Investigation of Lipoproteins Oxidation Mechanisms by the Analysis of Lipid Hydroperoxide Isomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PC 16:0/18:2;OOH and CE 18:2;OOH Isomers Standards
2.3. MS/MS and LC-MS/MS Analyses of PC 16:0/18:2;OOH and CE 18:2;OOH Isomers
2.4. LC-MS Analysis of PC 16:0/18:2 and CE 18:2
2.5. Preparation of Healthy Human Plasma and Lipoproteins
2.6. Extraction of PC 16:0/18:2;OOH (PC 16:0/18:2) and CE 18:2;OOH (CE 18:2)
2.7. Statistics
3. Results
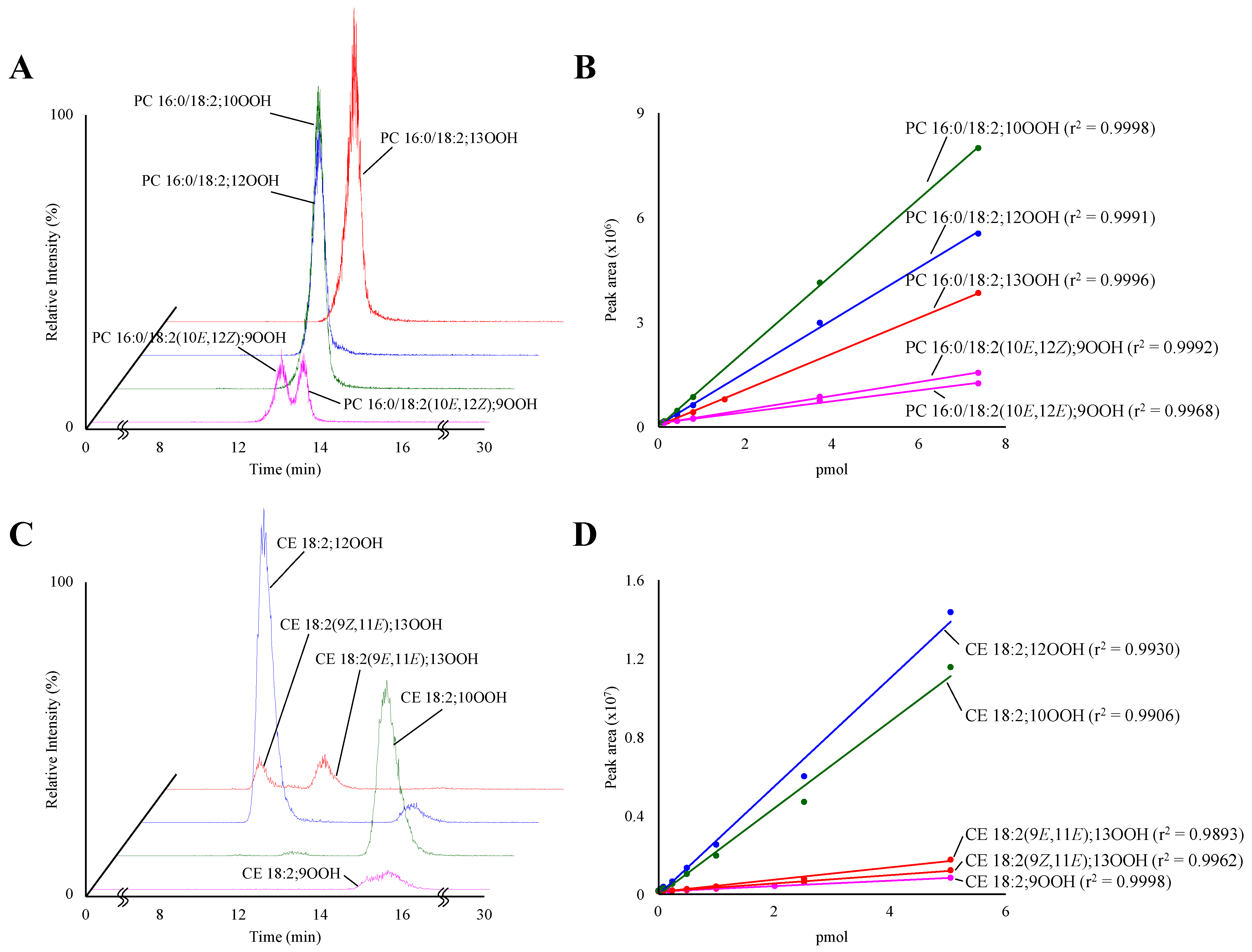
3.1. Development of the Analytical Methods for PC 16:0/18:2;OOH and CE 18:2;OOH Isomers
3.2. Analysis of PC 16:0/18:2;OOH and CE 18:2;OOH Isomers in Each Lipoprotein of Healthy Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHT | butylhydroxytoluene |
| CID | Collision-induced dissociation |
| EDTA | ethylenediaminetetraacetic acid |
| ESI | electrospray ionization |
| LOOH | lipid hydroperoxides |
| LOQ | limits of quantification |
| MxP | 2-methoxypropene |
| MRM | multiple reaction monitoring |
| SPE | solid phase extraction |
References
- Kinoshita, M.; Oikawa, S.; Hayasaka, K.; Sekikawa, A.; Nagashima, T.; Toyota, T.; Miyazawa, T. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin. Chem. 2000, 46, 822–828. [Google Scholar] [CrossRef]
- Chien, C.-T.; Chang, W.-T.; Chen, H.-W.; Wang, T.-D.; Liou, S.-Y.; Chen, T.-J.; Chang, Y.-L.; Lee, Y.-T.; Hsu, S.-M. Ascorbate supplement reduces oxidative stress in dyslipidemic patients undergoing apheresis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1111–1117. [Google Scholar] [CrossRef]
- Adachi, J.; Matsushita, S.; Yoshioka, N.; Funae, R.; Fujita, T.; Higuchi, S.; Ueno, Y. Plasma phosphatidylcholine hydroperoxide as a new marker of oxidative stress in alcoholic patients. J. Lipid Res. 2004, 45, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Nagashima, T.; Oikawa, S.; Hirayama, Y.; Tokita, Y.; Sekikawa, A.; Ishigaki, Y.; Yamada, R.; Miyazawa, T. Increase of serum phosphatidylcholine hydroperoxide dependent on glycemic control in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2002, 56, 19–25. [Google Scholar] [CrossRef]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Asai, A.; Nagao, M.; Nagashima, K.; Oikawa, S.; Miyazawa, T. Liquid chromatography–tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal. Biochem. 