Columbianadin Dampens In Vitro Inflammatory Actions and Inhibits Liver Injury via Inhibition of NF-κB/MAPKs: Impacts on ∙OH Radicals and HO-1 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cultivation and MTT Assay for Cell Viability
2.3. Detection of Hydroxyl Radicals
2.4. Immunoblotting Study
2.5. Confocal Microscopy Assay
2.6. Measurement of NO Production
2.7. Animals and Experimental Design
2.8. Histological Analysis
2.9. Measurement of Liver Function Enzymes
2.10. Statistical Evaluation
3. Results
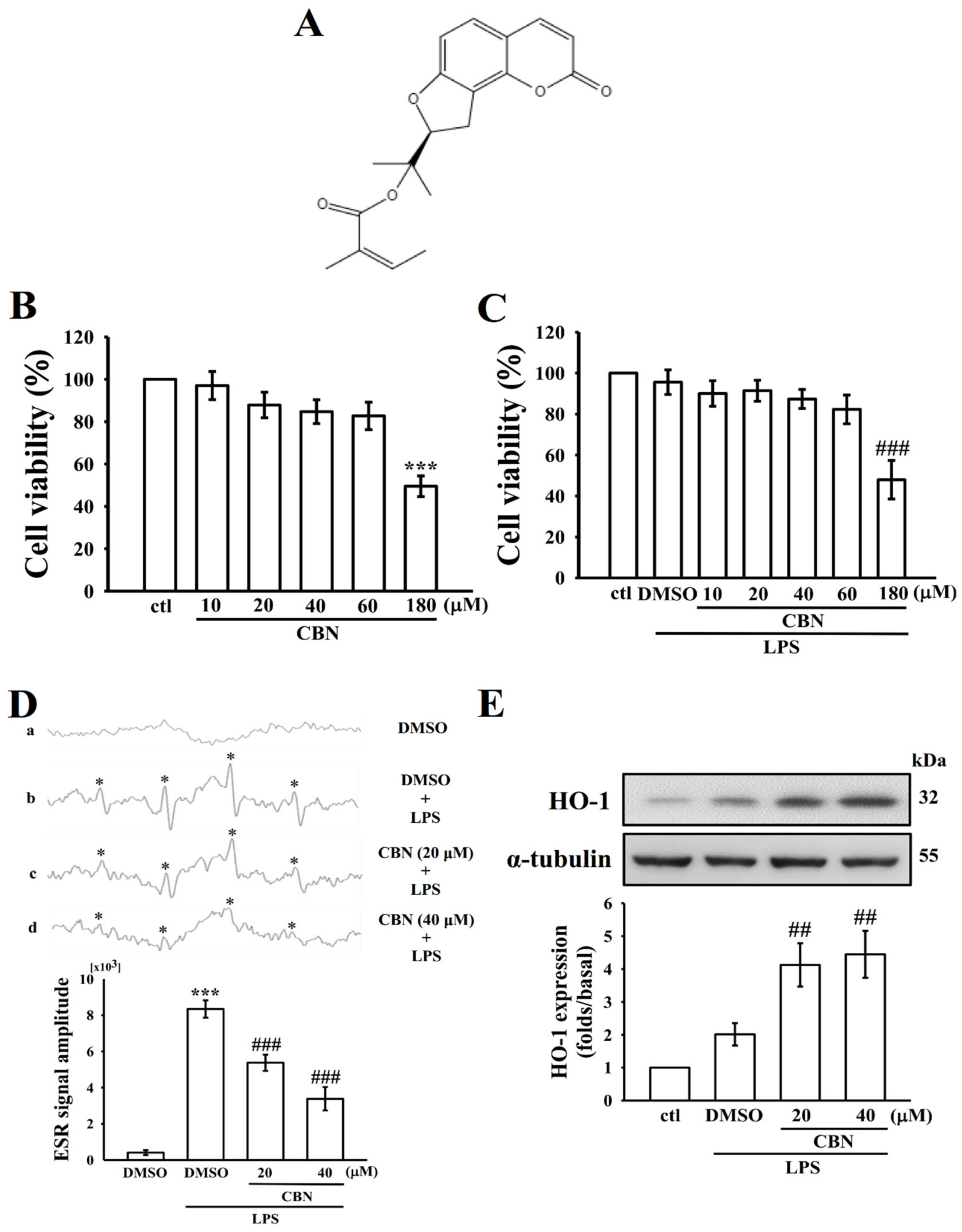
3.1. Effects of CBN on Cytotoxicity, •OH Radical Production, and HO-1 Expression
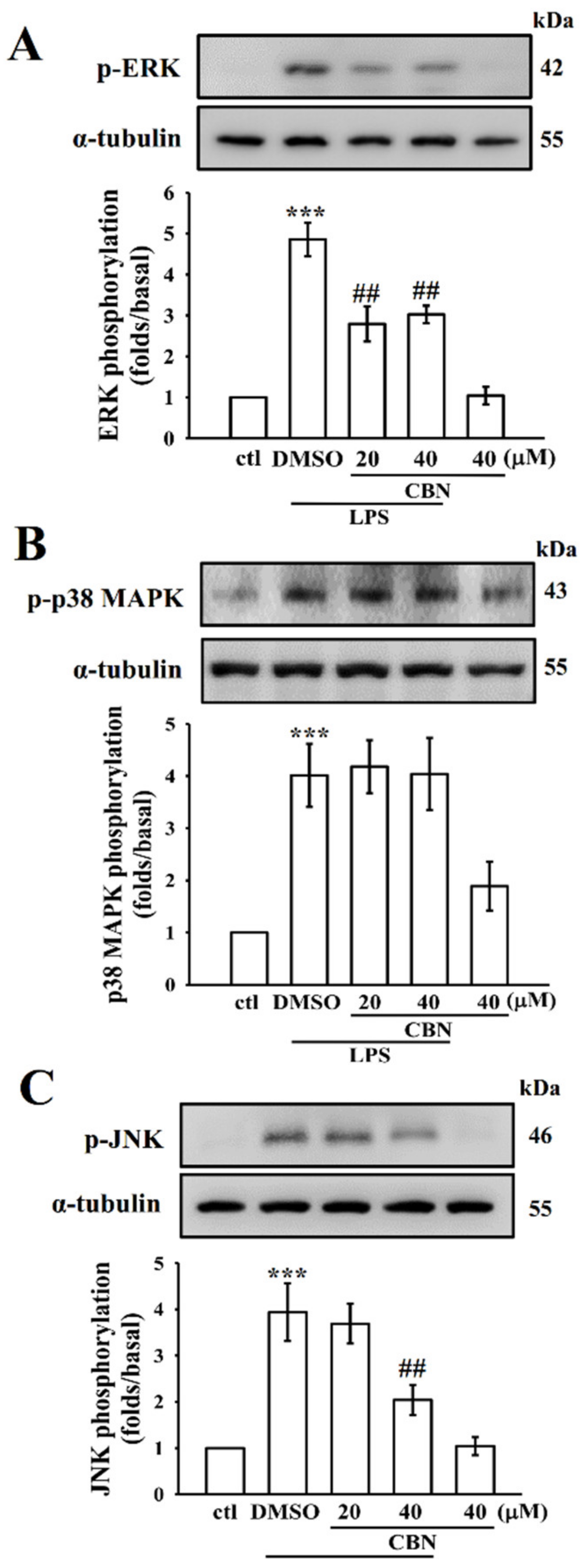
3.2. CBN Suppresses LPS-Induced MAPK Activation
3.3. CBN Regulates NF-κB Signaling Pathways
3.4. CBN Reduced the Expression of Inflammatory Mediators
3.4.1. Effects on NO and iNOS
3.4.2. Effects on Inflammatory Cytokines
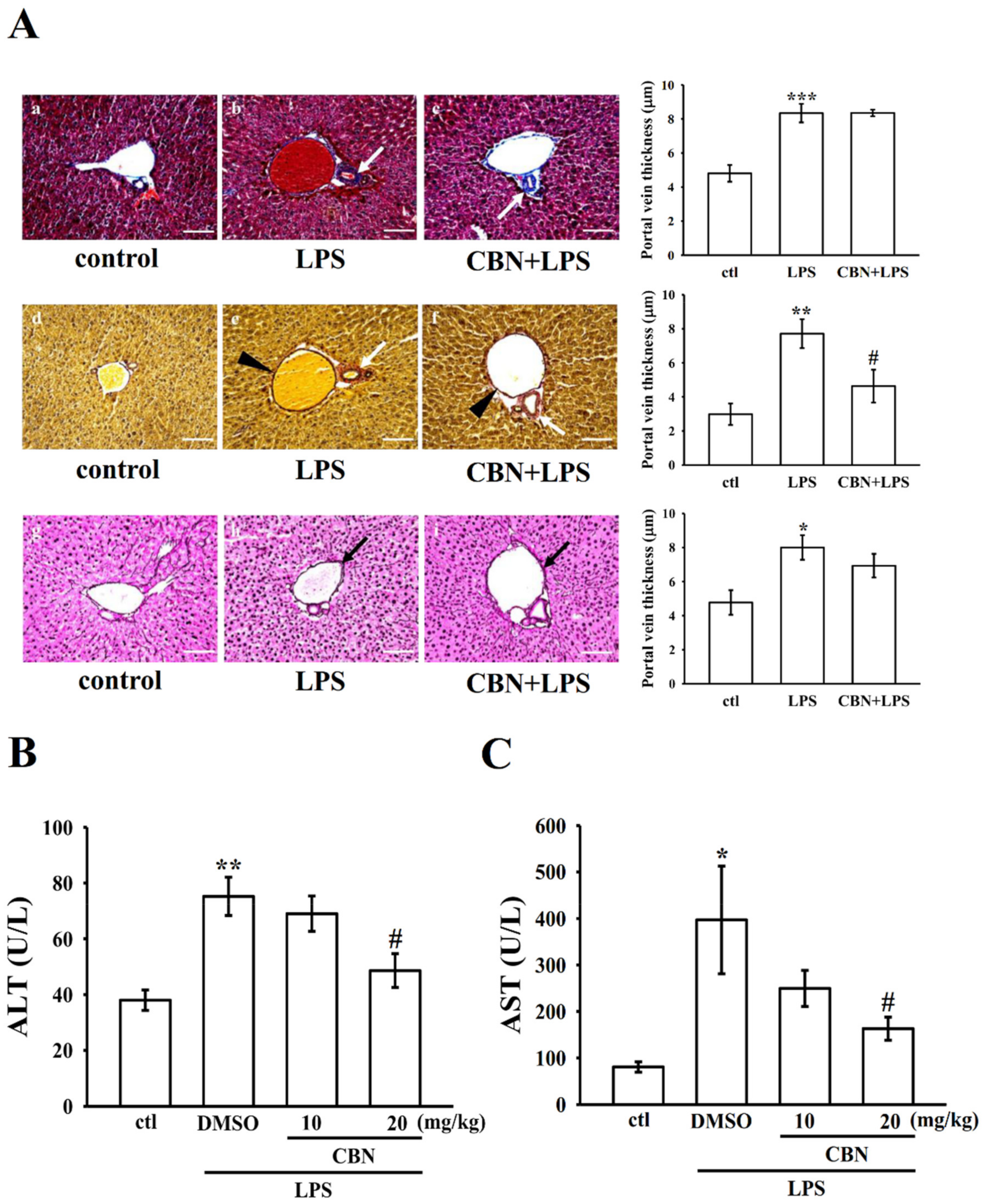
3.4.3. Effects of CBN on Hepatic Histopathology
3.5. CBN Reduces Liver Marker Enzymes ALT and AST
3.6. Effects of CBN on Liver MAPKs, NF-κB, Inflammatory Cytokines, and iNOS Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Kankala, S.; Kankala, R.K.; Gundepaka, P.; Thota, N.; Nerella, S.; Gangula, M.R.; Guguloth, H.; Kagga, M.; Vadde, R.; Vasam, C.S. Regio selective synthesis of isoxazole–mercaptobenzimidazole hybrids and their in vivo analgesic and anti-inflammatory activity studies. Bioorg. Med. Chem. Lett. 2013, 23, 1306–1309. [Google Scholar] [CrossRef]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-kappaB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef]
- Karin, M.; Delhase, M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signaling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 141–153. [Google Scholar]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Moret-Tatay, I.; Iborra, M.; Cerrillo, E.; Tortosa, L.; Nos, P.; Beltran, B. Possible biomarkers in blood for crohn’s disease: Oxidative stress and micrornas-current evidences and further aspects to unravel. Oxid. Med. Cell. Longev. 