Structural Characteristics of Crude Polysaccharides from 12 Selected Chinese Teas, and Their Antioxidant and Anti-Diabetic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Crude Polysaccharides from 12 Chinese Teas
2.3. Test of Structural Properties of TPs
2.3.1. Test of Molecular Weights (Mw), Polydispersities (Mw/Mn), and Compositional Monosaccharides
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy and Nuclear Magnetic Resonance (NMR) Analysis
2.4. In Vitro Antioxidant Assays
2.5. Evaluation of the Anti-Diabetic Activity of TPs
2.5.1. In Vitro α-Glucosidase Inhibitory Assay
2.5.2. In Vitro Antiglycation Assay
2.6. Statistical Analysis
3. Results
3.1. The Extraction Yields and Chemical Compositions of TPs
3.2. Structural Properties of TPs
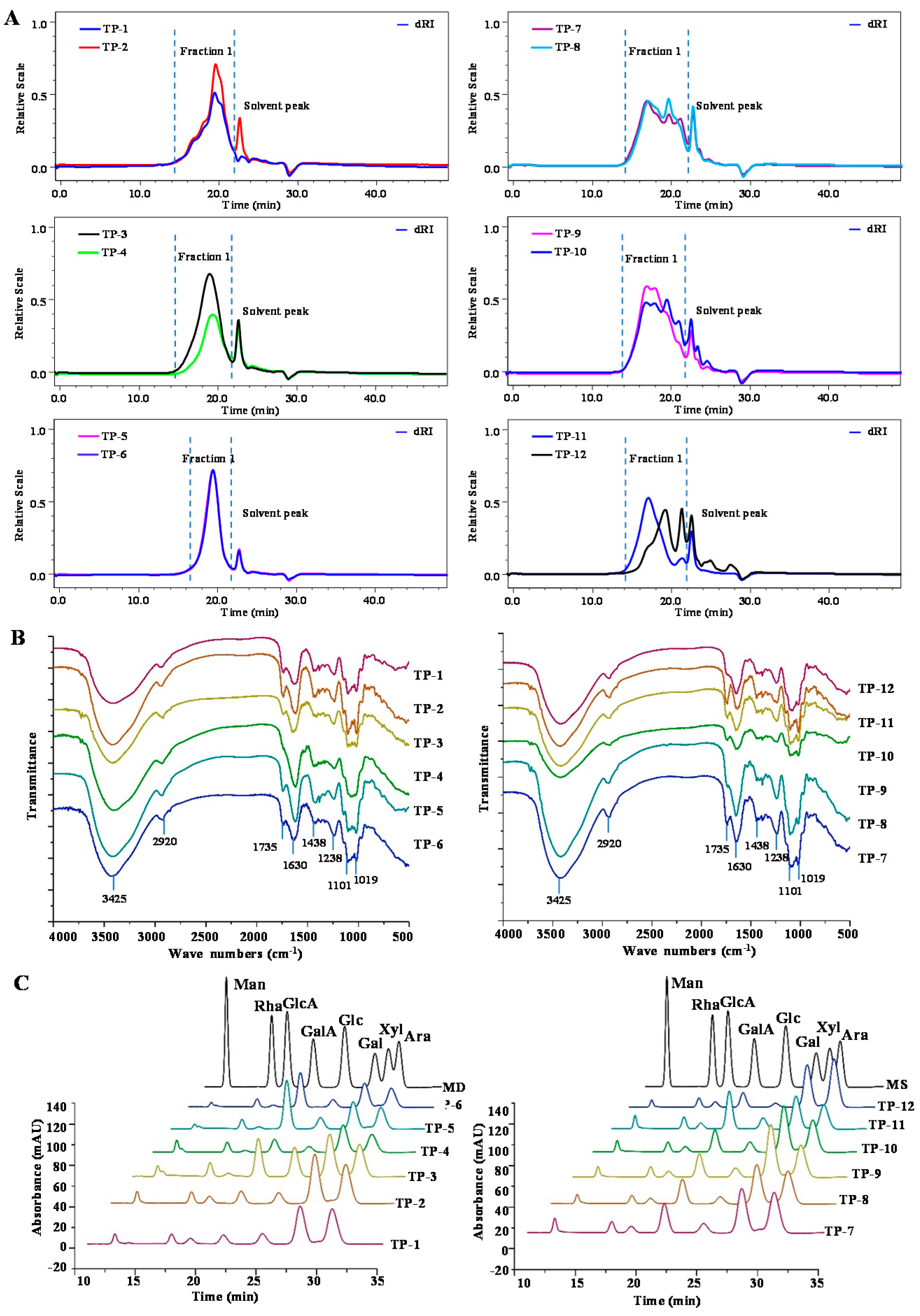
3.2.1. Molecular Weights of TPs
3.2.2. FT-IR Spectra of TPs
3.2.3. Compositional Monosaccharides of TPs
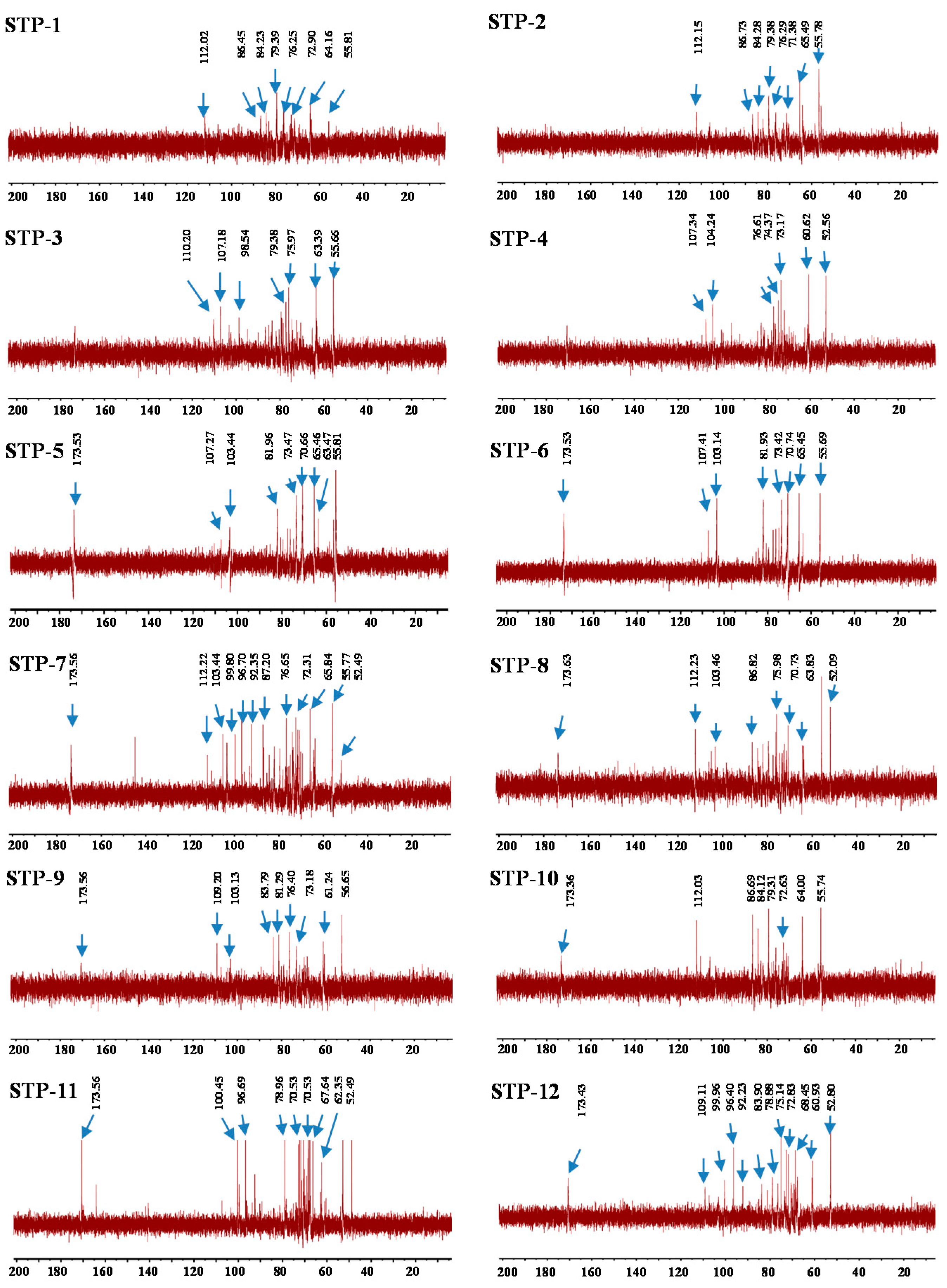
3.2.4. NMR Analysis
3.3. In Vitro Antioxidant Activities
3.4. In Vitro Anti-Diabetic Activities of TPs
3.4.1. In Vitro α-Glucosidase Inhibitory Activity
3.4.2. In Vitro Antiglycation Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocolloid. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Bio. 2017, 7, 352–357. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, C.N.; Cao, S.Y.; Tang, G.Y.; Gan, R.Y.; Li, H.B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1693–1705. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.; Mata, A.; Corke, H.; Gan, R.; Fang, Y. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocolloid. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Guo, Y.J.; Sun, L.Q.; Yu, B.Y.; Qi, J. An integrated antioxidant activity fingerprint for commercial teas based on their capacities to scavenge reactive oxygen species. Food Chem. 2017, 237, 645–653. [Google Scholar] [CrossRef]
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Li, H.B.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Du, W.H.; Peng, S.M.; Liu, Z.H.; Shi, L.; Tan, L.F.; Zou, X.Q. Hypoglycemic effect of the water extract of Pu-erh tea. J. Agric. Food. Chem. 2012, 60, 10126–10132. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, M.; Qu, Z.S.; Xie, B.J. Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate. J. Agric. Food Chem. 2007, 55, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Fu, Q.Y.