Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Cell Culture
2.3. RT (Reverse Transcription)-PCR
2.4. Northern Blot Analysis
2.5. Luciferase Promoter Assay
2.6. Immunofluorescence Analysis
2.7. Western Blot Analysis
2.8. Assay of System xc- Transport Activity
2.9. Hydrogen Peroxide (H2O2) Treatment and Glutathione Measurement
2.10. Data Analysis
3. Results
3.1. Se-Met-Induced Upregulation of Nrf2
3.2. Se-Met-Induced Upregulation of xCT Expression
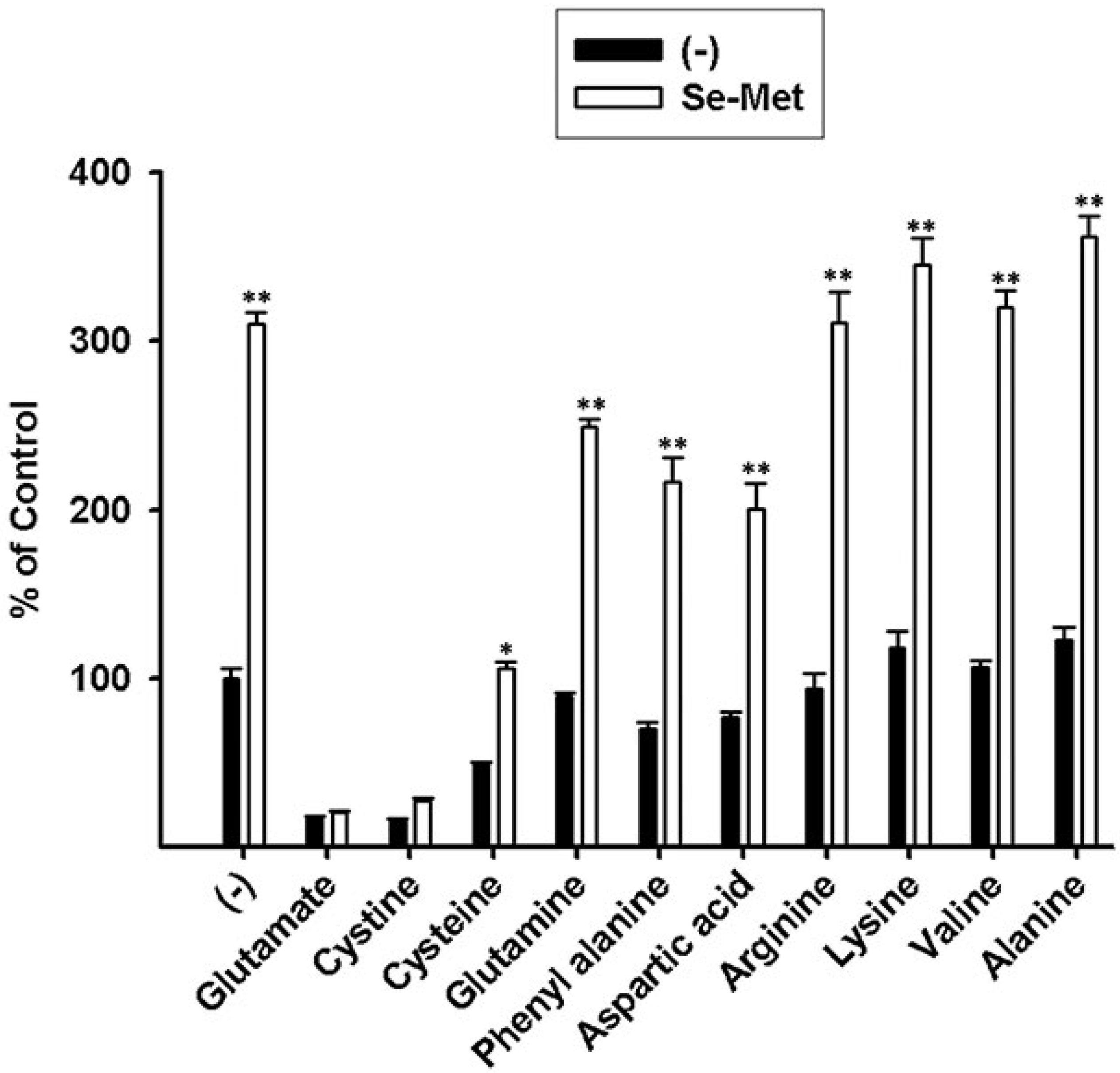
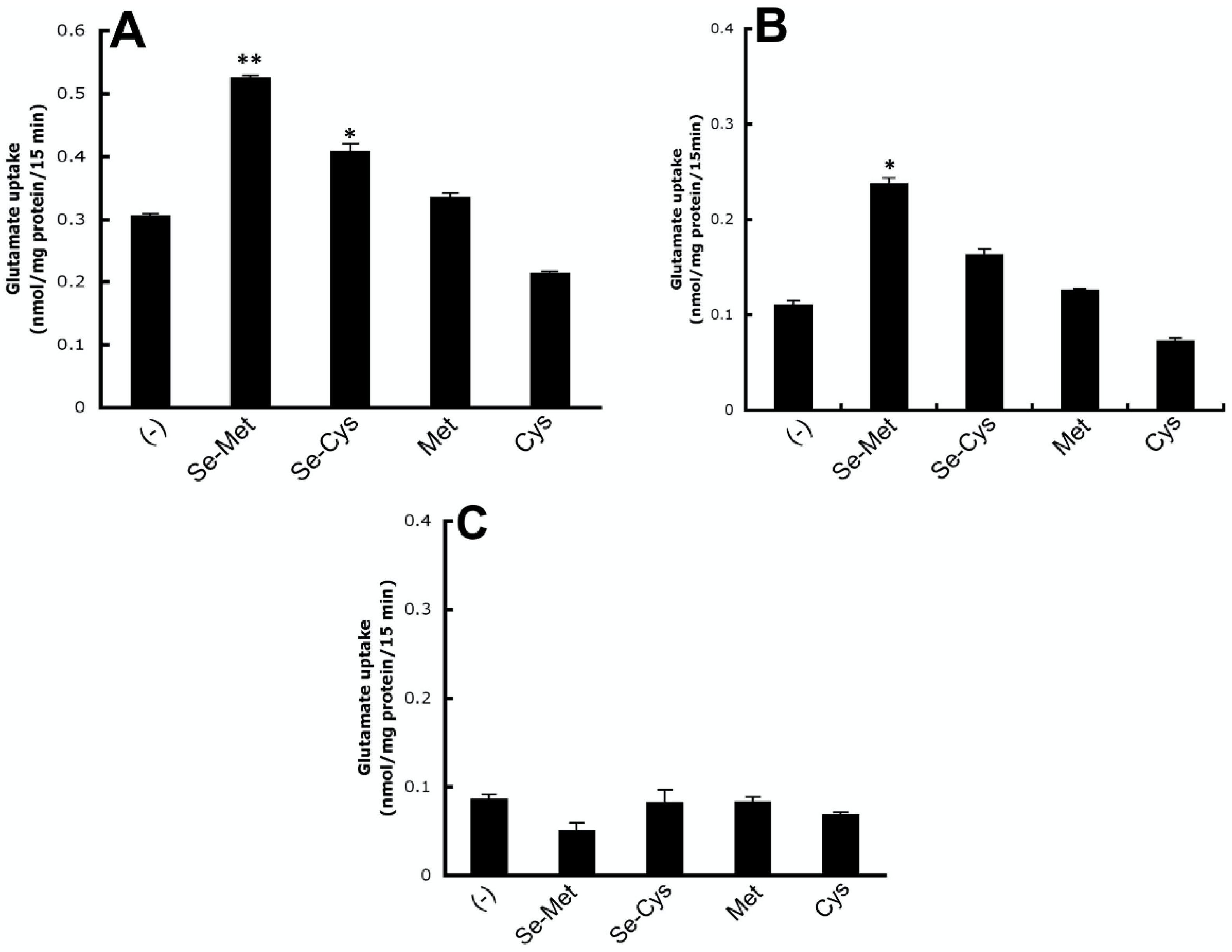
3.3. Characterization of Se-Met-Induced xc- Transport Activity
3.4. Kinetic Analysis of Se-Met-Induced System xc- Transport Activity
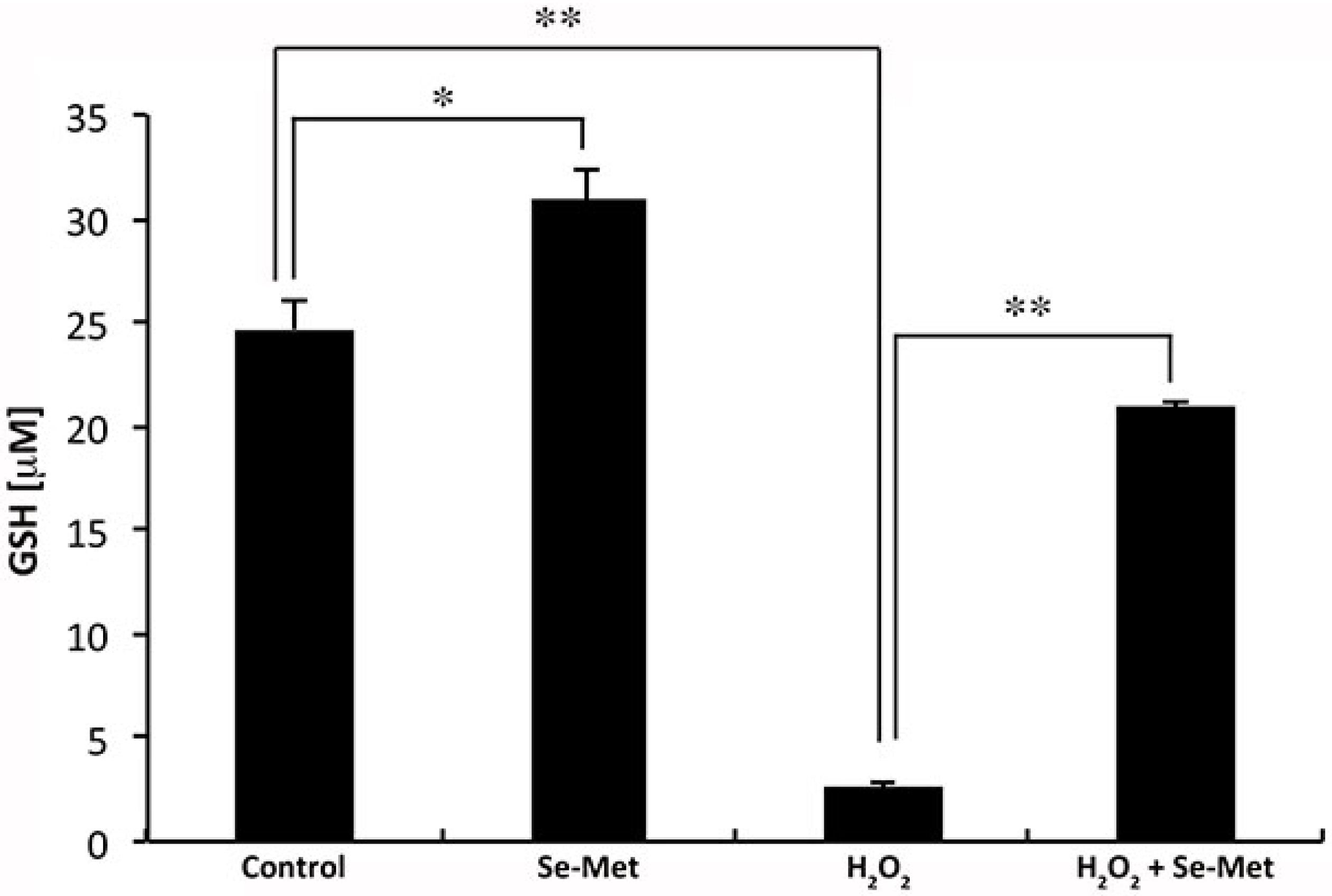
3.5. Influence of Se-Met on Glutathione Levels in ARPE-19 Cells with and without Oxidative Stress
3.6. Se-Met Induces System xc- Activity also in Primary Cultures of Mouse RPE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Battagliola, E.T.; D’Angelo, R.; Sidoti, A.; Donato, L. Expression of Pro-Angiogenic Markers Is Enhanced by Blue Light in Human RPE Cells. Antioxidants (Basel) 2020, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; D’Angelo, R.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; Scimone, C. Effects of A2E-Induced Oxidative Stress on Retinal Epithelial Cells: New Insights on Differential Gene Response and Retinal Dystrophies. Antioxidants (Basel) 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int. Rev. Cell Mol. Biol. 2012, 298, 135–177. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Martis, R.M.; Knight, L.J.; Donaldson, P.J.; Lim, J.C. Identification, Expression, and Roles of the Cystine/Glutamate Antiporter in Ocular Tissues. Oxid. Med. Cell. Longev. 2020, 2020, 4594606. [Google Scholar] [CrossRef]
- Bridges, C.C.; Kekuda, R.; Wang, H.; Prasad, P.D.; Mehta, P.; Huang, W.; Smith, S.B.; Ganapathy, V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 47–54. [Google Scholar]