2015, 2016, 2325162. [Google Scholar] [CrossRef] [Green Version]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. Oxidative stress marker in oral cancer: A review. J. Can. Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef]
- So, B.E.; Bach, T.T.; Paik, J.H.; Jung, S.K. Kmeria duperreana (Pierre) Dandy extract suppresses LPS-induced iNOS and NO via regulation of NF-κB pathways and p38 in murin macrophage RAW 264.7 cells. Prev. Nutr. Food Sci. 2020, 25, 166–172. [Google Scholar] [CrossRef]
- Manning, A.M.; Davis, R.J. Targeting JNK for therapeutic benefit: From junk to gold? Nat. Rev. Drug. Discov. 2003, 2, 554–565. [Google Scholar] [CrossRef]
- Hao, G.; Wang, Z.G.; Fu, W.Y.; Yang, Y. Research progress on effect of coumarins compounds in anti-tumor. Chin. J. Chin. Materia. Medica. 2008, 33, 2016–2019. [Google Scholar]
- Zhang, C.Y.; Zhang, B.G.; Yang, X.W. Studies on the chemical constituents of the root of Angelica pubescens f. biserrata. Pharm. J. Chin. People’s Lib. Arm. 2007, 23, 241–245. [Google Scholar]
- Li, R.Z.; He, Y.Q.; Qiao, M.; Xu, Y.; Zhang, Q.B.; Meng, J.R.; Gu, Y.; Ge, L.P. Studies of the active constituents of the Chinese drug ‘Duhuo’ Angelica pubescens. Acta Pharm. Sin. 1989, 24, 546–551. [Google Scholar]
- Chen, Y.F.; Tsai, H.Y.; Wu, T.S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Guo, Q.M.; Wang, Y. Absorption and transport of 6 coumarins isolated from the root of Angelica pubescens f. biserrata in human Caco-2 cell monolayer model. J. Chin. Integr. Med. 2008, 6, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.M.; Hsia, C.W.; Tsai, C.L.; Hsia, C.H.; Jayakumar, T.; Velusamy, M.; Sheu, J.R. Modulation of human platelet activation and in vivo vascular thrombosis by columbianadin: Regulation by integrin αIIbβ3 inside-out but not outside-in signals. J. Biomed. Sci. 2020, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.S.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.F.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef] [Green Version]