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; Ye, J.H.; Li, Q.S.; Polito, C.A.; Liang, Y.R. Tea polysaccharides and their bioactivities. Molecules 2016, 21, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelvana, E.; Karadagb, A.; Doganb, K.; Aksuc, S.; Tasc, A.; Akalınd, K.; Eklioğlue Atlı, Ö.; Alasalvar, C. In-vitro antidiabetic activities, chemical compositions, antioxidant activities, and toxicity of black tea polysaccharides as a potential source of dietary ingredients. J. Food Bioact. 2021, 13. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q.; Saeeduddin, M.; Ou, S.; Zeng, X.; Ye, H. Recent advances in tea polysaccharides: Extraction, purification, physicochemical characterization and bioactivities. Carbohydr. Polym. 2016, 153, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Polysaccharides from Chinese tea: Recent advance on bioactivity and function. Int. J. Biol. Macromol. 2013, 62, 76–79. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Andrae-Marobela, K.; Okatch, H.; Xiao, J. Tea polysaccharides as food antioxidants: An old woman’s tale? Food Chem. 2013, 138, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Guo, H.; Lin, S.; Lu, M.; Gong, J.D.B.; Wang, L.; Zhang, Q.; Lin, D.R.; Qin, W.; Wu, D.T. Characterization, in vitro binding properties, and inhibitory activity on pancreatic lipase of beta-glucans from different Qingke (Tibetan hulless barley) cultivars. Int. J. Biol. Macromol. 2018, 120, 2517–2522. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.Y.; Liu, L.; Wu, C.Y.; Liu, H.; Zhao, L.; Zhang, Q.; Liu, Y.T.; Li, S.Q.; Qin, W.; et al. Effects of sulfated modification on the physicochemical properties and biological activities of beta-glucans from Qingke (Tibetan hulless barley). Int. J. Biol. Macromol. 2019, 141, 41–50. [Google Scholar] [CrossRef]
- Nie, X.R.; Fu, Y.; Wu, D.T.; Huang, T.T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.R.; Chen, H.; Qin, W. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction optimization and effects of extraction methods on the chemical structures and antioxidant activities of polysaccharides from snow Chrysanthemum (Coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, F.; Wang, P.; Liu, X.; He, J.J.; Xian, M.L.; Zhao, L.; Qin, W.; Gan, R.Y.; Wu, D.T. Effects of drying methods on the physicochemical characteristics and bioactivities of polyphenolic-protein-polysaccharide conjugates from Hovenia dulcis. Int. J. Biol. Macromol. 2020, 148, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of Puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Huo, J.; Zhao, X.; Zheng, J.; Wei, X. Effect of different drying methods on chemical composition and bioactivity of tea polysaccharides. Int. J. Biol. Macromol. 2013, 62, 714–719. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shao, S.H.; Xu, P.; Chen, H.; Lin-Shiau, S.Y.; Deng, Y.T.; Lin, J.K. Fermentation process enhanced production and bioactivities of oolong tea polysaccharides. Food Res. Int. 2012, 46, 158–166. [Google Scholar] [CrossRef]
