Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Non-Pigmented Colonies and Streptomycin-Resistant Selection
2.3. Detection of Insertion Sequence Transposition
2.4. Transcriptomic Analysis by RNA-Seq
2.5. Quantitative Real-Time (qRT)-PCR
3. Results
3.1. Physiological Properties of Target Gene Disrupted and Complemented Mutants
3.2. Major Gene Categories Differentially Expressed in the Cystine Importer-Disrupted Mutant
3.3. Detection of IS Transposition
3.4. Streptomycin-Resistant Phenotypic Mutation
3.5. Gene Expression Levels of Redox Control-Related Genes and Transposases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSH | bacillithiol |
| Dps | DNA-binding protein for DNA protection in starved cell |
| IS | insertion sequence |
| MSH | mycothiol |
| RES | reactive electrophilic species |
| RONS | reactive oxygen/nitrogen species |
References
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Helmann, J.D. Bacillithiol, a new player in bacterial redox homeostasis. Antioxid. Redox. Signal. 2011, 15, 123–133. [Google Scholar] [CrossRef]
- Hung, J.; Cooper, D.; Turner, M.S.; Walsh, T.; Giffard, P.M. Cystine uptake prevents production of hydrogen peroxide by Lactobacillus fermentum BR11. FEMS Microbiol. Lett. 2003, 227, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Lo, R.; Turner, M.S.; Barry, D.G.; Sreekumar, R.; Walsh, T.P.; Giffard, P.M. Cystathionine gamma-lyase is a component of cystine-mediated oxidative defense in Lactobacillus reuteri BR11. J. Bacteriol. 2009, 191, 1827–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsu, I.; Kawano, Y.; Suzuki, M.; Morigasaki, S.; Saiki, K.; Yamazaki, S.; Nonaka, G.; Takagi, H. Uptake of L-cystine via an ABC transporter contributes defense of oxidative stress in the L-cystine export-dependent manner in Escherichia coli. PLoS ONE 2015, 10, e0120619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antelmann, H.; Helmann, J.D. Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 2011, 14, 1049–1063. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Rawat, M.; Maupin-Furlow, J.A. Redox and thiols in archaea. Antioxidants 2020, 9, 381. [Google Scholar] [CrossRef]
