Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propolis Powder Preparation
2.2. Total and Individual Polyphenols Quantification
2.3. Animal Experiments
2.4. Oral Glucose Tolerance Test
2.5. Biochemical Analyses
2.6. Cell Culture
2.7. RNA Extraction and Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Polyphenols Quantification in the Different Propolis Extract Powders
3.2. Propolis Extract Powder Supplementation Differentially Protects Against Diet-Induced Obesity
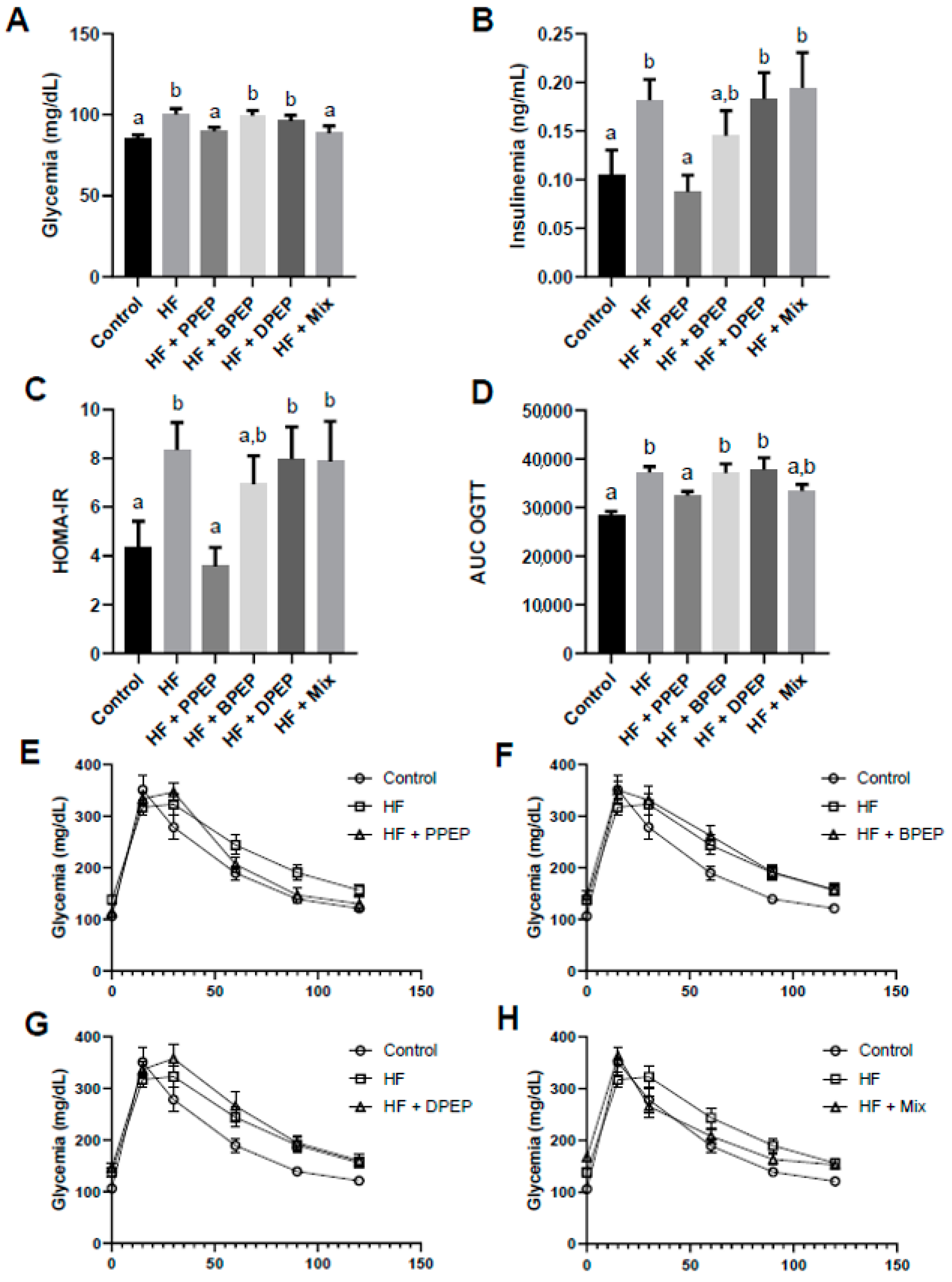
3.3. Poplar Propolis Extract Powder Supplementation Improves Glucose Homeostasis
3.4. Poplar Propolis Extract Powder Supplementation Modifies Gene Expression in Adipose Tissue
3.5. Polyphenols Present in Poplar Propolis Extract Powder Modify Gene expression in Adipocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Lotfy, M. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Cardinault, N.; Cayeux, M.O.; Percie du Sert, P. La propolis: Origine, composition et propriétés. Phytothérapie 2012, 10, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bankova, V.; Boudourova-Krasteva, G.; Sforcin, J.M.; Frete, X.; Kujumgiev, A.; Maimoni-Rodella, R.; Popov, S. Phytochemical Evidence for the Plant Origin of Brazilian Propolis from São Paulo State. Z. Naturforsch. C 1999, 54, 401–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazawa, S.; Yoneda, M.; Shibata, I.; Kanaeda, J.; Hamasaka, T.; Nakayama, T. Direct Evidence for the Plant Origin of Brazilian Propolis by the Observation of Honeybee Behavior and Phytochemical Analysis. Chem. Pharm. Bull. 2003, 51, 740–742. [Google Scholar] [CrossRef] [Green Version]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian Red Propolis: Botanical Origin and Comparative Analysis by High-Performance Liquid Chromatography–Photodiode Array Detection/Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef] [PubMed]
- Ichi, I.; Hori, H.; Takashima, Y.; Adachi, N.; Kataoka, R.; Okihara, K.; Hashimoto, K.; Kojo, S. The beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat diet. J. Food Sci. 2009, 74, H127–H131. [Google Scholar] [CrossRef]
- Sakai, T.; Ohhata, M.; Fujii, M.; Oda, S.; Kusaka, Y.; Matsumoto, M.; Nakamoto, A.; Taki, T.; Nakamoto, M.; Shuto, E. Brazilian Green Propolis Promotes Weight Loss and Reduces Fat Accumulation in C57BL/6 Mice Fed A High-Fat Diet. Biol. Pharm. Bull. 2017, 40, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Koya-Miyata, S.; Arai, N.; Mizote, A.; Taniguchi, Y.; Ushio, S.; Iwaki, K.; Fukuda, S. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol. Pharm. Bull. 2009, 32, 2022–2028. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, H.; Naoe, Y.; Kimura, S.; Miyamoto, T.; Okamoto, S.; Toda, C.; Shimamoto, Y.; Iwanaga, T.; Miyoshi, I. