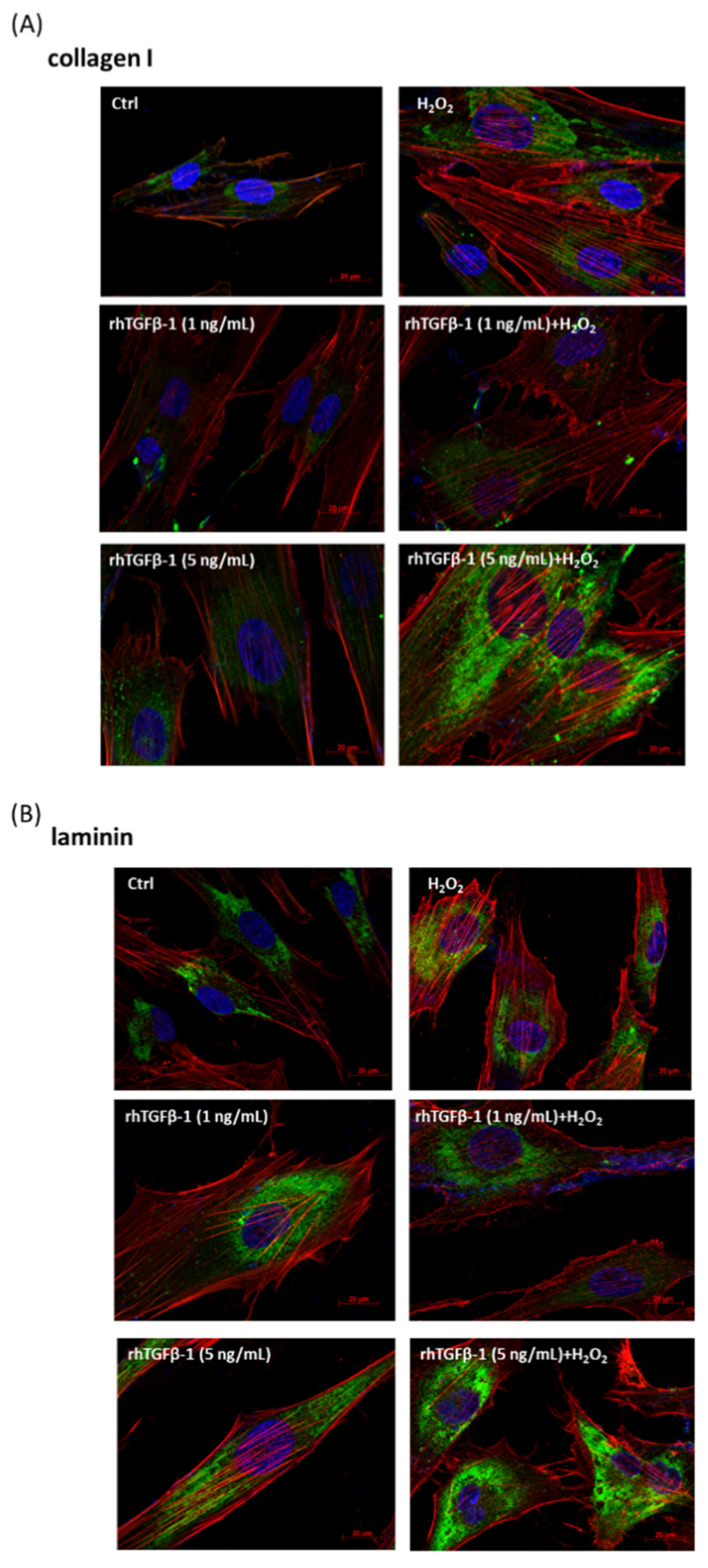
3.6. Immunofluorescence Analysis of rhTGF-β1 Protein-Treated HTMCs with or without H2O2 Exposure (0.5 mM for 1 h)
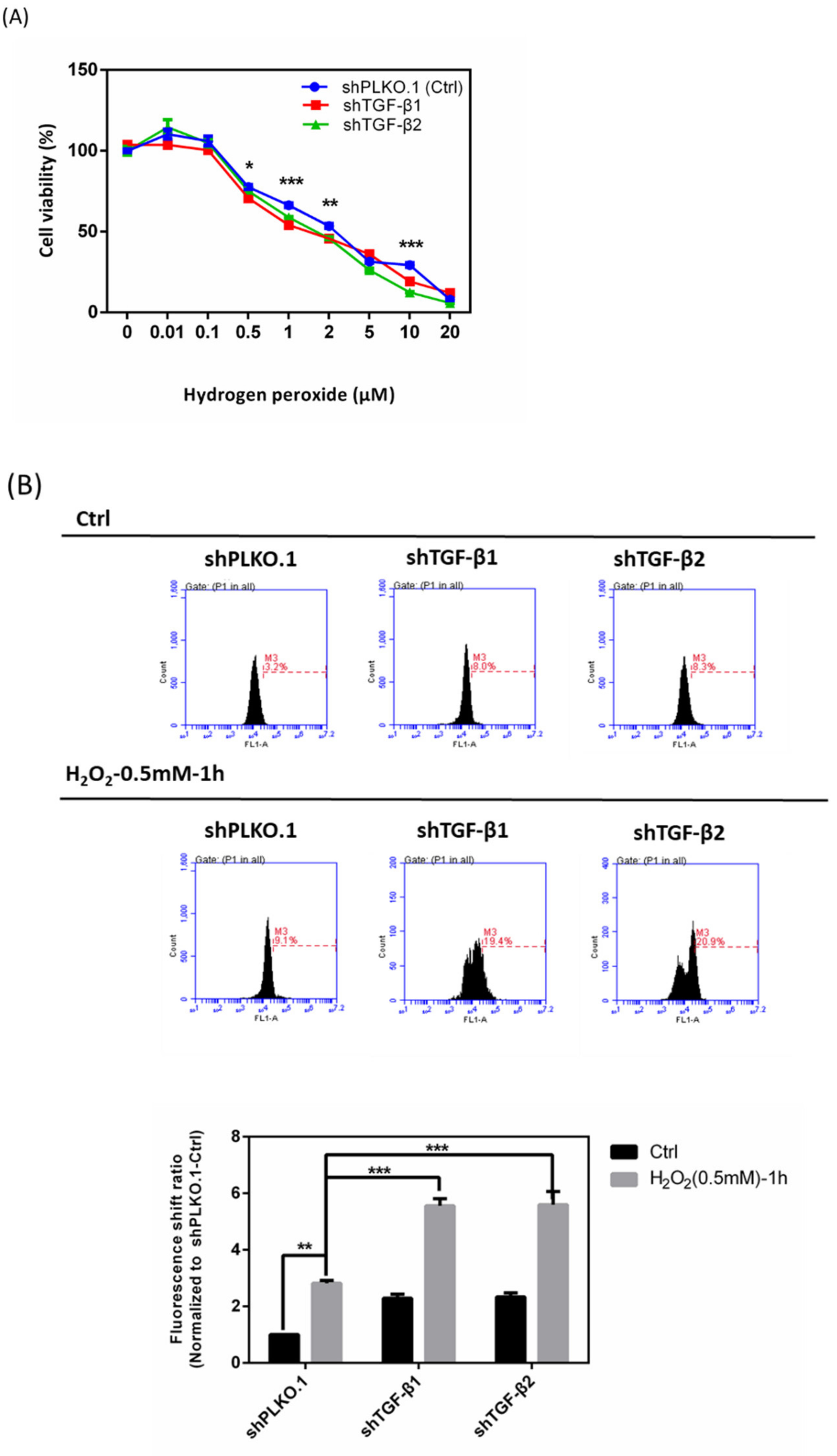
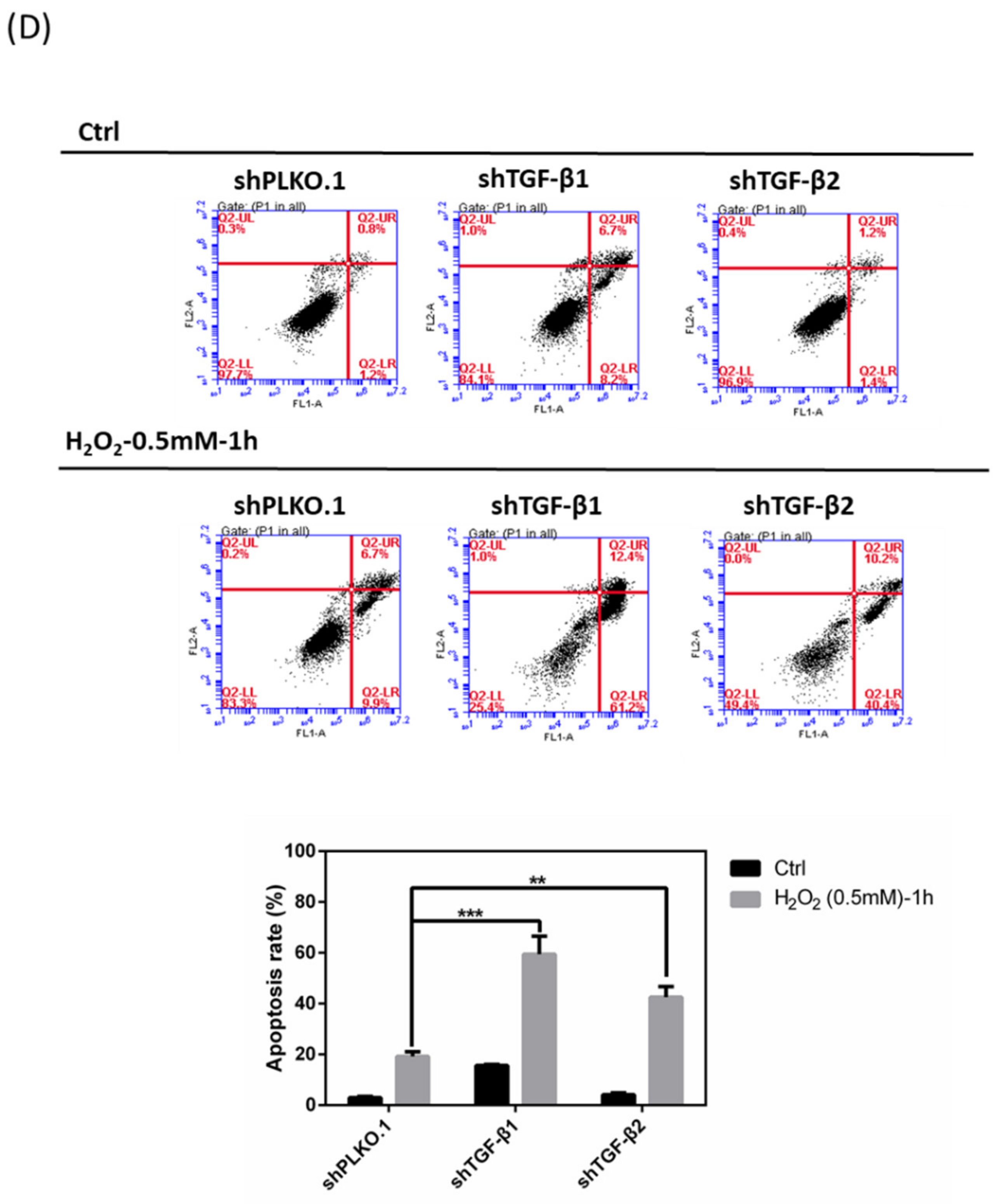
3.7. Effects of shTGF-β1/2 Knockdown in HTMCs with H2O2 Exposure
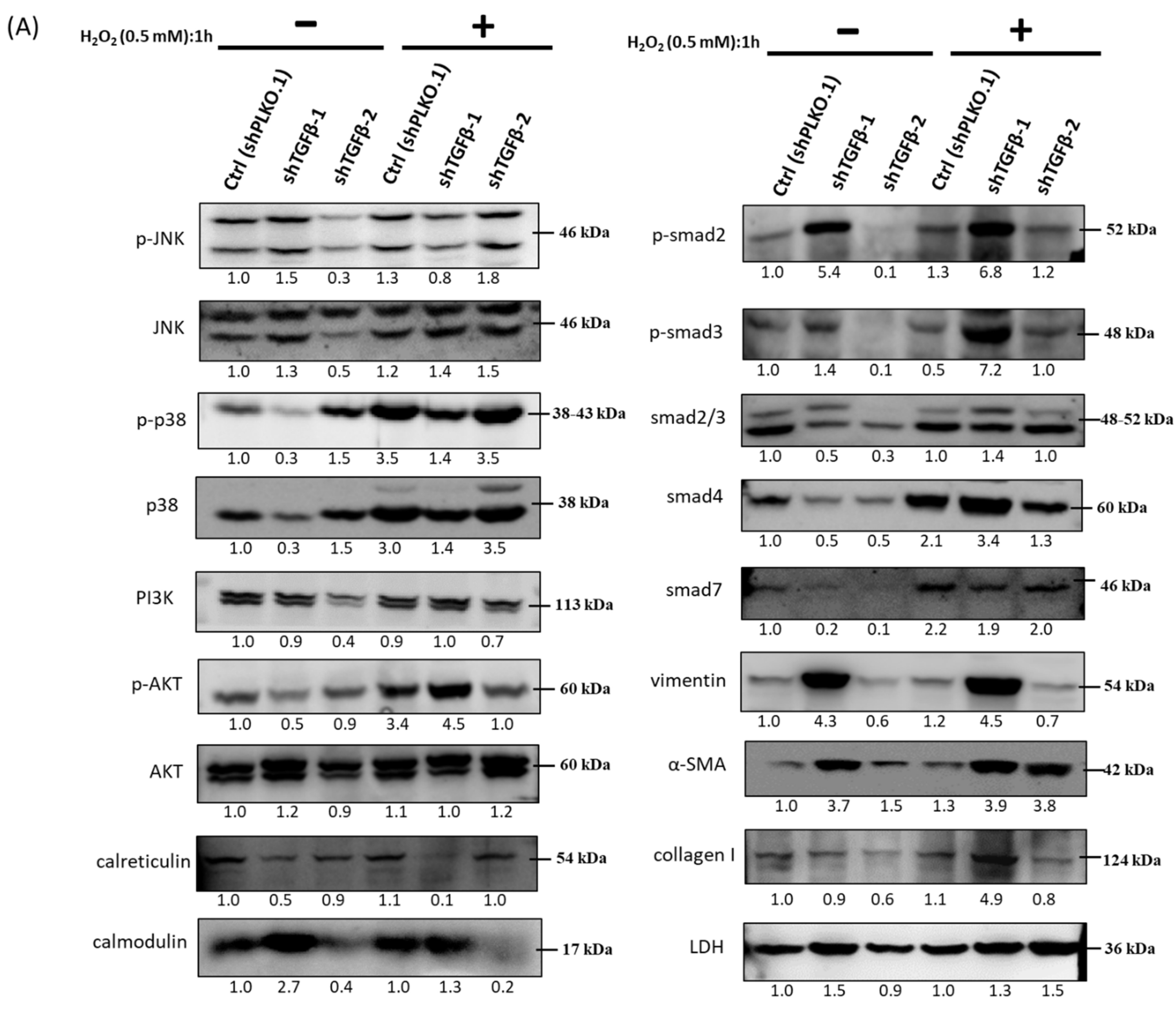
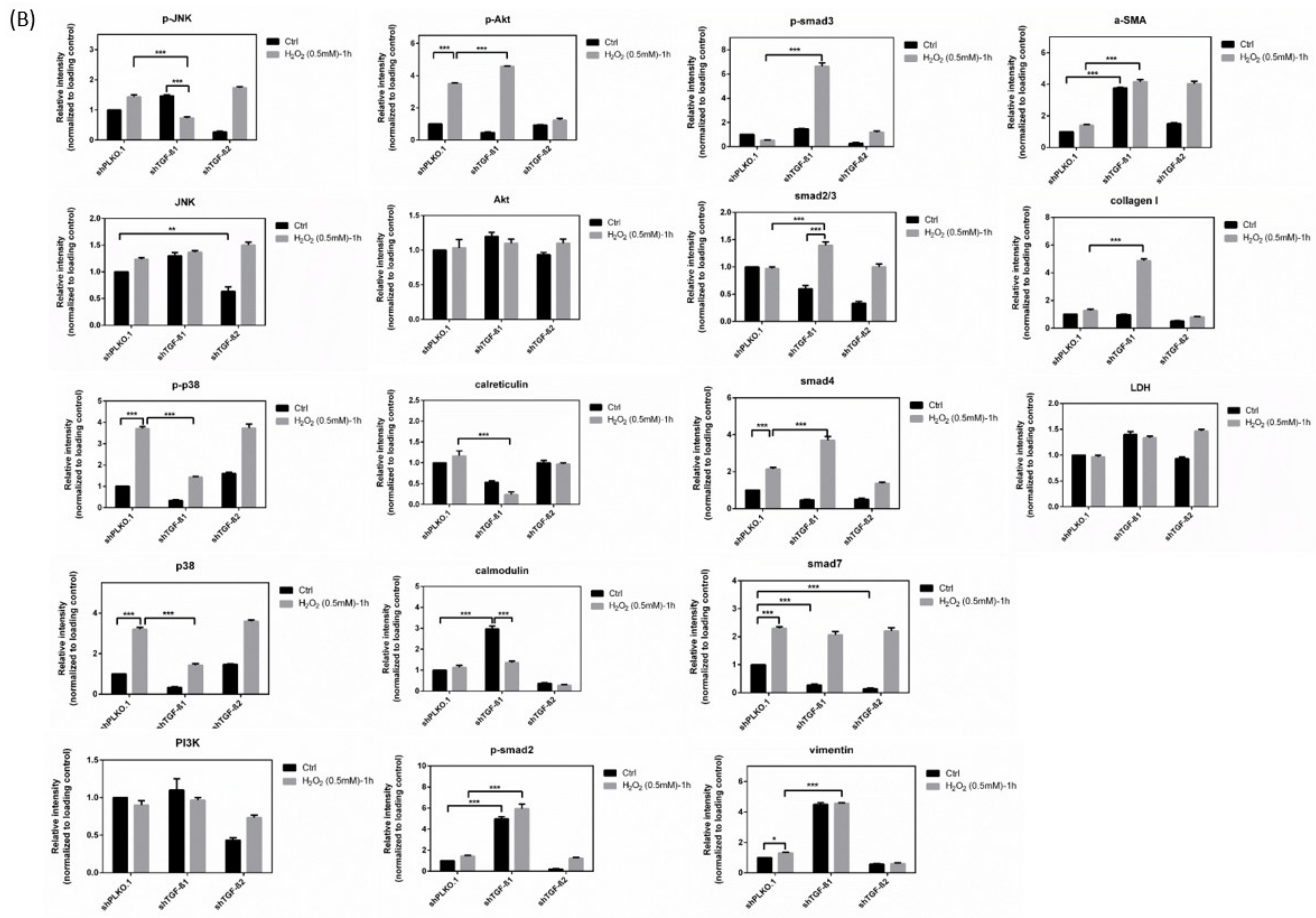
3.8. Immunoblot Analysis and Identification of shTGF-β1/2 Knockdown HTMCs in Oxidative Stress Pathways
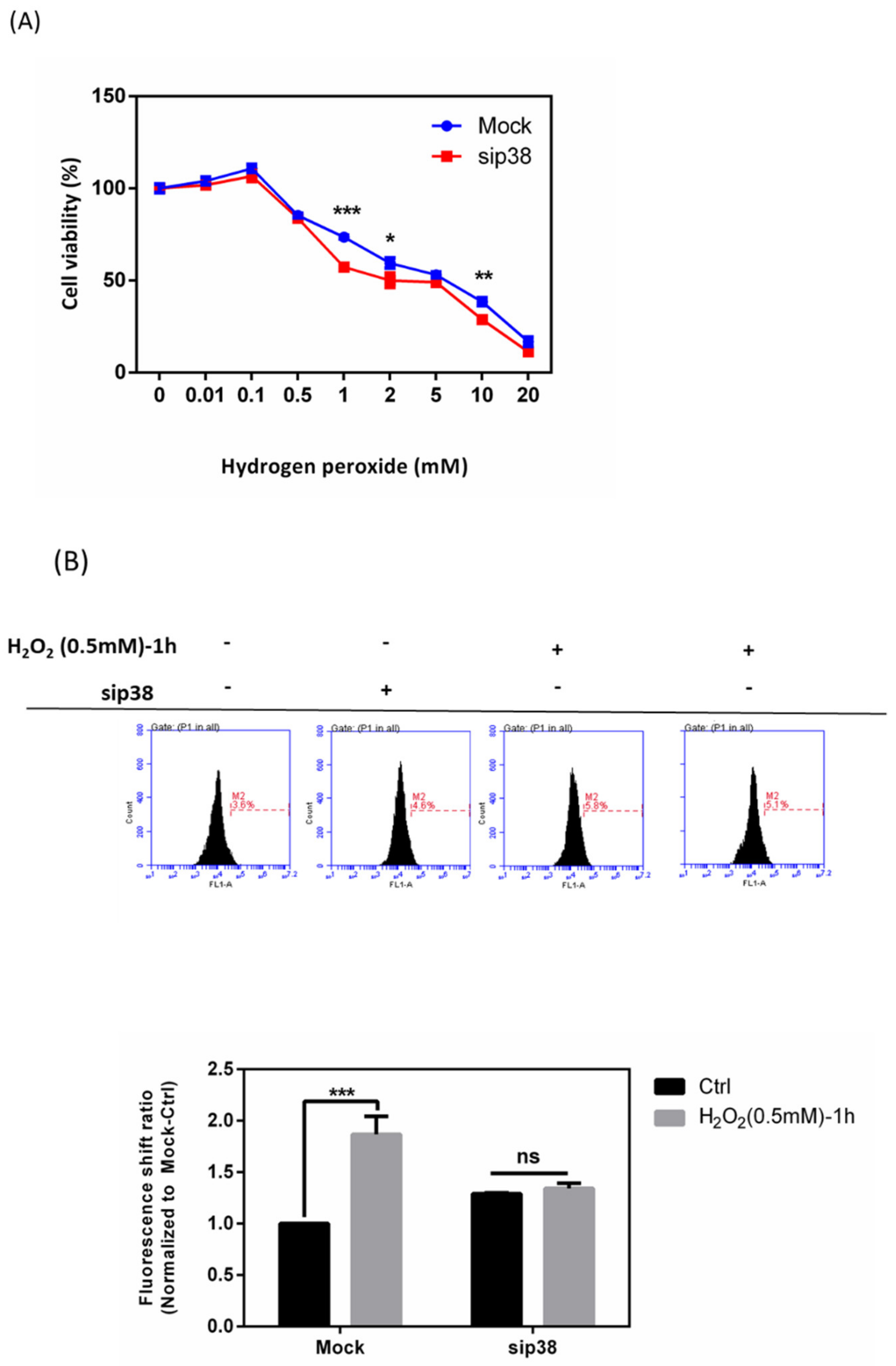
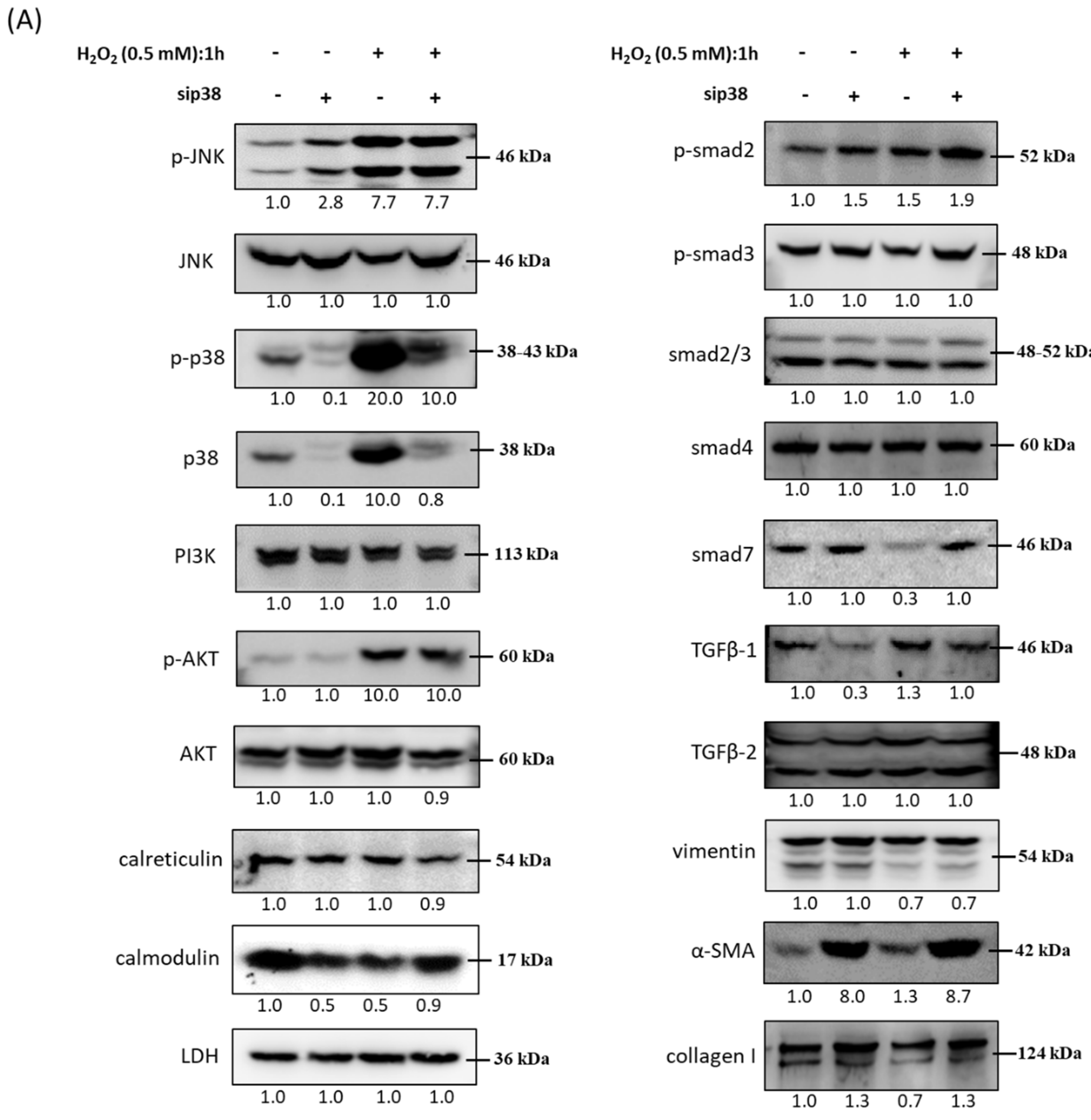
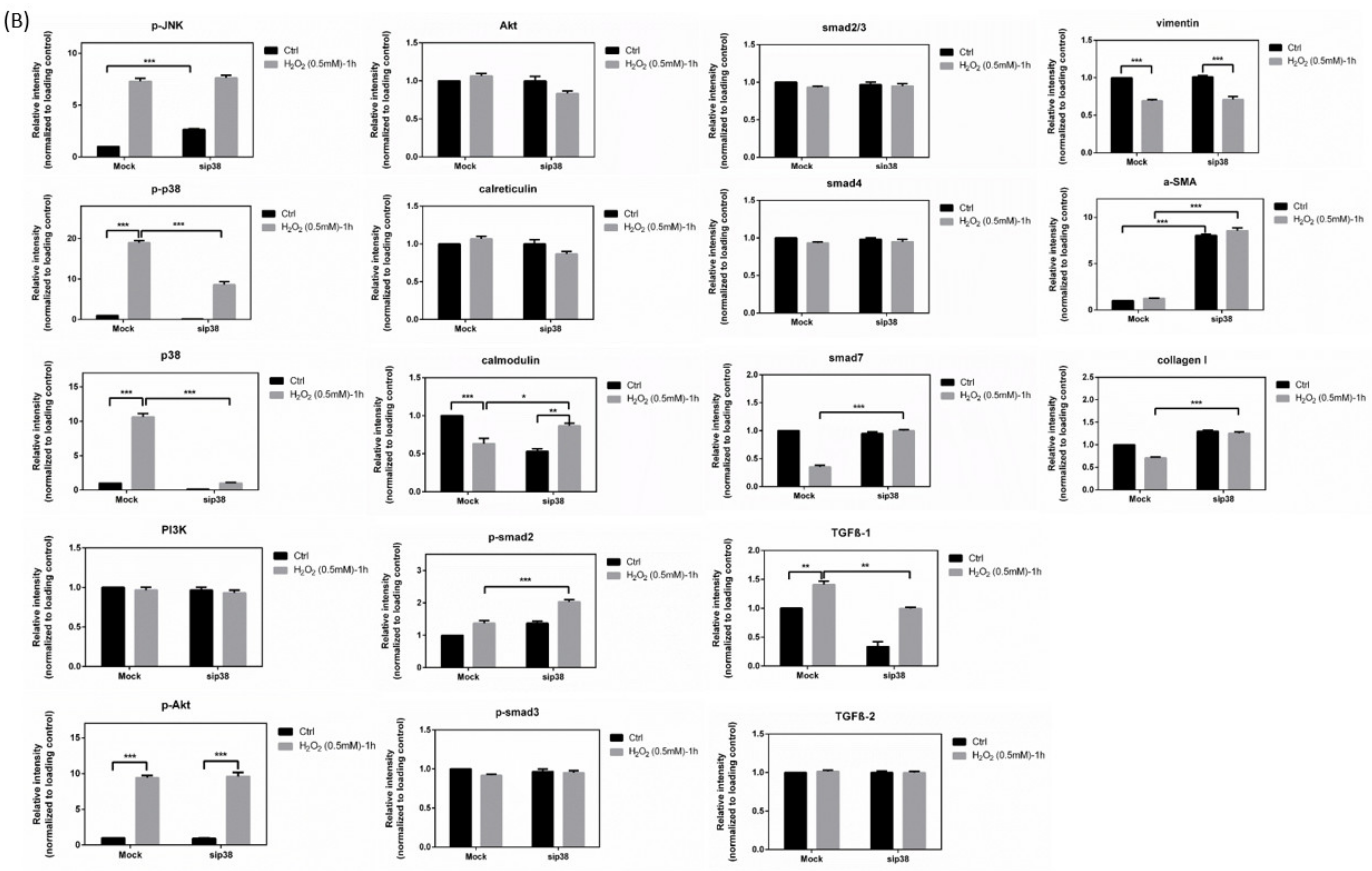
3.9. Effects of sip38 MAPK Knockdown in HTMCs with H2O2 Exposure
3.10. Immunoblot Analysis and Identification of sip38 MAPK Knockdown HTMCs in Oxidative Stress Pathways
3.11. A Hypothetical Model Detailing the Role of the TGF-β-Related Oxidative Stress Pathway in HTMCs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsukamoto, T.; Kajiwara, K.; Nada, S.; Okada, M. Src mediates TGF-beta-induced intraocular pressure elevation in glaucoma. J. Cell. Physiol. 2019, 234, 1730–1744. [Google Scholar] [CrossRef]
- Chhunchha, B.; Singh, P.; Stamer, W.D.; Singh, D.P. Prdx6 retards senescence and restores trabecular meshwork cell health by regulating reactive oxygen species. Cell Death Discov. 2017, 3, 17060. [Google Scholar] [CrossRef] [Green Version]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, Y.; Yue, B.Y. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: The trabecular meshwork. J. Cell. Physiol. 1999, 180, 182–189. [Google Scholar] [CrossRef]