- Imlay, J.A. Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Hillion, M.; Antelmann, H. Thiol-based redox switches in prokaryotes. Biol. Chem. 2015, 396, 415–444. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, E.; Bes, M.T.; González, A.; Peleato, M.L.; Fillat, M.F. Redox-based transcriptional regulation in prokaryotes: Revisiting model mechanisms. Antioxid. Redox Signal. 2019, 30, 1651–1696. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-G.; Lee, B.-J. How bacterial redox sensors transmit redox signals via structural changes. Antioxidants 2021, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Omelchenko, M.V.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Lapidus, A.; Copeland, A.; Kim, E.; Land, M.; et al. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE 2007, 2, e955. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.J. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, Y. Carotenoid biosynthesis in extremeophilic Deinococcus-Thermus bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef] [Green Version]
- Agapov, A.A.; Kulbachinskiy, A.V. Mechanisms of stress resistance and gene regulation in the radioresistant bacterium Deinococcus radiodurans. Biochemistry 2015, 80, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, Z.; Chen, X.; Zhang, W.; Lin, M.; Chen, M. Comparative proteomics analysis reveals new features of the oxidative stress response in the polyextremophilic bacterium Deinococcus radiodurans. Microorganisms 2020, 8, 451. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.C.; Nobre, M.F.; Rainey, F.A.; Silva, M.T.; Wait, R.; Burghardt, J.; Chung, A.P.; da Costa, M.S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int. J. Syst. Bacteriol. 