- Reddy, N.M.; Kleeberger, S.R.; Bream, J.H.; Fallon, P.G.; Kensler, T.W.; Yamamoto, M.; Reddy, S.P. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene 2008, 27, 5821–5832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kolle, P.; Tschoep, K.; Issels, R.D.; Daniel, P.T.; et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragones, G.; Rowan, S.; Francisco, S.G.; Yang, W.; Weinberg, J.; Taylor, A.; Bejarano, E. Glyoxalase System as a Therapeutic Target against Diabetic Retinopathy. Antioxidants (Basel) 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Rahmanto, A.S.; Pattison, D.I.; Davies, M.J. Photo-oxidation-induced inactivation of the selenium-containing protective enzymes thioredoxin reductase and glutathione peroxidase. Free Radic. Biol. Med. 2012, 53, 1308–1316. [Google Scholar] [CrossRef]
- Lane, H.W.; Strength, R.; Johnson, J.; White, M. Effect of chemical form of selenium on tissue glutathione peroxidase activity in developing rats. J. Nutr. 1991, 121, 80–86. [Google Scholar] [CrossRef]
- Dun, Y.; Mysona, B.; Van Ells, T.; Amarnath, L.; Ola, M.S.; Ganapathy, V.; Smith, S.B. Expression of the cystine-glutamate exchanger (xc-) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006, 324, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Jadeja, R.N.; Powell, F.L.; Jones, M.A.; Fuller, J.; Joseph, E.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Loss of NAMPT in aging retinal pigment epithelium reduces NAD(+) availability and promotes cellular senescence. Aging (Albany NY) 2018, 10, 1306–1323. [Google Scholar] [CrossRef]
- Gambhir, D.; Ananth, S.; Veeranan-Karmegam, R.; Elangovan, S.; Hester, S.; Jennings, E.; Offermanns, S.; Nussbaum, J.J.; Smith, S.B.; Thangaraju, M.; et al. GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2208–2217. [Google Scholar] [CrossRef]
- Martin, P.M.; Ananth, S.; Cresci, G.; Roon, P.; Smith, S.; Ganapathy, V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol. Vis. 2009, 15, 362–372. [Google Scholar]
- Gnana-Prakasam, J.P.; Thangaraju, M.; Liu, K.; Ha, Y.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Absence of iron-regulatory protein Hfe results in hyperproliferation of retinal pigment epithelium: Role of cystine/glutamate exchanger. Biochem. J. 2009, 424, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nomura, S.; Maebara, K.; Sato, K.; Tamba, M.; Bannai, S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 