2015, 471, 51–60. [Google Scholar] [CrossRef]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the levels of amyloid-β, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer’s disease: Possible interactions between amyloid-β and these lipids. J. Alzheimers Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1985, 23, 197–221. [Google Scholar] [CrossRef]
- Ito, J.; Mizuochi, S.; Nakagawa, K.; Kato, S.; Miyazawa, T. Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation. Anal. Chem. 2015, 87, 4980–4987. [Google Scholar] [CrossRef]
- Shimizu, N.; Bersabe, H.; Ito, J.; Kato, S.; Towada, R.; Eitsuka, T.; Kuwahara, S.; Miyazawa, T.; Nakagawa, K. Mass spectrometric discrimination of squalene monohydroperoxide isomers. J. Oleo Sci. 2017, 66, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Nakagawa, K.; Suzuki, Y.; Suzuki, K.; Mizuochi, S.; Miyazawa, T. Preparation of 13 or 9-hydroperoxy-9Z,11E (9E,11E) or 10E,12Z (10E,12E)-octadecadienoic phosphatidylcholine hydroperoxide. J. Oleo Sci. 2014, 63, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Shimizu, N.; Ogura, Y.; Otoki, Y.; Ito, J.; Sakaino, M.; Sano, T.; Kuwahara, S.; Takekoshi, S.; Imagi, J.; et al. Structural analysis of lipid hydroperoxides using mass spectrometry with alkali metals. J. Am. Soc. Mass Spectrom. 2021, 32, 2399–2409. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Kato, S.; Shimizu, N.; Hanzawa, Y.; Otoki, Y.; Ito, J.; Kimura, F.; Takekoshi, S.; Sakaino, M.; Sano, T.; Eitsuka, T.; et al. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography–tandem mass spectrometry. NPJ Sci. Food 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Itabe, H.; Yamamoto, H.; Imanaka, T.; Shimamura, K.; Uchiyama, H.; Kimura, J.; Sanaka, T.; Hata, Y.; Takano, T. Sensitive detection of oxidatively modified low density lipoprotein using a monoclonal antibody. J. Lipid Res. 1996, 37, 45–53. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1956, 226, 497–509. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011; pp. 1–23. [Google Scholar]