1997, 47, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Brim, H.; Venkateswaran, A.; Kostandarithes, H.M.; Fredrickson, J.K.; Daly, M.J. Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl. Environ. Microbiol. 2003, 69, 4575–4582. [Google Scholar] [CrossRef] [Green Version]
- Choo, K.; Kim, M.; Abdi Nansa, S.; Bae, M.K.; Lee, C.; Lee, S.-J. Redox potential change by the cystine importer affected on enzymatic antioxidant protection in Deinococcus geothermalis. Antonie Van Leeuwenhoek 2020, 113, 779–790. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.; Lim, S.; Sim, J.; Rhie, H.G.; Lee, S.-J. Oxidative stress response of Deinococcus geothermalis via a cystine importer. J. Microbiol. 2017, 55, 137–146. [Google Scholar] [CrossRef]
- Touchon, M.; Rocha, E.P. Causes of insertion sequences abundance in prokaryotic genomes. Mol. Biol. Evol. 2007, 24, 969–981. [Google Scholar] [CrossRef] [Green Version]
- Blesa, A.; Sanchez, M.; Sacristan-Horcajada, E.; Fuente, S.G.; Peiro, R.; Berenguer, J. Into the Thermus mobilome: Presence, diversity and recent activities of insertion sequences across Thermus spp. Microorganisms 2019, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Fayad, N.; Awad, M.K.; Mahillon, J. Diversity of Bacillus cereus sensu lato mobilome. BMC Genom. 2019, 20, 436. [Google Scholar] [CrossRef] [Green Version]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [Green Version]
- Siguier, P.; Gourbeyre, E.; Varani, A.; Ton-Hoang, B.; Chandler, M. Everyman’s guide to bacterial insertion sequences. Microbiol. Spectrum. 2015, 3, 555–590. [Google Scholar] [CrossRef] [Green Version]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, N.; Bae, M.K.; Choo, K.; Lee, S.