- Wang, N.; Chintala, S.K.; Fini, M.E.; Schuman, J.S. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat. Med. 2001, 7, 304–309. [Google Scholar] [CrossRef]
- Izzotti, A.; Bagnis, A.; Sacca, S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Sacca, S.C.; Izzotti, A.; Rossi, P.; Traverso, C. Glaucomatous outflow pathway and oxidative stress. Exp. Eye Res. 2007, 84, 389–399. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Zhong, W.; Yang, B.; Sun, L.; Zheng, Y. Oxidative stress in the trabecular meshwork (Review). Int. J. Mol. Med. 2016, 38, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zheng, Y. Oxidative stress and antioxidants in the trabecular meshwork. PeerJ 2019, 7, e8121. [Google Scholar] [CrossRef]
- Petrova, N.V.; Velichko, A.K.; Razin, S.V.; Kantidze, O.L. Small molecule compounds that induce cellular senescence. Aging Cell 2016, 15, 999–1017. [Google Scholar] [CrossRef] [Green Version]
- Fatma, N.; Kubo, E.; Toris, C.B.; Stamer, W.D.; Camras, C.B.; Singh, D.P. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic. Res. 2009, 43, 783–795. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Q.; Wang, J.; Liu, J.P.; Feng, H.; Liu, W.B.; Yan, Q.; Liu, Y.; Sun, S.M.; Deng, M.; Gong, L.; et al. hTERT extends proliferative lifespan and prevents oxidative stress-induced apoptosis in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2503–2513. [Google Scholar] [CrossRef] [Green Version]
