CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
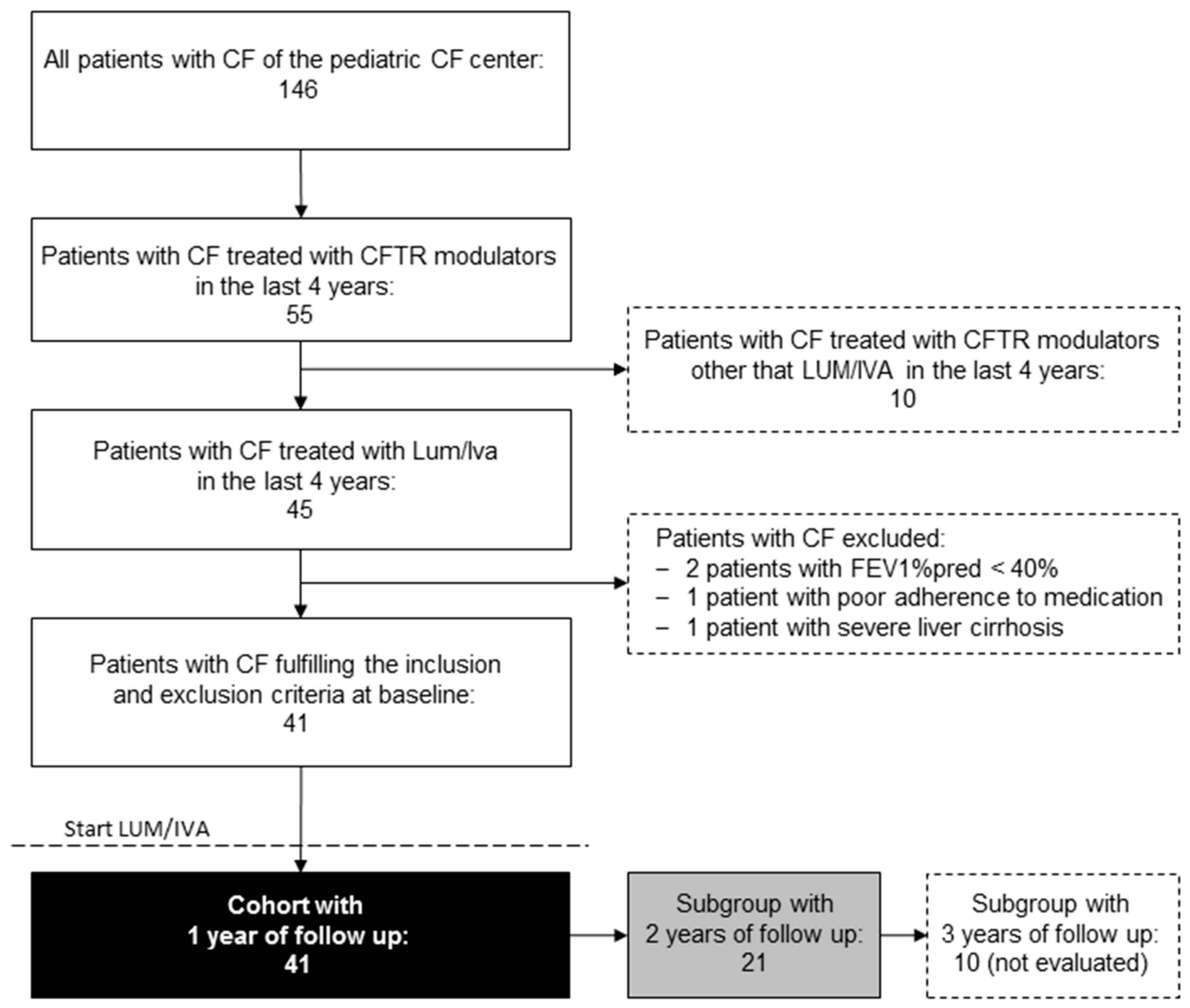
2.1. Study Population
2.2. Anthropometry, Lung Function Tests
2.3. Measurement of Biochemical Routine Parameters, Vitamin A, Vitamin E
2.4. Statistics
3. Results
3.1. Effect of Treatment with LUM/IVA on Anthropometry and Lung Function Tests
3.2. Effect of Treatment with LUM/IVA on Biochemical Parameters, Retinol, and Vitamin E
4. Discussion
4.1. CF Lung Disease under Lumacaftor/Ivacaftor Therapy
4.2. Abdominal CF Disease under Lumacaftor/Ivacaftor Therapy
4.3. Vitamin A (Retinol)
4.4. Vitamin E
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic Fibrosis. Nat. Rev. Dis. Primers 2015, 1, 15010. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Boucher, R.C. Pathophysiology of Cystic Fibrosis Lung Disease. In Cystic Fibrosis; Mall, M.A., Elborn, J.S., Eds.; European Respiratory Society: Sheffield, UK, 2014. [Google Scholar]
- Mall, M.A.; Hartl, D. CFTR: Cystic fibrosis and beyond. Eur. Respir. J. 2014, 44, 1042–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CFTR1-Database. Cystic Fibrosis Mutation Database. Available online: http://www.genet.sickkids.on.ca/StatisticsPage.html (accessed on 18 October 2020).