- Jun, M.S.; Ha, Y.M.; Kim, H.S.; Jang, H.J.; Kim, Y.M.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Lee, S.H.; et al. Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J. Ethnopharmacol. 2011, 133, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Martin, M.; Michalek, S.M.; Katz, J. Role of mitogen-activated protein kinases and NF-κB in the regulation of proinflammatory and anti-Inflammatory cytokines by Porphyromonas gingivalis Hemagglutinin B. Infect. Immun. 2005, 73, 3990–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology 2010, 52, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cidlowski, J.A. Glutathione efflux and cell death. Antioxid. Redox Signal. 2012, 17, 1694–1713. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Brune, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduc. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Zapelini, P.H.; Rezin, G.T.; Cardoso, M.R.; Ritter, C.; Klamt, F.; Moreira, J.C.F.; Streck, E.M.; Pizzol, F.D. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 2008, 8, 211–218. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative Stress, inflammation, and apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Ye, B.R.; Kim, E.A.; Kim, J.; Kim, M.S.; Lee, W.W.; Ahn, G.N.; Kang, N.; Jung, W.K.; Heo, S.J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Suzuki, M.; Nagashima, Y.; Suzuki, S.; Hashiba, T.; Tsuburai, T.; Ikehara, K.; Matsuse, T.; Ishigatsubo, Y. Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Hum. Gene Ther. 2001, 12, 967–979. [Google Scholar]