- Li, F.; Feng, K.L.; Yang, J.C.; He, Y.S.; Guo, H.; Wang, S.P.; Gan, R.Y.; Wu, D.T. Polysaccharides from dandelion (Taraxacum mongolicum) leaves: Insights into innovative drying techniques on their structural characteristics and biological activities. Int. J. Biol. Macromol. 2021, 167, 995–1005. [Google Scholar] [CrossRef]
- Mao, L.; Shao, S.; Sun, S.; Wang, Y.; Xu, P.; Cai, L. Purification, physicochemical characterization, and bioactivities of polysaccharides from Puerh tea. J. Food Nutr. Res. 2014, 2, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.Y.; Lin, H.X.; Li, J.; Wang, Y.X.; Cui, S.W.; Nie, S.P.; Xie, M.Y. Structural characterization of a highly branched polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2012, 87, 2416–2424. [Google Scholar] [CrossRef]
- Lin, X.; Ji, X.; Wang, M.; Yin, S.; Peng, Q. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: Structural characterizations and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 607–615. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Zhang, J.; Zhang, S.; Zhao, W.; Liu, T.; Wang, D. Isolation, purification, structural characteristic and antioxidative property of polysaccharides from A. cepa L. var. agrogatum Don. Food Sci. Hum. Well. 2020, 9, 71–79. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Q.; Zhao, J.; Mao, G.; Feng, W.; Chen, Y.; Li, Q.; Wu, X.; Yang, L.; Zhao, T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef]
- Hu, C.; Li, H.X.; Zhang, M.T.; Liu, L.F. Structure characterization and anticoagulant activity of a novel polysaccharide from Leonurus artemisia (Laur.) S. Y. Hu F. RSC Adv. 2020, 10, 2254–2266. [Google Scholar] [CrossRef] [Green Version]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Nifantiev, N.E.; Usov, A.I. Two fucosylated chondroitin sulfates from the sea cucumber Eupentacta fraudatrix. Carbohydr. Polym. 2017, 164, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Xu, Q.; Bian, G.; Guo, Q.; Fang, Z.; Wu, W. Structure characterization and immunomodulatory activity of a new neutral polysaccharide SMP-0b from Solanum muricatum. Int. J. Biol. Macromol. 