- Kerem, E.; Corey, M.; Kerem, B.; Durie, P.; Tsui, L.-C.; Levison, H. Clinical and genetic comparisons of patients with cystic fibrosis, with or without meconium ileus. J. Pediatr. 1989, 114, 767–773. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Baker, D.; Leung, K.G.; Reed, G.; Baker, S.S.; Borowitz, D. Evolution of Pancreatic Function during the First Year in Infants with Cystic Fibrosis. J. Pediatr. 2013, 162, 808–812. [Google Scholar] [CrossRef]
- Walkowiak, J.; Sands, D.; Nowakowska, A.; Piotrowski, R.; Zybert, K.; Herzig, K.H.; Milanowski, A. Early Decline of Pancreatic Function in Cystic Fibrosis Patients with Class 1 or 2 Cftr Mutations. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 199–201. [Google Scholar] [CrossRef]
- Kraisinger, M.; Hochhaus, G.; Stecenko, A.; Bowser, E.; Hendeles, L. Clinical Pharmacology of Pancreatic Enzymes in Patients with Cystic Fibrosis and In Vitro Performance of Microencapsulated Formulations. J. Clin. Pharmacol. 1994, 34, 158–166. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [Green Version]
- Feranchak, A.P.; Sontag, M.K.; Wagener, J.S.; Hammond, K.B.; Accurso, F.J.; Sokol, R.J. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J. Pediatr. 1999, 135, 601–610. [Google Scholar] [CrossRef]
- Lancellotti, L.; D’Orazio, C.; Mastella, G.; Mazzi, G.; Lippi, U. Deficiency of Vitamins E and a in Cystic Fibrosis Is Inde-pendent of Pancreatic Function and Current Enzyme and Vitamin Supplementation. Eur. J. Pediatr. 1996, 155, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.; Kerem, E.; Rivlin, J.; Bentur, L.; Stankiewicz, H.; Bdolach-Abram, T.; Wilschanski, M. Vitamins A and E and Pulmonary Exacerbations in Patients with Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Siwamogsatham, O.; Dong, W.; Binongo, J.N.; Chowdhury, R.; Alvarez, J.A.; Feinman, S.J.; Enders, J.; Tangpricha, V. Relationship Between Fat-Soluble Vitamin Supplementation and Blood Concentrations in Adolescent and Adult Patients with Cystic Fibrosis. Nutr. Clin. Pract. 2014, 29, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, K.P., Jr. Vitamin a Deficiency Disorders in Children and Women. Food Nutr. Bull. 2003, 24 (Suppl. S4), S78–S90. [Google Scholar] [CrossRef]
- Obeid, M.; Price, J.; Sun, L.; Scantlebury, M.H.; Overby, P.; Sidhu, R.; Chiriboga, C.A.; Quittell, L.M. Facial Palsy and Id-iopathic Intracranial Hypertension in Twins with Cystic Fibrosis and Hypovitaminosis A. Pediatr. Neurol. 2011, 44, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; A Tanumihardjo, S. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef]
- Suskind, D.L. Nutritional Deficiencies During Normal Growth. Pediatr. Clin. N. Am. 2009, 56, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Bieri, J.G.; Fratantoni, J.F.; Wood, R.E.; Di Sant’Agnese, P.A. The Occurrence and Effects of Human Vitamin E Deficiency. A study in pacients with cystic fibrosis. J. Clin. Investig. 1977, 60, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madarasi, A.; Lugassi, A.; Greiner, E.; Holics, K.; Biró, L.; Mozsáry, E. Antioxidant status in patients with cystic fibrosis. Ann. Nutr. Metab. 2000, 44, 207–211. [Google Scholar] [CrossRef]