- Thalhamer, T.; McGrath, M.A.; Harnett, M.M. MAPKs and their relevance to arthritis and inflammation. Rheumatology 2008, 47, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Fernandes, J.C.; Brunet, J.; Moldovan, F.; Schrier, D.; Flory, C.; Martel-Pelletier, J. In vivo selective inhibition of mitogen-activated protein kinase kinase ½ in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003, 48, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zhang, H.; Li, X.; Zhan, H.; Chen, F.; Han, L.; Cao, X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Intl. Immunopharmacol. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Brit. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Hong, P.; Zheng, X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef]
- Sheu, J.R.; Chen, Z.C.; Hsu, M.J.; Wang, S.H.; Jung, K.W.; Wu, W.F.; Pan, S.H.; Teng, R.D.; Yang, C.H.; Hsieh, C.Y. CME-1, a novel polysaccharide, suppresses iNOS expression in lipopolysaccharide-stimulated macrophages through ceramide-initiated protein phosphatase 2A activation. J. Cell Mol. Med. 2018, 22, 999–1013. [Google Scholar]
- Tirapelli, L.F.; Batalhão, M.E.; Jacob-Ferreira, A.L.; Tirapelli, D.P.; Carnio, E.C.; Tanus-Santos, J.E.; Queiroz, R.H.; Uyemura, S.A.; Padovan, C.M.; Tirapelli, C.R. Chronic ethanol consumption induces histopathological changes and increases nitric oxide generation in the rat liver. Tissue Cell. 2011, 43, 384–391. [Google Scholar] [CrossRef]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-Inflammatory effect of a polyphenol-enriched fraction from Acalypha wilkesiana on lipopolysaccharide-stimulated RAW 264.7 macrophages and acetaminophen-induced liver injury in mice. Oxid. Med. Cell. Longevit. 2018, 2018, 7858094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Hsu, A.C.Y.; Pan, H.; Gu, Y.; Zuo, X.; Dong, B.; Wang, Z.; Zheng, J.; Lu, J.; Zheng, R.; et al. Columbianadin suppresses lipopolysaccharide (LPS)-induced inflammation and apoptosis through the NOD1 pathway. Molecules 2019, 24, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Dan, C.; Jinjun, B.; Zi-Chun, H.; Lin, M.; Wei, C.; Xu, Z.; Ri, Z.; Shun, C.; Wen-Zhu, S.; Qing-Cai, J.; et al. Modulation