2004, 325, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Ananth, S.; Martin, P.M.; Roon, P.; Smith, S.B.; Sterneck, E.; Prasad, P.D.; Ganapathy, V. c/ebpdelta Null mouse as a model for the double knock-out of slc5a8 and slc5a12 in kidney. J. Biol. Chem. 2006, 281, 26769–26773. [Google Scholar] [CrossRef] [Green Version]
- Mysona, B.; Dun, Y.; Duplantier, J.; Ganapathy, V.; Smith, S.B. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Muller glial cells. Cell Tissue Res. 2009, 335, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, C.C.; Hu, H.; Miyauchi, S.; Siddaramappa, U.N.; Ganapathy, M.E.; Ignatowicz, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Induction of cystine-glutamate transporter xc- by human immunodeficiency virus type 1 transactivator protein tat in retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2906–2914. [Google Scholar] [CrossRef] [Green Version]
- Kursvietiene, L.; Mongirdiene, A.; Bernatoniene, J.; Sulinskiene, J.; Staneviciene, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants (Basel) 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.H. Involvement of Nrf2 in Ocular Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1703810. [Google Scholar] [CrossRef]
- Promsote, W.; Powell, F.L.; Veean, S.; Thounaojam, M.; Markand, S.; Saul, A.; Gutsaeva, D.; Bartoli, M.; Smith, S.B.; Ganapathy, V.; et al. Oral Monomethyl Fumarate Therapy Ameliorates Retinopathy in a Humanized Mouse Model of Sickle Cell Disease. Antioxid. Redox Signal. 2016, 25, 921–935. [Google Scholar] [CrossRef] [Green Version]
- Habib, E.; Linher-Melville, K.; Lin, H.X.; Singh, G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc(-) cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maenpaa, H.; Gegelashvili, G.; Tahti, H. Expression of glutamate transporter subtypes in cultured retinal pigment epithelial and retinoblastoma cells. Curr. Eye Res. 2004, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Albano, R.; Raddatz, N.J.; Hjelmhaug, J.; Baker, D.A.; Lobner, D. Regulation of System xc(-) by Pharmacological Manipulation of Cellular Thiols. Oxid. Med. Cell. Longev. 