- Winklhofer-Roob, B.M.; Tuchschmid, P.E.; Molinari, L.; Shmerling, D.H. Response to a single oral dose of all-rac-alpha-tocopheryl acetate in patients with cystic fibrosis and in healthy individuals. Am. J. Clin. Nutr. 1996, 63, 717–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winklhofer-Roob, B.M.; Hof, M.A.V.; Shmerling, D.H. Long-term oral vitamin E supplementation in cystic fibrosis patients: RRR-alpha-tocopherol compared with all-rac-alpha-tocopheryl acetate preparations. Am. J. Clin. Nutr. 1996, 63, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stead, R.J.; Muller, D.P.; Matthews, S.; E Hodson, M.; Batten, J.C. Effect of abnormal liver function on vitamin E status and supplementation in adults with cystic fibrosis. Gut 1986, 27, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Jih, K.-Y.; Lin, W.-Y.; Sohma, Y.; Hwang, T.-C. CFTR potentiators: From bench to bedside. Curr. Opin. Pharmacol. 2017, 34, 98–104. [Google Scholar] [CrossRef]
- Hanrahan, J.W.; Sato, Y.; Carlile, G.W.; Jansen, G.; Young, J.C.; Thomas, D.Y. Cystic Fibrosis: Proteostatic correctors of CFTR trafficking and alternative therapeutic targets. Expert Opin. Ther. Targets 2019, 23, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Mayer-Hamblett, N.; Rowe, S.M. Cystic Fibrosis: Emergence of Highly Effective Targeted Therapeutics and Potential Clinical Implications. Am. J. Respir. Crit. Care Med. 2020, 201, 1193–1208. [Google Scholar] [CrossRef]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstan, M.W.; McKone, E.F.; Moss, R.B.; Marigowda, G.; Tian, S.; Waltz, D.; Huang, X.; Lubarsky, B.; Rubin, J.; Millar, S.J.; et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir. Med. 2017, 5, 107–118. [Google Scholar] [CrossRef]
- Accurso, F.J.; Rowe, S.M.; Clancy, J.P.; Boyle, M.P.; Dunitz, J.M.; Durie, P.R.; Sagel, S.D.; Hornick, D.B.; Konstan, M.W.; Donaldson, S.H.; et al. Effect of Vx-770 in Persons with Cystic Fibrosis and the G551d-Cftr Mutation. N. Engl. J. Med. 2010, 363, 1991–2003. [Google Scholar] [CrossRef] [Green Version]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Graeber, S.Y.; Dopfer, C.; Naehrlich, L.; Gyulumyan, L.; Scheuermann, H.; Hirtz, S.; Wege, S.; Mairbäurl, H.; Dorda, M.; Hyde, R.; et al. Effects of Lumacaftor–Ivacaftor Therapy on Cystic Fibrosis Transmembrane Conductance Regulator Function in Phe508del Homozygous Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 197, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Graham-Maar, R.C.; Schall, J.I.; Zemel, B.S.; Stallings, V.A. Vitamin A intake and elevated serum retinol levels in children and young adults with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Quanjer, P.; Stanojevic, S.; Cole, T.; Baur, X.; Hall, G.; Culver, B.; Enright, P.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Stahl, M.; Wielpütz, M.O.; Graeber, S.Y.; Joachim, C.; Sommerburg, O.; Kauczor, H.-U.; Puderbach, M.; Eichinger, M.; Mall, M.A. Comparison of Lung Clearance Index and Magnetic Resonance Imaging for Assessment of Lung Disease in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2016, 195, 349–359. [Google Scholar] [CrossRef]
- Stahl, M.; Joachim, C.; Wielpütz, M.O.; Mall, M.A. Comparison of lung clearance index determined by washout of N2 and SF6 in infants and preschool children with cystic fibrosis. J. Cyst. Fibros. 2019, 18, 399–406. [Google Scholar] [CrossRef]
- Anagnostopoulou, P.; Latzin, P.; Jensen, R.; Stahl, M.; Harper, A.; Yammine, S.; Usemann, J.; Foong, R.E.; Spycher, B.; Hall, G.L.; et al. Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur. Respir. J. 2019, 55, 1901302. [Google Scholar] [CrossRef]