-J. Transposition of insertion sequences was triggered by oxidative stress in radiation-resistant bacterium Deinococcus geothermalis. Microorganisms 2019, 7, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Choo, K.; Lee, S.-J. Active transposition of insertion sequences by oxidative stress in Deinococcus geothermalis. Front. Microbiol. 2020, 11, 558747. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Bae, M.K.; Lee, S.-J. An anoxidant defense system in radiation-resistant bacterium Deinococcus geothermalis against oxidative stress. In Antioxidants—Benefits, Sources, Mechanisms of Action; InTechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tojo, S.; Tanaka, Y.; Ochi, K. Activation of antibiotic production in Bacillus spp. by cumulative drug resistance mutations. Antimicro. Agents Chemother 2015, 59, 7799–7804. [Google Scholar] [CrossRef] [Green Version]
- Gregory, S.T.; Dahlberg, A.E. Transposition of an insertion sequence, ISTth7, in the genome of the extreme thermophile Thermus thermophilus HB8. FEMS Microbiol. Lett. 2008, 289, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.L.; Rawat, M.; La Clair, J.J.; Jothivasan, V.K.; Budiarto, T.; Hamilton, C.J.; Claiborne, A.; Helmann, J.D.; Fahey, R.C. Bacillithiol is an antioxidant thiol produced in Bacilli. Nat. Chem. Biol. 2009, 5, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Mourenza, A.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative stress-generating antimicrobials, a novel strategy to overcome antibacterial resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Henard, C.A.; Bourret, T.J.; Song, M.; Vazquez-Torres, A. Control of redox balance by the stringent response regulatory protein promotes antioxidant defense of Salmonella. J. Biol. Chem. 2010, 285, 36785–36793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Minh, P.N.L.; Dötsch, A.; Hildbrand, F.; Panmanee, W.; Elfarash, A.; Schulz, S.; Plaisance, S.; Charlier, D.; Hassett, D.; et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res. 2012, 40, 4320–4333. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Omelchenko, M.V.; Beliaev, A.S.; Venkateswaran, A.; Stair, J.; Wu, L.; Thompson, D.K.; Xu, D.; Rogozin, I.; et al. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc. Natl. Acad. Soc. USA. 2003, 100, 4191–4196. [Google Scholar] [CrossRef] [Green Version]
- Luan, H.; Meng, N.; Fu, J.; Chen, X.; Xu, X.; Feng, Q.; Jiang, H.; Dai, J.; Yuan, X.; Lu, Y.; et al. Genome-wide transcriptome and antioxidant analyses on gamma-irradiated phases of Deinococcus radiodurans R1. PLoS ONE 2014, 9, e85649. [Google Scholar] [CrossRef]
- Lim, S.; Jung, J.-H.; Blanchard, L.; De Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.; Liu, W.; Chen, Y.; Liu, Y.; Han, J.; Geng, X.; Li, J.; Jiang, S.; Zhou, Z.; Zhang, W.; et al. RNA-Seq-based comparative transcriptome analysis highlights new features of the heat-stress response in the extremophilic bacterium Deinococcus radiodurans. Int. J. Mol. Sci. 2019, 20, 5603. [Google Scholar] [CrossRef] [Green Version]
- Stetina, M.; Behr, J.; Vogel, R.F. The transcriptional response of Lactobacillus sanfranciscensis DSM20451T and its tcyB mutant lacking a functional cystine transporter to diamide stress. Appl. Environ. Microbiol. 2014, 80, 4114–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpkins, J.A.; Rickel, K.E.; Madeo, M.; Ahlers, B.A.; Carlisle, G.B.; Nelson, H.J.; Cardillo, A.L.; Weber, E.A.; Vitiello, P.F.; Pearce, D.A.; et al. Disruption of a cystine transporter downregulates expression of genes involved in sulfur regulation and cellular respiration. Biol. Open 2016, 5, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Kharat, A.; Coursange, E.; Noirclerc-Savoye, M.; Lacoste, J.; Blot, M. IS1 transposition is enhanced by translation errors and by bacterial growth at extreme glucose levels. Acta Biochim. Pol. 2006, 53, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Narumi, I.; Cherdchu, K.; Kitayama, S.; Watanabe, H. The Deinococcus radiodurans uvrA gene: Identification of mutation sites in two mitomycin-sensitive strains and the first discovery of insertion sequence element from deinobacteria. Gene 1997, 198, 115–126. [Google Scholar] [CrossRef]
- Mennecier, S.; Servant, P.; Coste, G.; Bailone, A.; Sommer, S. Mutagenesis via IS transposition in Deinococcus radiodurans. Mol. Microbiol. 2006, 59, 317–325. [Google Scholar] [CrossRef]
- Pasternak, C.; Ton-Hoang, B.; Coste, G.; Bailone, A.; Chandler, M.; Sommer, S. Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. PLoS Genet. 2010, 6, e1000799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, C.; Dulermo, R.; Ton-Hoang, B.; Debuchy, R.; Siguier, P.; Coste, G.; Chandler, M.; Sommer, S. ISDra2 transposition in Deinococcus radiodurans is downregulated by TnpB. Mol. Microbiol. 2013, 88, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, C.; Hashimoto, M.; Honda, H.; Kato, J.-I. Effects on IS1 transposition frequency of a mutation in the ygjD gene involved in an essential tRNA modification in Escherichia coli. FEMS Microbiol. Lett. 2013, 347, 140–148. [Google Scholar]
- Craig, N. Transposition. In Escherichia coli and Salmonella. Cellular and Molecular Biology; Neidhardt, F.C., Ed.; American Society for Microbiology: Washington, DC, USA, 1996; Volume 2, pp. 2339–2362. [Google Scholar]
- Twiss, E.; Coros, A.M.; Tavakoli, N.P.; Derbyshire, K.M. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol. Microbiol. 2005, 57, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Taketani, T.; Tanabiki, M.; Ohara, M.; Kobayashi, J.; Ohshiro, T. Frequent transposition of multiple insertion sequences in Geobacillus kaustophilus HTA426. Front. Microbiol. 2021, 12, 650461. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.; Guerin, P.; Roche, D.; Cruveiller, S.; Pignol, D.; Vallenet, D.; Armengaud, J.; De Groot, A. Conservation and diversity of the IrrE/DdrO-controlled radiation response in radiation-resistant Deinococcus bacteria. MicrobiologyOpen 2017, 6, e477. [Google Scholar] [CrossRef] [PubMed]