- Fleenor, D.L.; Shepard, A.R.; Hellberg, P.E.; Jacobson, N.; Pang, I.H.; Clark, A.F. TGFbeta2-induced changes in human trabecular meshwork: Implications for intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2006, 47, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making sense of latent TGFbeta activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Zode, G.S.; Sethi, A.; Brun-Zinkernagel, A.M.; Chang, I.F.; Clark, A.F.; Wordinger, R.J. Transforming growth factor-beta2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol. Vis. 2011, 17, 1745–1758. [Google Scholar]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Fatma, N.; Kubo, E.; Sharma, P.; Beier, D.R.; Singh, D.P. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: Increased expression and activation of TGFbeta. Cell Death Differ. 2005, 12, 734–750. [Google Scholar] [CrossRef]
- Jiang, Z.; Seo, J.Y.; Ha, H.; Lee, E.A.; Kim, Y.S.; Han, D.C.; Uh, S.T.; Park, C.S.; Lee, H.B. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem. Biophys. Res. Commun. 2003, 309, 961–966. [Google Scholar] [CrossRef]
- Ghatak, S.; Hascall, V.C.; Markwald, R.R.; Feghali-Bostwick, C.; Artlett, C.M.; Gooz, M.; Bogatkevich, G.S.; Atanelishvili, I.; Silver, R.M.; Wood, J.; et al. Transforming growth factor beta1 (TGFbeta1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis. J. Biol. Chem. 2017, 292, 10490–10519. [Google Scholar] [CrossRef] [Green Version]
- Awai-Kasaoka, N.; Inoue, T.; Kameda, T.; Fujimoto, T.; Inoue-Mochita, M.; Tanihara, H. Oxidative stress response signaling pathways in trabecular meshwork cells and their effects on cell viability. Mol. Vis. 2013, 19, 1332–1340. [Google Scholar]