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: Possible involvement of immune cells in mesenteric adipose tissue. Adipocyte 2013, 2, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Wei, P.; Lu, Q.; Liu, R.; Ding, Y.; Zhang, J. Beneficial Effects of Poplar Buds on Hyperglycemia, Dyslipidemia, Oxidative Stress, and Inflammation in Streptozotocin-Induced Type-2 Diabetes. J. Immunol. Res. 2018, 2018, 7245956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.H.; Chien, Y.W.; Chang, M.L.; Hou, C.C.; Chan, C.H.; Tang, H.W.; Huang, H.Y. Taiwanese Green Propolis Ethanol Extract Delays the Progression of Type 2 Diabetes Mellitus in Rats Treated with Streptozotocin/High-Fat Diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw. Open 2018, 1, e183788. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Tourniaire, F.; Romier-Crouzet, B.; Lee, J.H.; Marcotorchino, J.; Gouranton, E.; Salles, J.; Malezet, C.; Astier, J.; Darmon, P.; Blouin, E.; et al. Chemokine Expression in Inflamed Adipose Tissue Is Mainly Mediated by NF-kappaB. PLoS ONE 2013, 8, e66515. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384 e312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrier, J.-F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef] [Green Version]
- Ruhl, R.; Landrier, J.F. Dietary regulation of adiponectin by direct and indirect lipid activators of nuclear hormone receptors. Mol. Nutr. Food Res. 2015, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Matsushige, K.; Basnet, P.; Hase, K.; Kadota, S.; Tanaka, K.; Namba, T. Propolis protects pancreatic β-cells against the toxicity of streptozotocin (STZ). Phytomedicine 1996, 3, 203–209. [Google Scholar] [CrossRef]
- Fuliang, H.; Hepburn, H.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol. Res. 2005, 51, 147–152. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Xuan, H.; Hu, F. Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid. Based Complement. Altern. Med. 2012, 2012, 981896. [Google Scholar] [CrossRef]
- Kitamura, H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules 2019, 24, 4394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2016, 58, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir-Knezevic, S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004, 54, 65–72. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Dalifard, J.; Defoort, C.; Svilar, L.; Martin, J.C.; Tourniaire, F.; Landrier, J.F. Gene Expression Pattern in Response to Cholecalciferol Supplementation Highlights Cubilin as a Major Protein of 25(OH)D Uptake in Adipocytes and Male Mice White Adipose Tissue. Endocrinology 2018, 159, 957–966. [Google Scholar] [CrossRef]
- Landrier, J.F.; Gouranton, E.; El Yazidi, C.; Malezet, C.; Balaguer, P.; Borel, P.; Amiot, M.J. Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology 2009, 150, 5318–5325. [Google Scholar] [CrossRef]
- Tourniaire, F.; Musinovic, H.; Gouranton, E.; Astier, J.; Marcotorchino, J.; Arreguin, A.; Bernot, D.; Palou, A.; Bonet, M.L.; Ribot, J.; et al. All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid. Res. 2016, 56, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Landrier, J.F.; Malezet-Desmoulins, C.; Reboul, E.; Marie Lorec, A.; Josephe Amiot, M.; Borel, P. Comparison of different vehicles to study the effect of tocopherols on gene expression in intestinal cells. Free Radic. Res. 2008, 42, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.C.; Tourniaire, F.; Landrier, J.F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid. Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cardinault, N.; Tourniaire, F.; Astier, J.; Couturier, C.; Perrin, E.; Dalifard, J.; Seipelt, E.; Mounien, L.; Letullier, C.; Bonnet, L.; et al. Poplar Propolis Ethanolic Extract Reduces Body Weight Gain and Glucose Metabolism Disruption in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2020, 64, 2000275. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Tao, L.; Chen, X.; Jones, T.J.; Wang, K.; Hu, F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients 2020, 12, 959. [Google Scholar] [CrossRef] [Green Version]
- Coppari, R.; Bjorbaek, C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 2012, 11, 692–708. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.H.; Seo, S.G.; Min, S.; Yang, H.; Lee, E.; Son, J.E.; Kwon, J.Y.; Yue, S.; Chung, M.Y.; Kim, K.H.; et al. Caffeic acid phenethyl ester, a major component of propolis, suppresses high fat diet-induced obesity through inhibiting adipogenesis at the mitotic clonal expansion stage. J. Agric. Food Chem. 2014, 62, 4306–4312. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Kuda, O.; Kopecky, J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: A key to lean phenotype. Biochim. Biophys. Acta 2013, 1831, 986–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrier, J.F.; Karkeni, E.; Marcotorchino, J.; Bonnet, L.; Tourniaire, F. Vitamin D modulates adipose tissue biology: Possible consequences for obesity? Proc. Nutr. Soc. 2016, 75, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Nna, V.U.; Abu Bakar, A.B.; Md Lazin, M.; Mohamed, M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2018, 120, 305–320. [Google Scholar] [CrossRef]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Shao, W.; Chiang, Y.; Foltz, W.; Zhang, Z.; Ling, W.; Fantus, I.G.; Jin, T. Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 2011, 54, 922–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Magd, N.F.A.; El-Mesery, M.; El-Karef, A.; El-Shishtawy, M.M. Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating NrF2 pathway. Life Sci. 2018, 193, 159–170. [Google Scholar] [CrossRef]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [Green Version]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Stahli, A.; Maheen, C.U.; Strauss, F.J.; Eick, S.; Sculean, A.; Gruber, R. Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int. J. Oral. Sci. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Nguyen, T.; Yang, C.S.; Pickett, C.B. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic. Biol. Med. 2004, 37, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef]
- Nair, S.; Li, W.; Kong, A.N. Natural dietary anti-cancer chemopreventive compounds: Redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol. Sin. 2007, 28, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [Green Version]



| Polyphenol (mg/100 g) | Poplar Propolis Extract | Baccharis Propolis Extract | Dalbergia Propolis Extract |
|---|---|---|---|
| Caffeic acid | 373 | 87 | Nm |
| Coumaric acid | 1654 | 728 | Nm |