- Robinson, P.D.; Latzin, P.; Ramsey, K.A.; Stanojevic, S.; Aurora, P.; Davis, S.D.; Gappa, M.; Hall, G.L.; Horsley, A.; Jensen, R.; et al. Assembly on Pediatrics. Preschool Multiple-Breath Washout Testing. An Official American Thoracic Society Technical State-ment. Am. J. Respir. Crit. Care Med. 2018, 197, e1–e19. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Welsh, M.J. AJRCCM: 100-YearAnniversary.Progress along the Pathway of Discovery Leading to Treatment and Cure of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Ratjen, F.; Hug, C.; Marigowda, G.; Tian, S.; Huang, X.; Stanojevic, S.; Milla, C.E.; Robinson, P.D.; Waltz, D.; Davies, J.C.; et al. Efficacy and Safety of Lumacaftor and Ivacaftor in Patients Aged 6–11 Years with Cystic Fibrosis Homozygous for F508del-Cftr: A Randomised, Placebo-Controlled Phase 3 Trial. Lancet Respir. Med. 2017, 5, 557–567. [Google Scholar] [CrossRef]
- Shaw, M.; Khan, U.; Clancy, J.P.; Donaldson, S.H.; Sagel, S.D.; Rowe, S.M.; Ratjen, F. Prospect Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Changes in Lci in F508del/F508del Patients Treated with Lumacaftor/Ivacaftor: Results from the Prospect Study. J. Cyst. Fibros. 2020, 19, 931–933. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Boutin, S.; Wielpütz, M.O.; Joachim, C.; Frey, D.L.; Wege, S.; Sommerburg, O.; Kauczor, H.-U.; Stahl, M.; Dalpke, A.H.; et al. Effects of Lumacaftor-Ivacaftor on Lung Clearance Index, Magnetic Resonance Imaging and Airway Microbiome in Phe508del Homozygous Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2021. [Google Scholar] [CrossRef]
- Levy, H.; Kalish, L.A.; Huntington, I.; Weller, N.; Gerard, C.; Silverman, E.K.; Celedon, J.C.; Pier, G.B.; Weiss, S.T. Inflammatory Markers of Lung Disease in Adult Patients with Cystic Fibrosis. Pediatr. Pulmonol. 2007, 42, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Boutin, S.; Depner, M.; Stahl, M.; Graeber, S.Y.; Dittrich, S.A.; Legatzki, A.; von Mutius, E.; Mall, M.; Dalpke, A.H. Comparison of Oropharyngeal Microbiota from Children with Asthma and Cystic Fibrosis. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; West, R.H.; Manson, M.E.; Ruddy, J.; Jiang, D.; Previs, S.F.; Sonawane, N.D.; Burgess, J.D.; Kelley, T.J. Increased plasma membrane cholesterol in cystic fibrosis cells correlates with CFTR genotype and depends on de novo cholesterol synthesis. Respir. Res. 2010, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- White, N.M.; Jiang, D.; Burgess, J.D.; Bederman, I.R.; Previs, S.F.; Kelley, T.J. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2007, 292, L476–L486. [Google Scholar] [CrossRef]
- Gelzo, M.; Iacotucci, P.; Sica, C.; Liguori, R.; Comegna, M.; Carnovale, V.; Russo, A.D.; Corso, G.; Castaldo, G. Influence of pancreatic status on circulating plasma sterols in patients with cystic fibrosis. Clin. Chem. Lab. Med. 2020, 58, 1725–1730. [Google Scholar] [CrossRef]
- Gelzo, M.; Iacotucci, P.; Caputo, M.; Cernera, G.; Comegna, M.; Carnovale, V.; Corso, G.; Castaldo, G. Lumacaftor/ivacaftor improves liver cholesterol metabolism but does not influence hypocholesterolemia in patients with cystic fibrosis. J. Cyst. Fibros. 2021, 20, e1–e6. [Google Scholar] [CrossRef]