2020, 155, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Shakhmatov, E.G.; Toukach, P.V.; Michailowa, C.; Makarova, E.N. Structural studies of arabinan-rich pectic polysaccharides from Abies sibirica L. Biological activity of pectins of A. sibirica. Carbohydr. Polym. 2014, 113, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jin, C.; Chen, H.; Wang, P.; Yu, M.; Ding, K. Structural characterization and anti-A549 lung cancer cells bioactivity of a polysaccharide from Houttuynia cordata. Int. J. Biol. Macromol. 2018, 120, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. NMR-spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 30, 3307–3330. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.J.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef]
- Siu, K.C.; Chen, X.; Wu, J.Y. Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J. Funct. Foods 2014, 11, 548–556. [Google Scholar] [CrossRef]
- Alasalvar, C.; Aksu, S.; Eklioglu, O.A.; Tas, A.; Pelvan, E.; Dogan, K.; Karadag, A. α-Glucosidase inhibitory activities, functional properties, and safety of green tea polysaccharides as a potential source of dietary supplement. J. Food Bioact. 2018, 3, 124–132. [Google Scholar]
- Fan, M.; Zhu, J.; Qian, Y.; Yue, W.; Xu, Y.; Zhang, D.; Yang, Y.; Gao, X.; He, H.; Wang, D. Effect of purity of tea polysaccharides on its antioxidant and hypoglycemic activities. J. Food Biochem. 2020, 44, e13277. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.P.; Zhang, Y.J.; Lin, Z.; Liang, Y.R. Processing and chemical constituents of Pu-erh tea: A review. Food Res. Int. 2013, 53, 608–618. [Google Scholar] [CrossRef]
- Maharjan, A.; Karkee, A.; Prajapati, M.; Shrestha, H.; Manandhar Shrestha, J.T. Adverse effects of oral hypoglycemic agents and adherence to them among patients with Type 2 diabetes mellitus in Nepal. J. Lumbini Med. Coll. 2017, 5, 34. [Google Scholar]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Kulkarni, M.J.; Korwar, A.M.; Mary, S.; Bhonsle, H.S.; Giri, A.P. Glycated proteome: From reaction to intervention. Proteom. Clin. Appl. 2013, 7, 155–170. [Google Scholar] [CrossRef]
- He, Y.X.; Jun, H.M.; Li, M.Q.; Hua, L.Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Grain Oil Sci. Technol. 2018, 1, 768–775. [Google Scholar]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fu, M.X.; Zhao, Y.X.; Li, H.; Li, H.B.; Wu, D.T.; Gan, R.Y. The chemical, structural, and biological properties of crude polysaccharides from sweet tea (Lithocarpus litseifolius (Hance) Chun) based on different extraction technologies. Foods 2021, 10, 1779. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Fu, M.X.; Gan, R.Y.; Hu, Y.C.; Peng, L.X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocolloid. 2022, 122, 107085. [Google Scholar] [CrossRef]