- Magerand, R.; Rey, P.; Blanchard, L.; De Groot, A. Redox signaling through zinc activates the radiation response in Deinococcus bacteria. Sci. Rep. 2021, 11, 4528. [Google Scholar] [CrossRef] [PubMed]

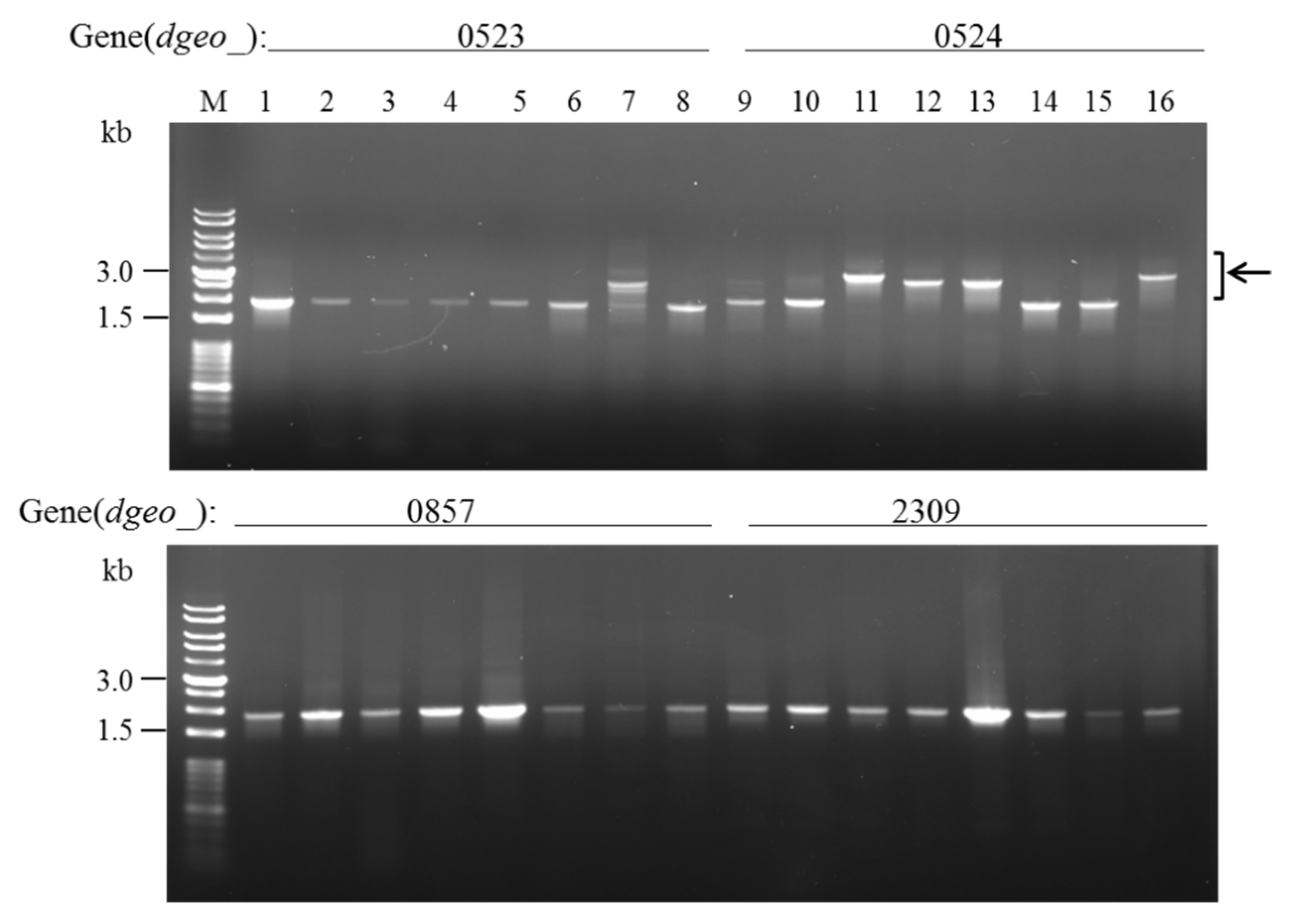
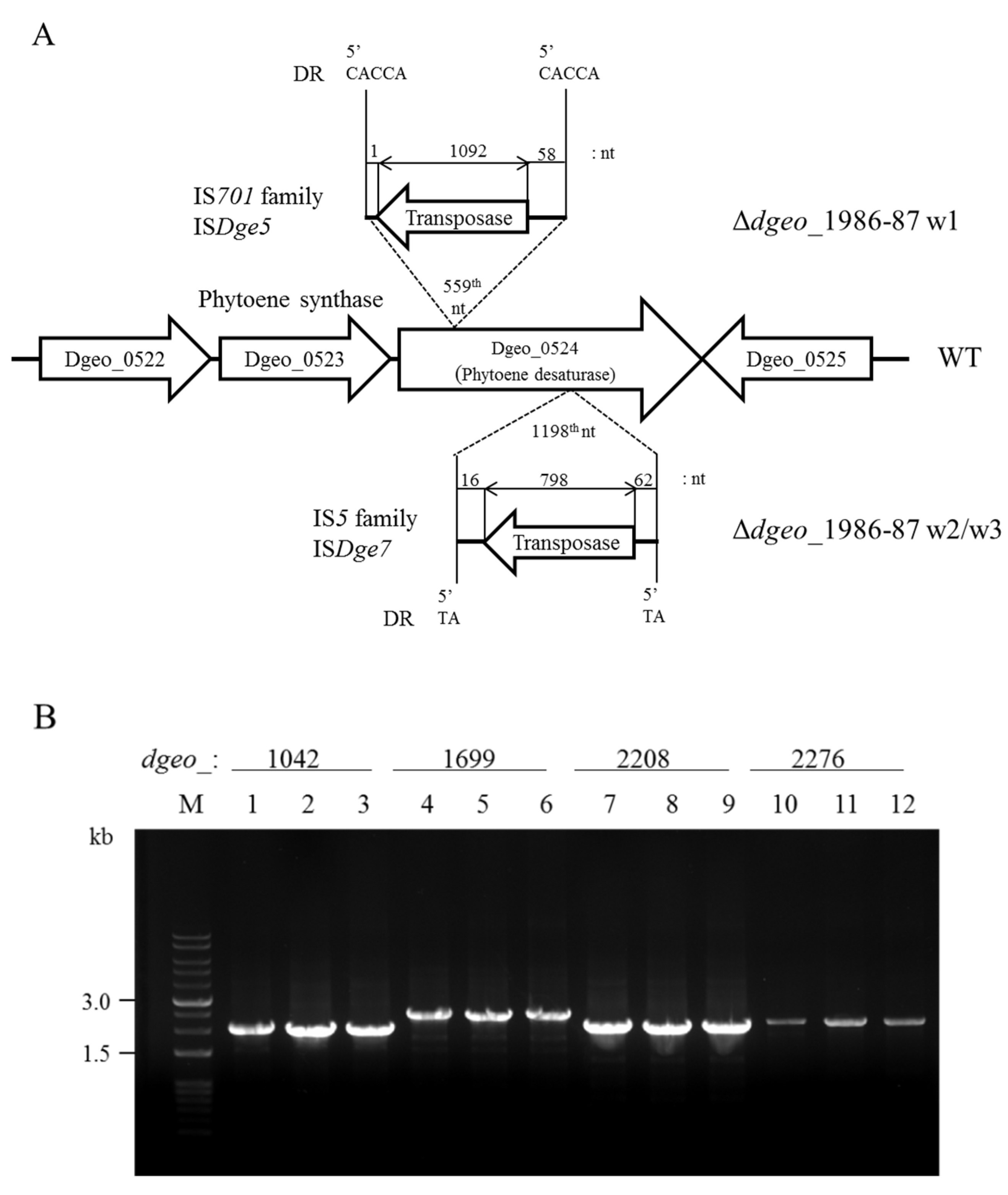

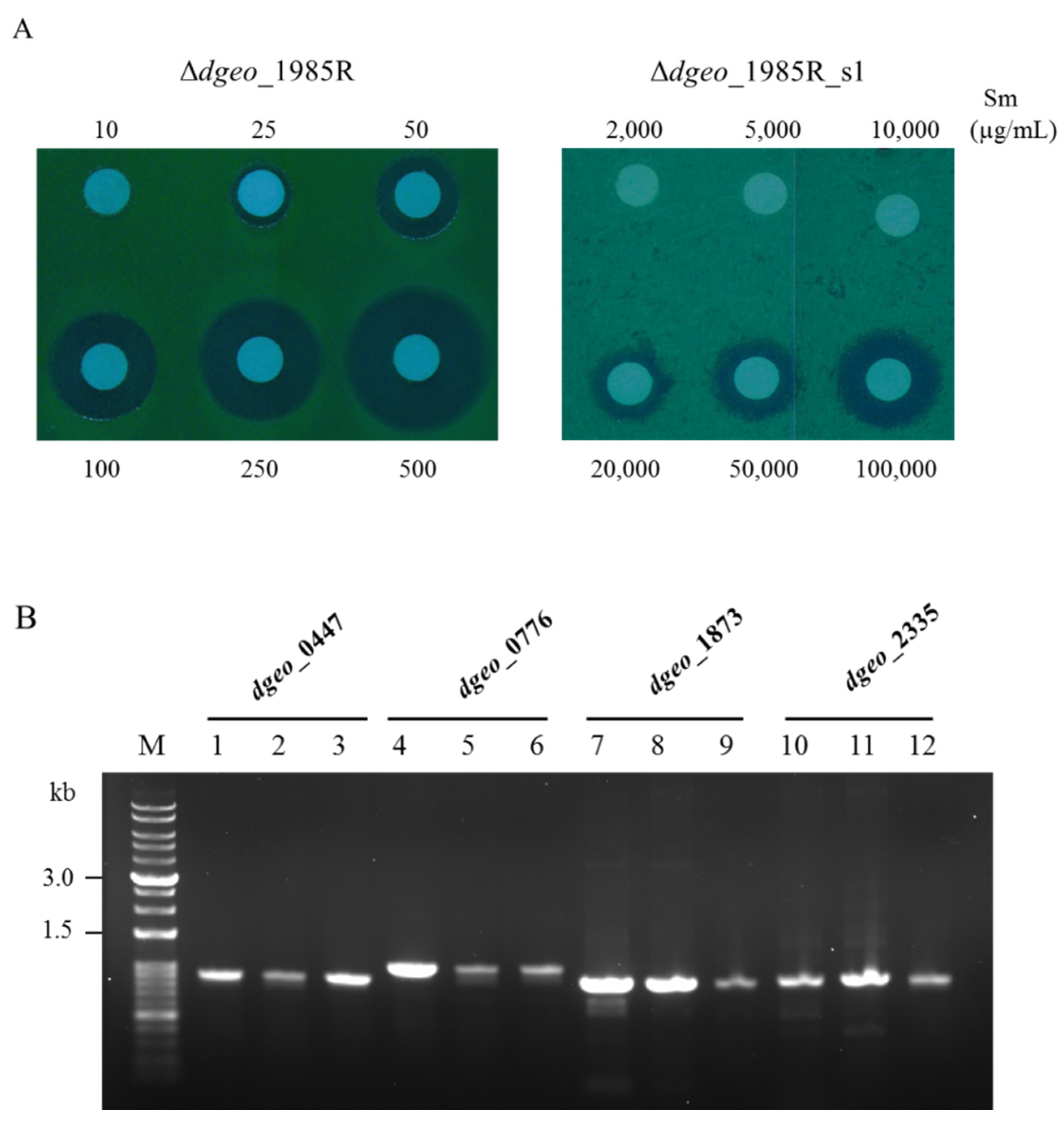

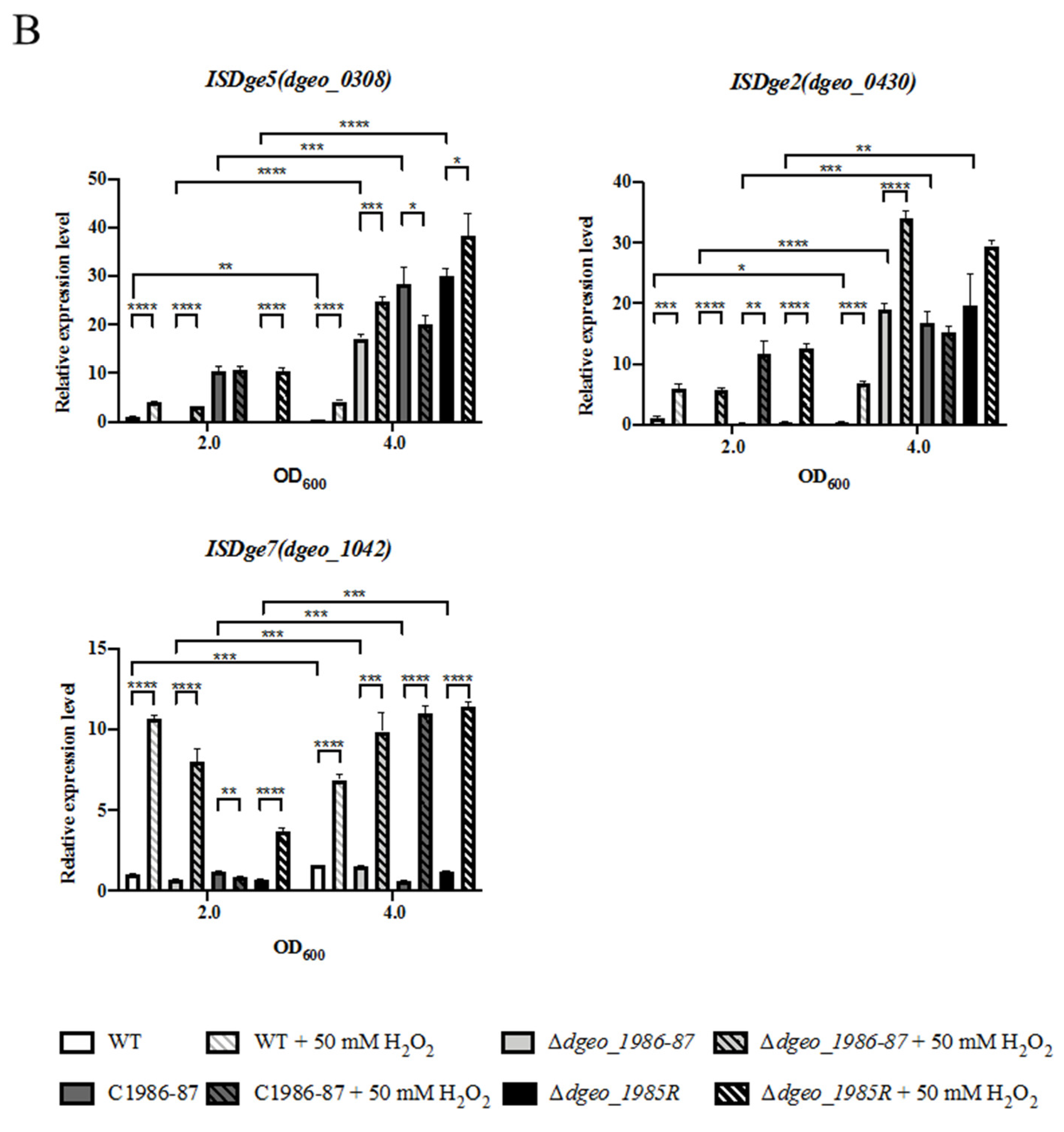
| Category | Gene | Fold Change | Function |
|---|---|---|---|
| Transposase (ISDge5, IS701 family) | dgeo_0308 | 4.82 | Transposase |
| dgeo_0464 | 3.71 | Transposase | |
| dgeo_0925 | 3.74 | Transposase | |
| dgeo_1807 | 6.15 | Transposase | |
| dgeo_2205 | 3.92 | Transposase | |
| dgeo_2430 | 4.93 | Transposase | |
| dgeo_2823 | 4.55 | Transposase | |
| dgeo_2659 | 4.65 | Transposase | |
| Sigma factor | dgeo_1346 | 4.13 | Sigma 70 |
| dgeo_1519 | 3.26 | Sigma factor | |
| Regulator | dgeo_0527 | 3.35 | HxlR family |
| dgeo_1148 | 3.18 | MarR family | |
| dgeo_1956 | 6.36 | SugE family | |
| dgeo_2329 | 8.36 | AraC family | |
| ABC transporter | dgeo_0543 | 3.34 | ABC transporter |
| dgeo_0647-48 | 6.96–7.06 | ABC transporter | |
| dgeo_0954-56 | 4.20–9.76 | Chromate transporter | |
| dgeo_1413 | 5.91 | ABC transporter | |
| dgeo_1805 | 8.03 | ABC transporter | |
| dgeo_2443 | 4.69 | Iron permease | |
| dgeo_2581-82 | 3.60–3.25 | cation transporter | |
| MFS transporter | dgeo_0249 | 3.75 | MFS transporter |
| dgeo_0530 | 6.41 | MFS efflux | |
| dgeo_1968 | 5.57 | MFS transporter | |
| dgeo_2330 | 3.22 | MFS efflux pump | |
| GCN5 family | dgeo_0369-70 | 9.60–12.53 | GCN5 family regulator |
| dgeo_2125 | 3.73 | GCN5 family acetyltransferase | |