- Milne, G.L.; Seal, J.R.; Havrilla, C.M.; Wijtmans, M.; Porter, N.A. Identification and analysis of products formed from phospholipids in the free radical oxidation of human low density lipoproteins. J. Lipid Res. 2005, 46, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.; Chiba, H.; Hui, S.-P. Oxidized lipid species in lipoproteins: Significance and analytical considerations. Med. Mass Spectrom. 2020, 4, 2–23. [Google Scholar]
- MacMillan, D.K.; Murphy, R.C. Analysis of lipid hydroperoxides and long-chain conjugated keto acids by negative ion electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 1190–1201. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Lu, Y.; Yang, R.; Gotlinger, K.H.; Petasis, N.P.; Serhan, C.N. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J. Am. Soc. Mass Spectrom. 2007, 18, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Derogis, P.B.M.C.; Freitas, F.P.; Marques, A.S.F.; Cunha, D.; Appolinario, P.P.; Paula, F.; Lourenço, T.C.; Murgu, M.; Mascio, P.D.; Medeiros, M.H.G.; et al. The development of a specific and sensitive LC-MS-based method for the detection and quantification of hydroperoxy- and hydroxydocosahexaenoic acids as a tool for lipidomic analysis. PLoS ONE 2013, 8, e77561. [Google Scholar] [CrossRef] [Green Version]
- Wheelan, P.; Zirrolli, J.A.; Murphy, R.C. Analysis of hydroxy fatty acids as pentafluorobenzyl ester, trimethylsilyl ether derivatives by electron ionization gas chromatography/mass spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.L.; Porter, N.A. Separation and identification of phospholipid peroxidation products. Lipids 2001, 36, 1265–1275. [Google Scholar] [CrossRef]
- Seal, J.R.; Porter, N.A. Liquid chromatography coordination ion-spray mass spectrometry (LC-CIS-MS) of docosahexaenoate ester hydroperoxides. Anal. Bioanal. Chem. 2004, 378, 1007–1013. [Google Scholar] [CrossRef]
- Yin, H.; Porter, N.A. Identification of intact lipid peroxides by Ag+ coordination ion-spray mass spectrometry (CIS-MS). Methods Enzymol. 2007, 433, 193–211. [Google Scholar]