| No. | Tea Name | Production Place | Fermentation Degree | Category |
|---|---|---|---|---|
| TP-1 | Dianhong Congou Black Tea | Kunming, Yunnan | Deep-fermented | Black tea |
| TP-2 | Yichang Congou Black Tea | Yichang, Hubei | Deep-fermented | Black tea |
| TP-3 | Fuzhuan Brick Tea | Anhua, Hubei | Post-fermented | Dark tea |
| TP-4 | Pu-erh Tea | Pu’er, Yunnan | Post-fermented | Dark tea |
| TP-5 | Fenghuang Shuixian Tea | Chao’an, Guangdong | Semi-fermented | Oolong tea |
| TP-6 | Wuyi Rock Tea | Wuyishan, Fujian | Semi-fermented | Oolong tea |
| TP-7 | Dianqing Tea | Kunming, Yunnan | Non-fermented | Green tea |
| TP-8 | Lushan Yunwu Tea | Jiujiang, Jiangxi | Non-fermented | Green tea |
| TP-9 | Gongmei White Tea | Nanping, Fujian | Mild-fermented | White tea |
| TP-10 | White Peony Tea | Nanping, Fujian | Mild-fermented | White tea |
| TP-11 | Weishan Maojian Tea | Ningxiang, Hunan | Light-fermented | Yellow tea |
| TP-12 | Yuan’an Luyuan Tea | Yichang, Hubei | Light-fermented | Yellow tea |
| No. | Extraction Yields (%) | Total Polysaccharides (%) | Protein Contents (%) | Degree of Esterification (%) | Total Uronic Acids (%) | TPC (mg GAE/g) |
|---|---|---|---|---|---|---|
| TP-1 | 1.81 ± 0.12 f | 78.97 ± 1.01 de | 5.23 ± 0.34 de | 12.43 ± 0.45 g | 16.04 ± 0.70 i | 92.88 ± 7.34 b |
| TP-2 | 2.37 ± 0.19 e | 83.26 ± 0.93 b | 3.42 ± 0.31 g | 15.94 ± 0.69 f | 18.33 ± 0.65 h | 53.41 ± 5.92 d |
| TP-3 | 5.65 ± 0.23 b | 82.39 ± 0.88 bc | 5.05 ± 0.42 de | 25.16 ± 0.72 e | 42.71 ± 0.99 c | 65.50 ± 5.59 c |
| TP-4 | 6.38 ± 0.28 a | 63.51 ± 0.76 g | 11.73 ± 0.76 a | - | 20.57 ± 0.42 g | 162.43 ± 9.43 a |
| TP-5 | 5.49 ± 0.35 b | 75.31 ± 1.09 f | 5.30 ± 0.45 de | 35.37 ± 0.51 c | 47.39 ± 1.12 a | 51.31 ± 7.86 d |
| TP-6 | 5.31 ± 0.29 b | 77.13 ± 1.22 ef | 4.71 ± 0.43 de | 36.68 ± 0.46 b | 44.27 ± 1.07 b | 55.41 ± 4.55 d |
| TP-7 | 3.05 ± 0.21 cd | 88.44 ± 1.36 a | 3.84 ± 0.30 fg | 34.59 ± 0.55 c | 33.36 ± 0.74 d | 13.54 ± 3.05 fg |
| TP-8 | 3.26 ± 0.22 c | 86.87 ± 1.73 a | 4.46 ± 0.36 ef | 8.99 ± 0.45 h | 33.15 ± 0.98 d | 14.84 ± 2.67 fg |
| TP-9 | 3.09 ± 0.22 cd | 82.22 ± 1.28 bc | 7.96 ± 0.52 c | 30.73 ± 0.44 d | 29.96 ± 0.86 e | 20.83 ± 2.62 ef |
| TP-10 | 2.58 ± 0.27 e | 81.83 ± 1.65 bc | 3.60 ± 0.39 g | 31.80 ± 0.69 d | 27.68 ± 0.63 f | 25.63 ± 2.83 e |