of TNF-α mRNA stability by human antigen R and miR181s in sepsis-induced immunoparalysis. EMBO Mol. Med. 2015, 7, 140–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajuwon, O.R.; Oguntibeju, O.O.; Marnewick, J.L. Amelioration of lipopolysaccharide-induced liver injury by aqueous rooibos (Aspalathus linearis) extract via inhibition of pro-inflammatory cytokines and oxidative stress. BMC Complement. Altern. Med. 2014, 14, 392. [Google Scholar] [CrossRef] [Green Version]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, L.N.; Cui, Y.L.; Jiang, H.L. Protective effect of danhong injection on acute hepatic failure induced by lipo-polysaccharide and d-galactosamine in mice. Evid. Based Complement. Alternat. Med. 2014, 2014, 153902. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakumar, T.; Hou, S.-M.; Chang, C.-C.; Fong, T.-H.; Hsia, C.-W.; Chen, Y.-J.; Huang, W.-C.; Saravanabhavan, P.; Manubolu, M.; Sheu, J.-R.; et al. Columbianadin Dampens In Vitro Inflammatory Actions and Inhibits Liver Injury via Inhibition of NF-κB/MAPKs: Impacts on ∙OH Radicals and HO-1 Expression. Antioxidants 2021, 10, 553. https://doi.org/10.3390/antiox10040553
Jayakumar T, Hou S-M, Chang C-C, Fong T-H, Hsia C-W, Chen Y-J, Huang W-C, Saravanabhavan P, Manubolu M, Sheu J-R, et al. Columbianadin Dampens In Vitro Inflammatory Actions and Inhibits Liver Injury via Inhibition of NF-κB/MAPKs: Impacts on ∙OH Radicals and HO-1 Expression. Antioxidants. 2021; 10(4):553. https://doi.org/10.3390/antiox10040553
Chicago/Turabian StyleJayakumar, Thanasekaran, Shaw-Min Hou, Chao-Chien Chang, Tsorng-Harn Fong, Chih-Wei Hsia, Yen-Jen Chen, Wei-Chieh Huang, Periyakali Saravanabhavan, Manjunath Manubolu, Joen-Rong Sheu, and et al. 2021. "Columbianadin Dampens In Vitro Inflammatory Actions and Inhibits Liver Injury via Inhibition of NF-κB/MAPKs: Impacts on ∙OH Radicals and HO-1 Expression" Antioxidants 10, no. 4: 553. https://doi.org/10.3390/antiox10040553