- Ammar, D.A.; Hamweyah, K.M.; Kahook, M.Y. Antioxidants Protect Trabecular Meshwork Cells From Hydrogen Peroxide-Induced Cell Death. Transl. Vis. Sci. Technol. 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Suri, F.; Yazdani, S.; Elahi, E. LTBP2 knockdown and oxidative stress affect glaucoma features including TGFbeta pathways, ECM genes expression and apoptosis in trabecular meshwork cells. Gene 2018, 673, 70–81. [Google Scholar] [CrossRef]
- Prendes, M.A.; Harris, A.; Wirostko, B.M.; Gerber, A.L.; Siesky, B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br. J. Ophthalmol. 2013, 97, 680–686. [Google Scholar] [CrossRef]
- Yang, X.; Hondur, G.; Li, M.; Cai, J.; Klein, J.B.; Kuehn, M.H.; Tezel, G. Proteomics Analysis of Molecular Risk Factors in the Ocular Hypertensive Human Retina. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5816–5830. [Google Scholar] [CrossRef]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef]
- Inoue-Mochita, M.; Inoue, T.; Fujimoto, T.; Kameda, T.; Awai-Kasaoka, N.; Ohtsu, N.; Kimoto, K.; Tanihara, H. p38 MAP kinase inhibitor suppresses transforming growth factor-beta2-induced type 1 collagen production in trabecular meshwork cells. PLoS ONE 2015, 10, e0120774. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.; Narumiya, S.; Honda, Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001, 42, 137–144. [Google Scholar]
- Rao, P.V.; Deng, P.F.; Kumar, J.; Epstein, D.L. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1029–1037. [Google Scholar]
- Shiryaev, A.; Moens, U. Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: Menage a trois or menage a quatre? Cell. Signal. 2010, 22, 1185–1192. [Google Scholar] [CrossRef]