| TP-11 | 2.69 ± 0.18 de | 80.49 ± 1.66 cd | 5.45 ± 0.42 d | 46.46 ± 0.80 a | 42.27 ± 0.82 c | 11.64 ± 1.31 g |
| TP-12 | 3.23 ± 0.25 c | 82.35 ± 1.48 bc | 9.34 ± 0.67 b | 15.31 ± 0.37 f | 20.37 ± 0.59 g | 12.34 ± 1.88 g |
| Mw × 104 (Da) | Mw/Mn | Monosaccharides and Molar Ratios | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | GalA | Glc | Gal | Xyl | Ara | |||
| TP-1 | 21.20 (± 0.66%) gh | 2.48 (± 1.07%) | 0.17 | 0.26 | 0.21 | 0.82 | 0.30 | 1.05 | 0.04 | 1.00 |
| TP-2 | 23.88 (± 1.35%) e | 1.47 (± 1.90%) | 0.19 | 0.28 | 0.24 | 1.01 | 0.28 | 1.23 | 0.05 | 1.00 |
| TP-3 | 27.69 (± 0.44%) d | 2.19 (± 0.67%) | 0.19 | 0.39 | 0.15 | 3.71 | 0.90 | 1.26 | - | 1.00 |
| TP-4 | 20.24 (± 0.60%) h | 1.93 (± 0.98%) | 0.39 | 0.51 | 0.12 | 2.41 | 0.34 | 1.49 | - | 1.00 |
| TP-5 | 9.16 (± 0.62%) k | 2.19 (± 1.00%) | 0.18 | 0.41 | 0.11 | 7.41 | 0.56 | 1.24 | 0.06 | 1.00 |
| TP-6 | 12.48 (± 0.57%) j | 2.16 (± 0.87%) | 0.18 | 0.37 | 0.12 | 5.49 | 0.38 | 1.17 | - | 1.00 |
| TP-7 | 23.29 (± 0.60%) ef | 3.53 (± 1.71%) | 0.21 | 0.25 | 0.19 | 2.33 | 0.24 | 1.07 | 0.05 | 1.00 |
| TP-8 | 16.73 (± 0.61%) i | 2.88 (± 1.96%) | 0.17 | 0.23 | 0.20 | 2.42 | 0.22 | 1.17 | 0.05 | 1.00 |
| TP-9 | 73.34 (± 0.71%) a | 2.13 (± 1.17%) | 0.21 | 0.29 | 0.22 | 2.33 | 0.29 | 1.60 | 0.05 | 1.00 |
| TP-10 | 49.13 (± 0.92%) b | 2.53 (± 1.61%) | 0.20 | 0.28 | 0.22 | 2.20 | 0.31 | 1.38 | 0.07 | 1.00 |
| TP-11 | 43.06 (± 0.54%) c | 2.06 (± 0.95%) | 0.35 | 0.46 | 0.30 | 5.04 | 0.46 | 1.32 | - | 1.00 |
| TP-12 | 22.47 (± 0.96%) fg | 3.08 (± 1.71%) | 0.08 | 0.18 | 0.14 | 0.97 | 0.09 | 0.86 | 0.02 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Fu, M.-X.; Wu, D.-T.; Zhao, Y.-X.; Li, H.; Li, H.-B.; Gan, R.-Y. Structural Characteristics of Crude Polysaccharides from 12 Selected Chinese Teas, and Their Antioxidant and Anti-Diabetic Activities. Antioxidants 2021, 10, 1562. https://doi.org/10.3390/antiox10101562
Guo H, Fu M-X, Wu D-T, Zhao Y-X, Li H, Li H-B, Gan R-Y. Structural Characteristics of Crude Polysaccharides from 12 Selected Chinese Teas, and Their Antioxidant and Anti-Diabetic Activities. Antioxidants. 2021; 10(10):1562. https://doi.org/10.3390/antiox10101562
Chicago/Turabian StyleGuo, Huan, Meng-Xi Fu, Ding-Tao Wu, Yun-Xuan Zhao, Hang Li, Hua-Bin Li, and Ren-You Gan. 2021. "Structural Characteristics of Crude Polysaccharides from 12 Selected Chinese Teas, and Their Antioxidant and Anti-Diabetic Activities" Antioxidants 10, no. 10: 1562. https://doi.org/10.3390/antiox10101562