- Gelzo, M.; Di Taranto, M.D.; Sica, C.; Boscia, A.; Papagni, F.; Fortunato, G.; Corso, G.; Russo, A.D. Age-related changes of cholestanol and lathosterol plasma concentrations: An explorative study. Lipids Health Dis. 2019, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Van de Peppel, I.P.; Bodewes, F.; Verkade, H.J.; Jonker, J.W. Bile Acid Homeostasis in Gastrointestinal and Metabolic Complications of Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Woestenenk, J.W.; Broos, N.; Stellato, R.K.; Arets, H.G.; Van Der Ent, C.K.; Houwen, R.H.; Information, P.E.K.F.C. Vitamin A intake and serum retinol levels in children and adolescents with cystic fibrosis. Clin. Nutr. 2016, 35, 654–659. [Google Scholar] [CrossRef]
- Brei, C.; Simon, A.; Krawinkel, M.B.; Naehrlich, L. Individualized vitamin A supplementation for patients with cystic fibrosis. Clin. Nutr. 2013, 32, 805–810. [Google Scholar] [CrossRef]
- Geubel, A.P.; de Galocsy, C.; Alves, N.; Rahier, J.; Dive, C. Liver Damage Caused by Therapeutic Vitamin a Admin-istration: Estimate of Dose-Related Toxicity in 41 Cases. Gastroenterology 1991, 100, 1701–1709. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Vitamin E: The shrew waiting to be tamed. Free Radic. Biol. Med. 2009, 46, 543–554. [Google Scholar] [CrossRef]
- Sapiejka, E.; Krzyżanowska-Jankowska, P.; Wenska-Chyży, E.; Szczepanik, M.; Walkowiak, D.; Cofta, S.; Pogorzelski, A.; Skorupa, W.; Walkowiak, J. Vitamin E status and its determinants in patients with cystic fibrosis. Adv. Med. Sci. 2018, 63, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Schall, J.I.; Zemel, B.S.; Stallings, V.A. Vitamin E status in children with cystic fibrosis and pancreatic insufficiency. J. Pediatr. 2006, 148, 556–559. [Google Scholar] [CrossRef]
- Leonard, S.W.; Good, C.K.; Gugger, E.T.; Traber, M.G. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am. J. Clin. Nutr. 2004, 79, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Richelle, M.; Enslen, M.; Hager, C.; Groux, M.; Tavazzi, I.; Godin, J.P.; Berger, A.; Metairon, S.; Quaile, S.; Piguet-Welsch, C.; et al. Both Free and Esterified Plant Sterols Reduce Cholesterol Absorption and the Bioavail-ability of Beta-Carotene and Alpha-Tocopherol in Normocholesterolemic Humans. Am. J. Clin. Nutr. 2004, 80, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Gelfond, D.; Heltshe, S.; Ma, C.; Rowe, S.M.; Frederick, C.; Uluer, A.; Sicilian, L.; Konstan, M.; Tullis, E.; Roach, C.R.N.; et al. Impact of CFTR Modulation on Intestinal pH, Motility, and Clinical Outcomes in Patients with Cystic Fibrosis and the G551D Mutation. Clin. Transl. Gastroenterol. 2017, 8, e81. [Google Scholar] [CrossRef]
- Hacquebard, M.; Carpentier, Y.A. Vitamin E: Absorption, plasma transport and cell uptake. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 133–138. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Vitamin E and Drug Metabolism. Biochem. Biophys. Res. Commun. 2003, 305, 737–740. [Google Scholar] [CrossRef]

| Clinical Parameter | Median (Range) |
|---|---|
| Age [years] | 6.6 (2.1–21.8) |
| Sex, female, n (%) | 20 (48.8%) |
| CFTR-Genotype: F508del/F508del, n (%) | 41 (100%) |
| Pancreatic insufficiency (PI), n (%) | 41 (100%) |
| BMI [kg/m2] | 15.8 (13.7–23.47) |
| Albumin [g/L] | 45.1 (29.4–50.2) |
| Creatinine [mg/dL] | 0.34 (0.19–0.74) |
| CrP [mg/L] | 2.0 (2.0–17.2) |