2015, 2015, 269371. [Google Scholar] [CrossRef] [Green Version]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Militante, J.D.; Lombardini, J.B. Taurine: Evidence of physiological function in the retina. Nutr. Neurosci. 2002, 5, 75–90. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Plestina-Borjan, I.; Katusic, D.; Medvidovic-Grubisic, M.; Supe-Domic, D.; Bucan, K.; Tandara, L.; Rogosic, V. Association of age-related macular degeneration with erythrocyte antioxidant enzymes activity and serum total antioxidant status. Oxid. Med. Cell. Longev. 2015, 2015, 804054. [Google Scholar] [CrossRef]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B. Role of the Nrf2 signaling system in health and disease. Clin. Genet. 2014, 86, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef] [Green Version]
- Sheck, L.; Davies, J.; Wilson, G. Selenium and ocular health in New Zealand. N. Z. Med. J. 2010, 123, 85–94. [Google Scholar]
- Flohe, L. Selenium, selenoproteins and vision. Dev. Ophthalmol. 2005, 38, 89–102. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Loef, M.; Schrauzer, G.N.; Walach, H. Selenium and Alzheimer’s disease: A systematic review. J. Alzheimers Dis. 2011, 26, 81–104. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.A.; Kawai, K.; Kupka, R.; Fawzi, W.W. Role of selenium in HIV infection. Nutr. Rev. 2010, 68, 671–681. [Google Scholar] [CrossRef]
- Dridi, S.; Hirano, Y.; Tarallo, V.; Kim, Y.; Fowler, B.J.; Ambati, B.K.; Bogdanovich, S.; Chiodo, V.A.; Hauswirth, W.W.; Kugel, J.F.; et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 13781–13786. [Google Scholar] [CrossRef] [Green Version]
- Filomeni, G.; Piccirillo, S.; Rotilio, G.; Ciriolo, M.R. p38(MAPK) and ERK1/2 dictate cell death/survival response to different pro-oxidant stimuli via p53 and Nrf2 in neuroblastoma cells SH-SY5Y. Biochem. Pharmacol. 2012, 83, 1349–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, S.; Markesbery, W.R.; Shao, C.; Lovell, M.A. Seleno-L-methionine protects against beta-amyloid and iron/hydrogen peroxide-mediated neuron death. Antioxid. Redox Signal. 2007, 9, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.R.; Kelley, M.R.; Smith, M.L. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 14548–14553. [Google Scholar] [CrossRef] [Green Version]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicastro, H.L.; Dunn, B.K. Selenium and prostate cancer prevention: Insights from the selenium and vitamin E cancer prevention trial (SELECT). Nutrients 2013, 5, 1122–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoefig, C.S.; Renko, K.; Kohrle, J.; Birringer, M.; Schomburg, L. Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J. Nutr. Biochem. 2011, 22, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Yazici, A.; Sogutlu-Sari, E.; Yay, A.; Aksit, H.; Kilic, A.; Aksit, D.; Yildiz, O.; Ermis, S.S. The protective effect of selenium in cisplatin-related retinotoxicity. Cutan. Ocul. Toxicol. 2014, 33, 327–332. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. The putative therapeutic value of high-dose selenium in proliferative retinopathies may reflect down-regulation of VEGF production by the hypoxic retina. Med. Hypotheses 2005, 64, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, M.A.; Ghirlanda, G.; Silveri, N.G.; Giardina, B.; Franconi, F.; Santini, S.A. Potential therapeutic effect of antioxidants in experimental diabetic retina: A comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic. Res. 2003, 37, 323–330. [Google Scholar] [CrossRef]
- Head, K.A. Natural therapies for ocular disorders, part one: Diseases of the retina. Altern. Med. Rev. 1999, 4, 342–359. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananth, S.; Miyauchi, S.; Thangaraju, M.; Jadeja, R.N.; Bartoli, M.; Ganapathy, V.; Martin, P.M. Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina. Antioxidants 2021, 10, 9. https://doi.org/10.3390/antiox10010009
Ananth S, Miyauchi S, Thangaraju M, Jadeja RN, Bartoli M, Ganapathy V, Martin PM. Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina. Antioxidants. 2021; 10(1):9. https://doi.org/10.3390/antiox10010009
Chicago/Turabian StyleAnanth, Sudha, Seiji Miyauchi, Muthusamy Thangaraju, Ravirajsinh N. Jadeja, Manuela Bartoli, Vadivel Ganapathy, and Pamela M. Martin. 2021. "Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina" Antioxidants 10, no. 1: 9. https://doi.org/10.3390/antiox10010009