- Chowdhury, R.; Chowdhury, S.; Roychoudhury, P.; Mandal, C.; Chaudhuri, K. Arsenic induced apoptosis in malignant melanoma cells is enhanced by menadione through ROS generation, p38 signaling and p53 activation. Apoptosis 2009, 14, 108–123. [Google Scholar] [CrossRef]
- Kaczara, P.; Sarna, T.; Burke, J.M. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic. Biol. Med. 2010, 48, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Tamai, K.; Mizushima, T.; Wu, X.; Inoue, A.; Ota, M.; Yokoyama, Y.; Miyoshi, N.; Haraguchi, N.; Takahashi, H.; Nishimura, J.; et al. Photodynamic Therapy Using Indocyanine Green Loaded on Super Carbonate Apatite as Minimally Invasive Cancer Treatment. Mol. Cancer Ther. 2018, 17, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Li, J.M.; Chou, H.C.; Wang, S.H.; Wu, C.L.; Chen, Y.W.; Lin, S.T.; Chen, Y.H.; Chan, H.L. Hyaluronic acid-dependent protection against UVB-damaged human corneal cells. Environ. Mol. Mutagen. 2013, 54, 429–449. [Google Scholar] [CrossRef]
- Xia, Y.F.; Chang, J.; Yang, J.F.; Ouyang, W.; Pitt, B.; Billiar, T.; Zhang, L.M. Non-canonical Wnt signaling contributes to ventilator-induced lung injury through upregulation of WISP1 expression. Int. J. Mol. Med. 2019, 43, 1217–1228. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.T.; Chang, Y.Y.; Chen, Y.C.; Liao, P.L.; Yang, D.J. Litchi (Litchi chinensis Sonn.) flower proanthocyanidin fraction exhibited protective efficacy to suppress nickel-induced expression for vascular endothelial growth factor in HepG2 cells. J. Food Biochem. 