- Ito, J.; Nakagawa, K.; Kato, S.; Hirokawa, T.; Kuwahara, S.; Nagai, T.; Miyazawa, T. A novel chiral stationary phase HPLC-MS/MS method to discriminate between enzymatic oxidation and auto-oxidation of phosphatidylcholine. Anal. Bioanal. Chem. 2016, 408, 7785–7793. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Vila, A.; Korytowski, W.; Girotti, A.W. Spontaneous transfer of phospholipid and cholesterol hydroperoxides between cell membranes and low-density lipoprotein: Assessment of reaction kinetics and prooxidant effects. Biochem. 2002, 41, 13705–13716. [Google Scholar] [CrossRef]
- Thiagarajan, A.N.; Chand, P.; Bhat, B.V.; Sridhar, M.G. Assessment of oxidative stress in babies under phototherapy for neonatal jaundice. Int. J. Adv. Med. Health Res. 2014, 1, 66–68. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Basque, M.C.; Stephenson, V.C.; Vinqvist, M.R. Photooxidations initiated or sensitized by biological molecules: Singlet oxygen versus radical peroxidation in micelles and human blood plasma. Photochem. Photobiol. 2003, 78, 248–255. [Google Scholar] [CrossRef]
- Karlsson, H.; Kontush, A.; James, R.W. Functionality of HDL: Antioxidation and detoxifying effects. High Density Lipoproteins 2015, 224, 207–228. [Google Scholar]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Christison, J.K.; Rye, K.A.; Stocker, R. Exchange of oxidized cholesteryl linoleate between LDL and HDL mediated by cholesteryl ester transfer protein. J. Lipid Res. 1995, 36, 2017–2026. [Google Scholar] [CrossRef]
- Chowaniec, Z.; Skoczyńska, A. Plasma lipid transfer proteins: The role of PLTP and CETP in atherogenesis. Adv. Clin. Exp. Med. 2018, 27, 429–436. [Google Scholar] [CrossRef]

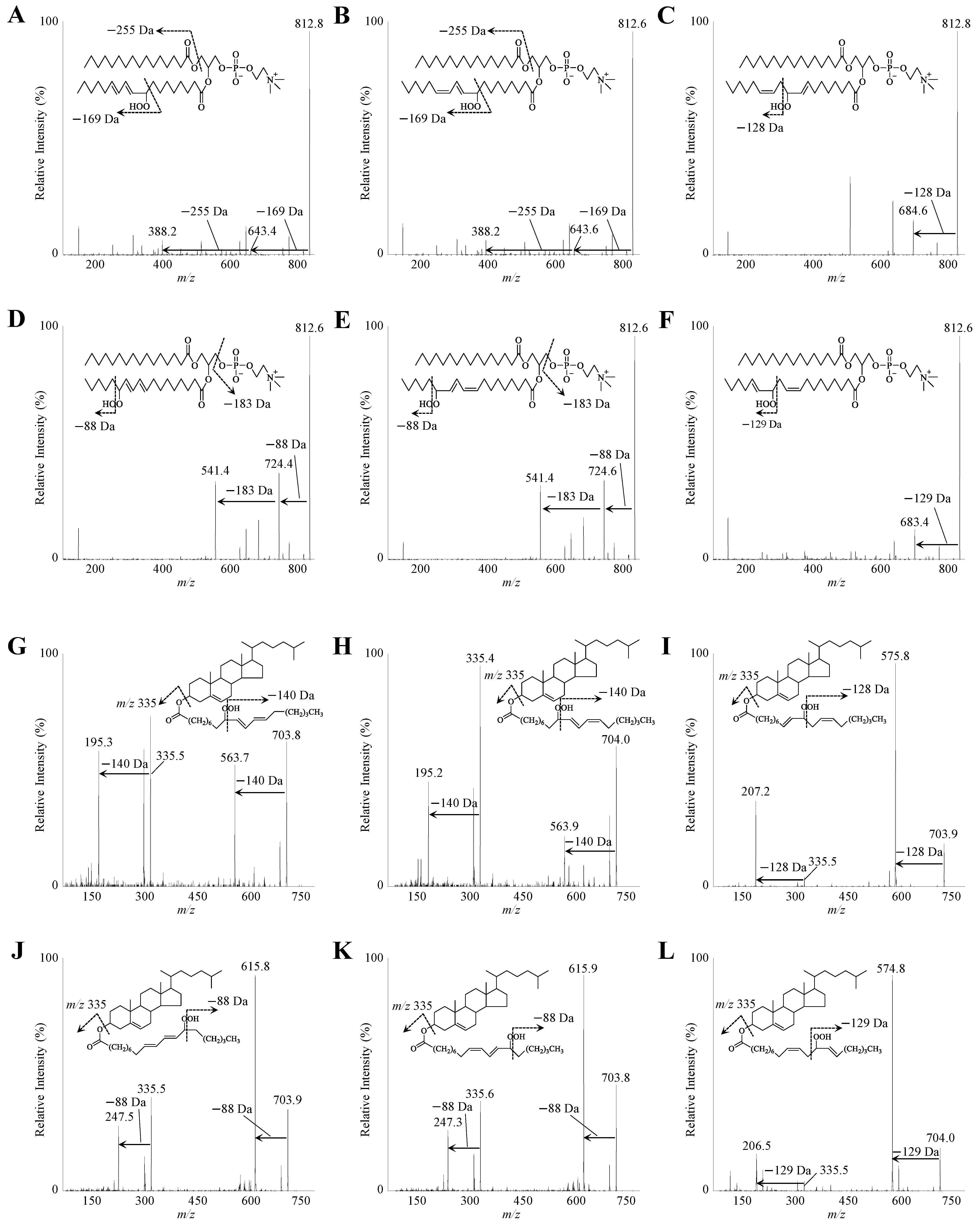

| PC 16:0/18:2 (µM) | The ratio of PC 16:0/18:2;OOH to PC 16:0/18:2 (×10−3%) | PC 16:0/18:2(10E,12Z);9OOH /PC 16:0/18:2(10E,12E);9OOH | |||||
| PC 16:0/18:2(10E,12Z);9OOH | PC 16:0/18:2(10E,12E);9OOH | PC 16:0/18:2;10OOH | PC 16:0/18:2;12OOH | PC 16:0/18:2(9Z,11E);13OOH + PC 16:0/18:2(9E,11E);13OOH | |||
| CM (n = 9) | 0.8 | - | - | - | - | - | - |
| 0.3 | - | - | - | - | - | - | |
| 0.5 | - | - | - | - | - | - | |
| 3.6 | - | - | - | - | 58.4 | - | |
| 0.2 | - | - | - | - | - | - | |
| 1.1 | - | - | - | - | 10 | - | |
| 1.1 | - | - | - | - | - | - | |