| dgeo_2313 | 11.2 | GCN5 family acetyltransferase | |
| Enzyme | dgeo_0071 | 5.01 | Peptidase M29 |
| dgeo_0334 | 4.68 | Carboxypeptidase | |
| dgeo_0431 | 5 | Phage integrase | |
| dgeo_0528 | 8.8 | NADH dehydrogenase | |
| dgeo_0570 | 3.68 | Proline tRNA ligase | |
| dgeo_0824 | 3.34 | Exonuclease | |
| dgeo_0909 | 3.45 | NADH-quinone oxidoreductase | |
| dgeo_1337 | 6.43 | Methylenetetrahydrofolate reductase | |
| dgeo_1407-08 | 7.49–9.32 | Ferredoxin nitrite reductase/sulfate adenylyltransferase | |
| dgeo_1714 | 10.78 | Cysteine tRNA ligase | |
| dgeo_2583 | 3.49 | NrdH redoxin | |
| dgeo_2681 | 3.63 | Zn dependent hydrolase | |
| dgeo_2801 | 3.14 | WYL domain protein |
| Loci | Gene | Fold | Function |
|---|---|---|---|
| Chromosome | dgeo_1056 | 0.22 | Nuclease inhibitor |
| dgeo_1081 | 0.27 | 3-hydroxyisobutyrate dehydrogenase | |
| dgeo_1535-36 | 0.17 | Thioesterase/pterin dehydratase | |
| dgeo_2122 | 0.07 | Peptide ABC transporter | |
| dgeo_2245 | 0.27 | Citrate synthase | |
| Plasmid 1 | dgeo_2704 | 0.25 | Cytochrome D subunit |
| dgeo_2619 | 0.2 | RpiR family regulator | |
| dgeo_2561 | 0.25 | Peptidase C39 | |
| dgeo_2515 | 0.29 | DNA binding protein | |
| Plasmid 2 | dgeo_3003-04 | 0.12 | Thiamine biosynthesis/ferric reductase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Lee, C.; Shin, E.; Lee, S.-J. Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis. Antioxidants 2021, 10, 1623. https://doi.org/10.3390/antiox10101623
Ye Q, Lee C, Shin E, Lee S-J. Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis. Antioxidants. 2021; 10(10):1623. https://doi.org/10.3390/antiox10101623
Chicago/Turabian StyleYe, Qianying, Chanjae Lee, Eunjung Shin, and Sung-Jae Lee. 2021. "Influence of Redox Imbalances on the Transposition of Insertion Sequences in Deinococcus geothermalis" Antioxidants 10, no. 10: 1623. https://doi.org/10.3390/antiox10101623