| IgG [g/L] (n = 39) | 9.36 (4.75–21.79) |
| GGT [U/L] | 11.0 (7.0–36.0) |
| AST [U/L] | 31.0 (12.0–111.0) |
| ALT [U/L] | 24 (1.7–190) |
| AP [U/L] | 287 (109–475) |
| Before Start LUM/IVA | 1 Year after Start LUM/IVA | p Value | |
|---|---|---|---|
| BMI z-score (mean ± SD) | −0.24 ± 0.89 | −0.04 ± 0.96 | 0.0432 * |
| FEV1%pred (n = 34) | 84.9 (40.6–122.8) | 87.6 (33.8–110.3) | 0.8428 |
| LCI (n = 39) | 9.00 (5.63–21.12) | 7.54 (5.11–21.58) | 0.0018 ** |
| CrP [mg/L] | 2.0 (2.0–17.2) | 2.0 (2.0–47.0) | 0.7422 |
| IgG [g/L] (n = 39) | 9.36 (4.75–21.79) | 8.13 (3.19–19.17) | 0.0001 *** |
| INR (n = 39) | 1.01 (0.94–1.25) | 1.06 (0.93–1.48) | 0.0001 *** |
| 25-OH-Cholecalciferol [nmol/L] (n = 40) | 63.9 (22.0–154.0) | 76.3 (23.0–134.5) | 0.1954 |
| Retinol [µmol/L] | 1.23 (0.51–2.50) | 1.33 (0.34–2.56) | 0.3874 |
| Vitamin E [µmol/L] | 22.0 (12.7–39.7) | 19.5 (4.8–45.6) | 0.0020 * |
| Cholesterol [mmol/L] | 3.36 (2.15–4.88) | 3.27 (1.51–4.45) | 0.0209 * |
| Vitamin E/Cholesterol [µmol/mmol] | 6.45 (3.43–11.23) | 5.96 (1.78–11.88) | 0.0152 * |
| Before Start LUM/IVA | 1 Year after Start LUM/IVA | 2 Year after Start LUM/IVA | p Value | |
|---|---|---|---|---|
| Patient number, N cohort (N female, %) | 21 | |||
| (11, 52.4%) | ||||
| Age at start of LUM/IVA (years) | 7.6 | |||
| (4.2–14.8) | ||||
| BMI z-score (mean ± SD) | −0.14 ± 0.83 | 0.16 ± 0.73 | 0.24 ± 0.63 | 0.0003 *** |
| FEV1%pred | 89.6 | 90.7 | 87.4 | 0.9731 |
| (57.1–116.5) | (59.9–110.3) | (58.2–128.0) | ||
| LCI (n = 20) | 9.44 | 8.19 | 7.92 | 0.0556 |
| (6.24–14.03) | (5.11–15.39) | (6.34–15.15) | ||
| CrP [mg/L] | 2.0 | 2.0 | 2.0 | 0.3679 |
| (2.0–2.0) | (2.0–9.0) | (2.0–13.2) | ||
| IgG [g/L] | 9.36 | 8.13 | 9.36 | 0.0006 *** |
| (4.75–13.87) | (3.70–13.87) | (4.75–12.92) | ||
| INR (n = 19) | 1.02 | 1.01 | 1.01 | 0.4791 |
| (0.96–1.18) | (0.97–1.14) | (0.98–1.13) | ||
| 25-OH-Cholecalciferol [nmol/L] (n = 20) | 61.0 | 67.9 | 73.1 | 0.3160 |
| (22.0–118.3) | (23.0–125.0) | (19.0–134.8) | ||
| Retinol [µmol/L] | 1.23 | 1.42 | 1.60 | 0.0140 * |
| (0.79–2.27) | (0.80–2.25) | (0.85–2.49) | ||
| Vitamin E [µmol/L] | 20.9 | 18.2 | 16.7 | 0.0085 ** |
| (14.5–31.9) | (5.58–33.4) | (3.2–30.5) | ||
| Cholesterol [mmol/L] | 3.43 | 3.20 | 3.21 | 0.0945 |
| (2.51–4.13) | (2.04–4.22) | (1.91–4.18) | ||
| Vitamin E/Cholesterol [µmol/mmol] | 6.15 | 5.81 | 5.52 | 0.0268 * |
| (3.75–9.74) | (1.78–11.88) | (1.00–9.95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerburg, O.; Hämmerling, S.; Schneider, S.P.; Okun, J.; Langhans, C.-D.; Leutz-Schmidt, P.; Wielpütz, M.O.; Siems, W.; Gräber, S.Y.; Mall, M.A.; et al. CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis. Antioxidants 2021, 10, 483. https://doi.org/10.3390/antiox10030483
Sommerburg O, Hämmerling S, Schneider SP, Okun J, Langhans C-D, Leutz-Schmidt P, Wielpütz MO, Siems W, Gräber SY, Mall MA, et al. CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis. Antioxidants. 2021; 10(3):483. https://doi.org/10.3390/antiox10030483
Chicago/Turabian StyleSommerburg, Olaf, Susanne Hämmerling, S. Philipp Schneider, Jürgen Okun, Claus-Dieter Langhans, Patricia Leutz-Schmidt, Mark O. Wielpütz, Werner Siems, Simon Y. Gräber, Marcus A. Mall, and et al. 2021. "CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis" Antioxidants 10, no. 3: 483. https://doi.org/10.3390/antiox10030483