| 0.9 | - | - | - | - | - | - | |
| 0.4 | - | - | - | - | - | - | |
| VLDL (n = 9) | 5.7 | - | - | - | - | 13.4 | - |
| 2.9 | - | - | - | - | 29.2 | - | |
| 5.8 | - | - | - | - | 42.3 | - | |
| 21.9 | 18 | - | - | - | 28.4 | - | |
| 4.5 | - | - | - | - | 25.6 | - | |
| 3.2 | - | - | - | - | 22.2 | - | |
| 14.9 | 18.5 | - | - | - | 27.3 | - | |
| 13.8 | 26 | - | - | - | 41 | - | |
| 6.5 | - | - | - | - | 27.2 | - | |
| LDL (n = 9) | 27.8 | 9.4 | - | - | - | 14.8 | - |
| 35.6 | 18.7 | 14.9 | - | - | 35.6 | - | |
| 22.9 | 12.8 | - | - | - | 23.6 | - | |
| 32.4 | 44 | 31.5 | - | - | 82.2 | - | |
| 28.7 | 10.8 | - | - | - | 20.4 | - | |
| 16.8 | 56.1 | 27.9 | - | - | 98.2 | - | |
| 41.7 | 75.2 | 44.1 | 0.9 | 0.7 | 138.5 | - | |
| 27.5 | 9 | - | - | - | 15.6 | - | |
| 31.7 | 58.8 | 33.4 | - | - | 112.3 | - | |
| Mean ± SD(The ratio) | 32.8 ± 25.8 | - | - | - | 60.1 ± 47.9 | - | |
| HDL (n = 9) | 63.6 | 13.7 | 8.3 | - | - | 23.3 | 1.7 |
| 45.1 | 68.7 | 33.9 | 0.8 | 0.7 | 120.5 | 2 | |
| 64.5 | 32.7 | 18.6 | 0.5 | - | 57.4 | 1.8 | |
| 28.7 | 93.7 | 47 | 1.3 | 1 | 168.3 | 2 | |
| 21.4 | 101.7 | 65.1 | - | - | 175.8 | 1.6 | |
| 32.7 | 19.4 | 10.9 | - | - | 34 | 1.8 | |
| 37.2 | 164.9 | 66.6 | 2.1 | 1.6 | 270 | 2.5 | |
| 39.2 | 78.1 | 45.5 | 0.9 | 0.6 | 135.1 | 1.7 | |
| 51.6 | 74.6 | 40.4 | 1 | 0.7 | 127.6 | 1.8 | |
| Mean ± SD(The ratio) | 71.9 ± 47.1 | 37.4 ± 21.5 | - | - | 123.6 ± 78.1 | - | |
| Significance (LDL vs. HDL) | <0.05 | - | - | - | 0.05 | - | |
| CE 18:2 (µM) | The ratio of CE 18:2;OOH to CE 18:2 (×10−3%) | CE 18:2(9Z,11E);13OOH /CE 18:2(9E,11E);13OOH | |||||
| CE 18:2(10E,12Z);9OOH + CE 18:2(10E,12E);9OOH | CE 18:2;10OOH | CE 18:2;12OOH | CE 18:2(9Z,11E);13OOH | CE 18:2(9E,11E);13OOH | |||
| CM (n = 9) | 16.8 | - | - | - | 10.1 | - | - |
| 2.5 | - | - | - | - | - | - | |
| 4.5 | - | - | - | - | - | - | |
| 14.3 | - | - | - | - | - | - | |
| 3.7 | - | - | - | - | - | - | |
| 18.5 | - | - | - | - | - | - | |
| 17.6 | - | 1 | - | 13 | - | - | |
| 6.9 | - | - | - | - | - | - | |
| 4.4 | - | - | - | - | - | - | |
| VLDL (n = 9) | 56.3 | - | - | 0.5 | 4.1 | 2 | - |
| 15.4 | - | - | - | 11 | - | - | |
| 35.3 | 10.1 | 1 | 0.8 | 9.7 | - | - | |