2019, 43, e12882. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ho, Y.J.; Chou, H.C.; Liao, E.C.; Tsai, Y.T.; Wei, Y.S.; Lin, L.H.; Lin, M.W.; Wang, Y.S.; Ko, M.L.; et al. TGF-beta1 signaling protects retinal ganglion cells from oxidative stress via modulation of the HO-1/Nrf2 pathway. Chem. Biol. Interact. 2020, 331, 109249. [Google Scholar] [CrossRef]
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-beta/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huai, G.; Wang, H.; Liu, Y.; Qi, P.; Shi, W.; Peng, J.; Yang, H.; Deng, S.; Wang, Y. Mutual regulation of the Hippo/Wnt/LPA/TGFbeta signaling pathways and their roles in glaucoma (Review). Int. J. Mol. Med. 2018, 41, 1201–1212. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, A.P.; Duan, X.M.; Hu, G.H.; Song, G.L.; Zuo, M.L.; Yang, Z.B. Upregulation of NOX2 and NOX4 Mediated by TGF-beta Signaling Pathway Exacerbates Cerebral Ischemia/Reperfusion Oxidative Stress Injury. Cell. Physiol. Biochem. 2018, 46, 2103–2113. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Park, S.R.; Cho, S.; Yu, S.L.; Lee, H.Y.; Park, C.G.; Kang, J.; Jung, D.Y.; Park, M.H.; Hwang, W.M.; et al. TGF-beta-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018, 73, 962–972. [Google Scholar] [CrossRef]
- Hazlewood, R.J.; Chen, Q.; Clark, F.K.; Kuchtey, J.; Kuchtey, R.W. Differential effects of angiotensin II type I receptor blockers on reducing intraocular pressure and TGFbeta signaling in the mouse retina. PLoS ONE 2018, 13, e0201719. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.L.; Chang, W.T.; Hong, M.Y.; Hung, K.C.; Chuang, C.C. Prevention of TGF-beta-induced early liver fibrosis by a maleic acid derivative antioxidant through suppression of ROS, inflammation and hepatic stellate cells activation. PLoS ONE 2017, 12, e0174008. [Google Scholar] [CrossRef] [Green Version]
- Tellios, N.; Belrose, J.C.; Tokarewicz, A.C.; Hutnik, C.; Liu, H.; Leask, A.; Motolko, M.; Iijima, M.; Parapuram, S.K. TGF-beta induces phosphorylation of phosphatase and tensin homolog: Implications for fibrosis of the trabecular meshwork tissue in glaucoma. Sci. Rep. 2017, 7, 812. [Google Scholar] [CrossRef] [Green Version]
- Fisichella, V.; Giurdanella, G.; Platania, C.B.; Romano, G.L.; Leggio, G.M.; Salomone, S.; Drago, F.; Caraci, F.; Bucolo, C. TGF-beta1 prevents rat retinal insult induced by amyloid-beta (1-42) oligomers. Eur. J. Pharmacol. 2016, 787, 72–77. [Google Scholar] [CrossRef]
- Inoue, T.; Ohbayashi, T.; Fujikawa, Y.; Yoshida, H.; Akama, T.O.; Noda, K.; Horiguchi, M.; Kameyama, K.; Hata, Y.; Takahashi, K.; et al. Latent TGF-beta binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum. Mol. Genet. 2014, 23, 5672–5682. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Casalena, G.; Daehn, I.; Bottinger, E. Transforming growth factor-beta, bioenergetics, and mitochondria in renal disease. Semin. Nephrol. 2012, 32, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, X.; Huang, J.; Zhong, Y.; Mao, Z.; Xiao, H.; Li, M.; Zhuo, Y. Inhibition of p38 mitogen-activated protein kinase phosphorylation decrease tert-butyl hydroperoxide-induced apoptosis in human trabecular meshwork cells. Mol. Vis. 2012, 18, 2127–2136. [Google Scholar]