| Ferulic acid | 381 | 28 | Nm |
| Apigenin | 137 | Nd | Nm |
| CAPE | 246 | Nd | Nm |
| Quercetin | 94 | 213 | Nm |
| Kaempferol | 368 | 288 | Nm |
| Galangin | 1598 | Nd | Nm |
| Cinnamic acid | 789 | Nd | Nm |
| Pinocembrin | 2349 | 73 | Nm |
| Chrysin | 1850 | Nd | Nm |
| Artepillin C | Nd | 1503 | Nm |
| Formononetin | Nm | Nm | 1010 |
| Biochanin A | Nm | Nm | 167 |
| Liquiritigenin | Nm | Nm | 1075 |
| Vestitol | Nm | Nm | 9741 |
| Medicarpin | Nm | Nm | 4317 |
| Total polyphenols (g/100 g extract powder) | 41.1 | 20 | 29.5 |
| Gene Name | Accesion Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| Tnfα | NM_013693.3 | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Pparα | NM_011144.6 | CTGAGACCCTCGGGGAAC | AAACGTCAGTTCACAGGGAAG |
| Mcad | NM_007382.5 | TGTCGAACACAACACTCGAAA | CTGCTGTTCCGTCAACTCAA |
| Lcad | NM_007381.4 | TGGGGACTTGCTCTCAACA | GGCCTGTGCAATTGGAGTA |
| Ccl2 | NM_011333.3 | CATCCACGTGTTGGCTCA | GATCATCTTGCTGGTGAATGAGT |
| Ccl5 | NM_013653.3 | TGCAGAGGACTCTGAGACAGC | GAGTGGTGTCCGAGCCATA |
| Hmox1 | NM_010442.2 | AGGCTAAGACCGCCTTCCT | TGTGTTCCTCTGTCAGCATCA |
| Gclc | NM_010295.2 | AGATGATAGAACACGGGAGGAG | TGATCCTAAAGCGATTGTTCTTC |
| Gclm | NM_008129.4 | TGACTCACAATGACCCGAAA | TCAATGTCAGGGATGCTTTCT |
| Nqo1 | NM_008706.5 | AGCGTTCGGTATTACGATCC | AGTACAATCAGGGCTCTTCTCG |
| 18S | NR_003278.3 | CGCCGCTAGAGGTGAAATTCT | CATTCTTGGCAAATGCTTTCG |
| Control | HF | HF + PPEP | HF + BPEP | HF + DPEP | HF + Mix | |
|---|---|---|---|---|---|---|
| TG (mmol/L) | 1.195 ± 0.11 a | 1.092 ± 0.12 a,c | 0.93 ± 0.03 a | 0.822 ± 0.13 b,c | 0.92 ± 0.05 a | 1.15 ± 0.08 a |
| NEFA (mmol/L) | 1.075 ± 0.12 a | 1.041 ± 0.09 a | 0.94 ± 0.07 a | 1.005 ± 0.007 a | 1.052 ± 0.115 a | 1.071 ± 0.08 a |
| Β-OH butyrate (mmol/L) | 2.319 ± 0.15 a | 4.051 ± 0.35 b | 2.44 ± 0.26 a | 3.13 ± 0.48 a,b | 3.26 ± 0.41 b | 2.89 ± 0.30 a |
| ALAT (UI) | 2479 ± 313 a | 2225 ± 400 a | 2592 ± 529 a | 2659 ± 64 3 a | 1980 ± 263 a | 2175 ± 732 a |
| Adiponectin (ng/mL) | 6836 ± 370 a | 5072 ± 776 b,c | 4712 ± 287 b | 6369 ± 609 a | 6860 ± 576 a | 6401 ± 508 a,c |
| Leptin (pg/mL) | 1660 ± 658 a | 12029 ± 2961 b,c | 2900 ± 2038 a | 8323 ± 2246 a,c | 11196 ± 2995 b | 9774 ± 3368 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinault, N.; Tourniaire, F.; Astier, J.; Couturier, C.; Bonnet, L.; Seipelt, E.; Karkeni, E.; Letullier, C.; Dlalah, N.; Georgé, S.; et al. Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice. Antioxidants 2021, 10, 411. https://doi.org/10.3390/antiox10030411
Cardinault N, Tourniaire F, Astier J, Couturier C, Bonnet L, Seipelt E, Karkeni E, Letullier C, Dlalah N, Georgé S, et al. Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice. Antioxidants. 2021; 10(3):411. https://doi.org/10.3390/antiox10030411
Chicago/Turabian StyleCardinault, Nicolas, Franck Tourniaire, Julien Astier, Charlene Couturier, Lauriane Bonnet, Eva Seipelt, Esma Karkeni, Claire Letullier, Naima Dlalah, Stephane Georgé, and et al. 2021. "Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice" Antioxidants 10, no. 3: 411. https://doi.org/10.3390/antiox10030411