| 213.9 | 8.3 | 0.5 | 0.4 | 8.3 | 0.6 | - | |
| 32.4 | - | 0.7 | 0.8 | 8.8 | - | - | |
| 21.2 | - | - | - | - | - | - | |
| 96.6 | 4.4 | - | - | 3.9 | - | - | |
| 126 | 3 | - | - | 2.1 | 0.9 | - | |
| 52.5 | - | 0.8 | 1 | 12.2 | - | - | |
| LDL (n = 9) | 1738.2 | 3.2 | 1.6 | 0.1 | 6.6 | 0.3 | 22 |
| 2131.1 | 2.9 | 0.6 | 0.2 | 12.5 | 0.5 | 25 | |
| 1185 | 3.1 | 0.3 | 0.3 | 12.9 | 0.7 | 18.4 | |
| 1860.5 | 3.8 | 0.1 | 0.1 | 4.1 | 0.3 | 13.7 | |
| 2017.5 | 3.9 | 0.1 | 0.1 | 4.4 | 0.8 | 5.5 | |
| 1204.1 | 6 | 0.2 | 0.3 | 7.2 | 1 | 7.2 | |
| 2783.5 | 6 | 0.1 | 0.3 | 10.4 | 0.8 | 13 | |
| 1710.2 | 5.3 | 0.2 | 0.2 | 6.3 | 1 | 6.3 | |
| 1877.6 | 6.7 | 0.2 | 0.3 | 8.4 | 1.2 | 7 | |
| Mean ± SD(The ratio) | 4.54 ± 1.45 | 0.38 ± 0.49 | 0.19 ± 0.09 | 8.09 ± 3.23 | 0.71 ± 0.32 | - | |
| HDL (n = 9) | 1226.7 | 7.1 | 0.3 | 0.4 | 9.2 | 1.2 | 7.7 |
| 1105.3 | 5 | 0.1 | 0.1 | 6.2 | 1 | 6.2 | |
| 1165.8 | 6.8 | 0.2 | 0.3 | 8.2 | 1.3 | 6.3 | |
| 480.3 | 7.9 | 0.3 | 0.5 | 11.5 | 1.8 | 6.4 | |
| 473 | 6 | 0.3 | 0.2 | 7 | 0.5 | 14 | |
| 644.8 | 7.2 | 0.4 | 0.3 | 7.8 | 0.7 | 11.1 | |
| 1017.1 | 6.6 | 0.2 | 0.2 | 7.5 | 1 | 7.5 | |
| 835.2 | 6 | 0.1 | 0.2 | 7.8 | 1.4 | 5.6 | |
| 1158.7 | 5.9 | 0.1 | 0.1 | 6.4 | 1.1 | 5.8 | |
| Mean ± SD(The ratio) | 6.50 ± 0.86 | 0.22 ± 0.09 | 0.27 ± 0.12 | 7.94 ± 1.63 | 1.11 ± 0.37 | - | |
| Significance (LDL vs. HDL) | <0.05 | n.s. | n.s. | n.s. | <0.05 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, S.; Osuka, Y.; Khalifa, S.; Obama, T.; Itabe, H.; Nakagawa, K. Investigation of Lipoproteins Oxidation Mechanisms by the Analysis of Lipid Hydroperoxide Isomers. Antioxidants 2021, 10, 1598. https://doi.org/10.3390/antiox10101598
Kato S, Osuka Y, Khalifa S, Obama T, Itabe H, Nakagawa K. Investigation of Lipoproteins Oxidation Mechanisms by the Analysis of Lipid Hydroperoxide Isomers. Antioxidants. 2021; 10(10):1598. https://doi.org/10.3390/antiox10101598
Chicago/Turabian StyleKato, Shunji, Yusuke Osuka, Saoussane Khalifa, Takashi Obama, Hiroyuki Itabe, and Kiyotaka Nakagawa. 2021. "Investigation of Lipoproteins Oxidation Mechanisms by the Analysis of Lipid Hydroperoxide Isomers" Antioxidants 10, no. 10: 1598. https://doi.org/10.3390/antiox10101598