- Zhu, D.H.; Wu, J.; Spee, C.; Ryan, S.J.; Hinton, D.R. BMP4 Mediates Oxidative Stress-induced Retinal Pigment Epithelial Cell Senescence and Is Overexpressed in Age-related Macular Degeneration. J. Biol. Chem. 2009, 284, 9529–9539. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Chou, H.C.; Chang, S.J.; Liao, E.C.; Tsai, Y.T.; Wei, Y.S.; Li, J.M.; Lin, L.H.; Lin, M.W.; Chen, Y.J.; et al. Proteomic Analysis of Various Rat Ocular Tissues after Ischemia-Reperfusion Injury and Possible Relevance to Acute Glaucoma. Int. J. Mol. Sci 2017, 18, 334. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Chou, H.-C.; Ho, Y.-J.; Chang, S.-J.; Liao, E.-C.; Wei, Y.-S.; Lin, M.-W.; Wang, Y.-S.; Chien, Y.-A.; Yu, X.-R.; et al. Characterization of TGF-β by Induced Oxidative Stress in Human Trabecular Meshwork Cells. Antioxidants 2021, 10, 107. https://doi.org/10.3390/antiox10010107
Chen H-Y, Chou H-C, Ho Y-J, Chang S-J, Liao E-C, Wei Y-S, Lin M-W, Wang Y-S, Chien Y-A, Yu X-R, et al. Characterization of TGF-β by Induced Oxidative Stress in Human Trabecular Meshwork Cells. Antioxidants. 2021; 10(1):107. https://doi.org/10.3390/antiox10010107
Chicago/Turabian StyleChen, Hsin-Yi, Hsiu-Chuan Chou, Yi-Jung Ho, Shing-Jyh Chang, En-Chi Liao, Yu-Shan Wei, Meng-Wei Lin, Yi-Shiuan Wang, Yu-An Chien, Xin-Ru Yu, and et al. 2021. "Characterization of TGF-β by Induced Oxidative Stress in Human Trabecular Meshwork Cells" Antioxidants 10, no. 1: 107. https://doi.org/10.3390/antiox10010107




