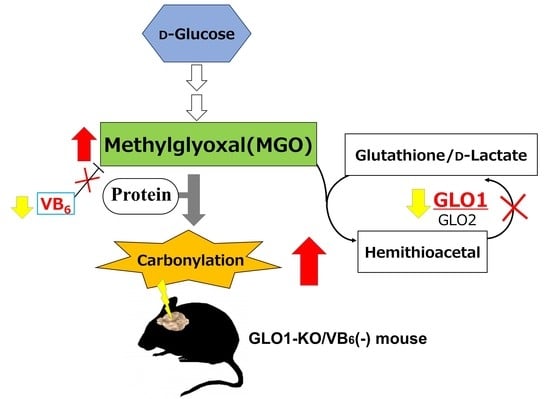
Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Generation of Glo1-KO Mice and VB6-Deficit Diet
2.3. Sample Preparation
2.4. Western Blot Analysis
2.5. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.6. Immunoprecipitation Analysis
2.7. Measurement of Creatine Kinase (CK) Activity in HIP Homogenates
2.8. Statistical Analysis
3. Results
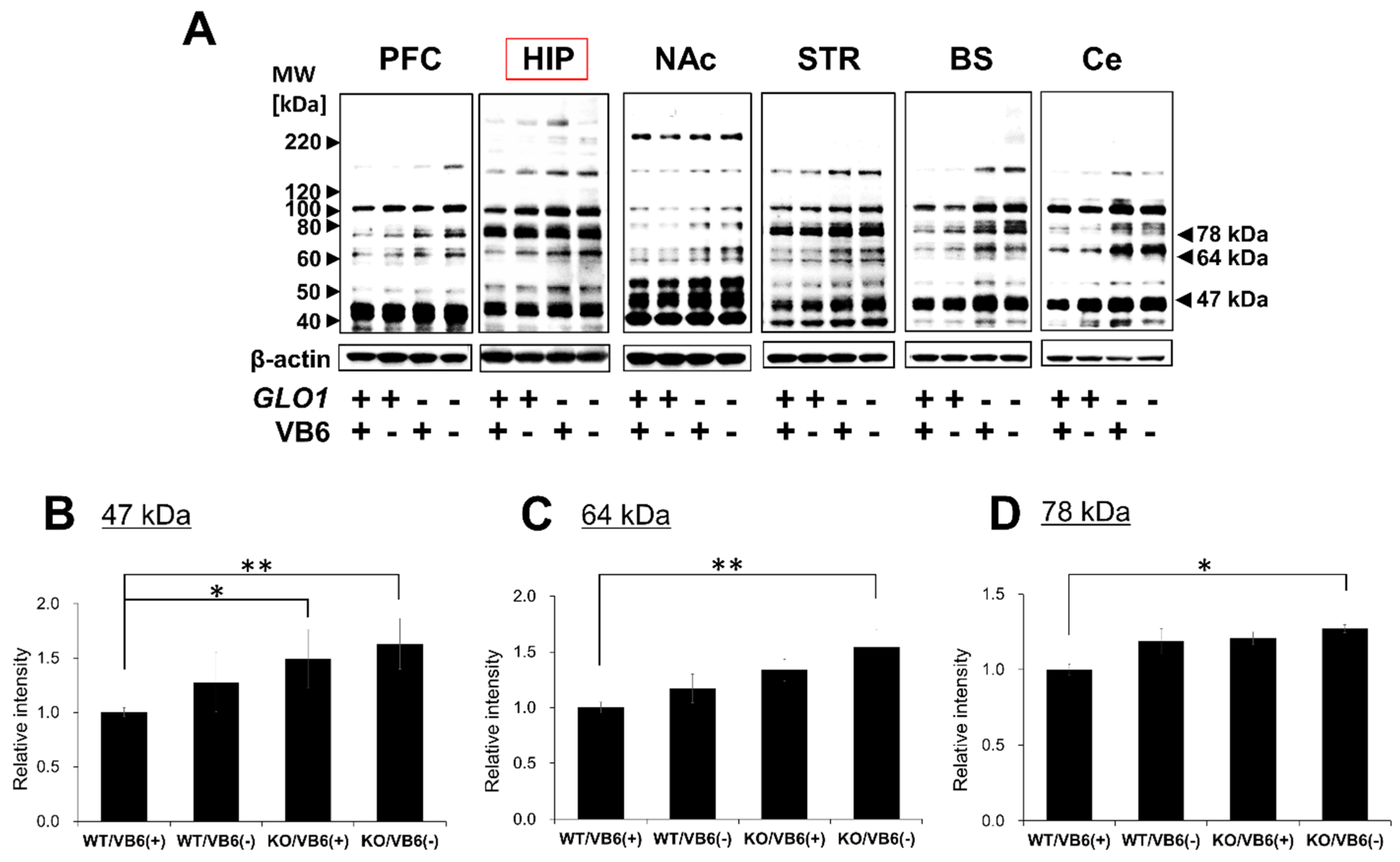
3.1. Accumulation of Various AGE-Modified Proteins in Six Different Regions of the KO/VB6(−) Mice Brain
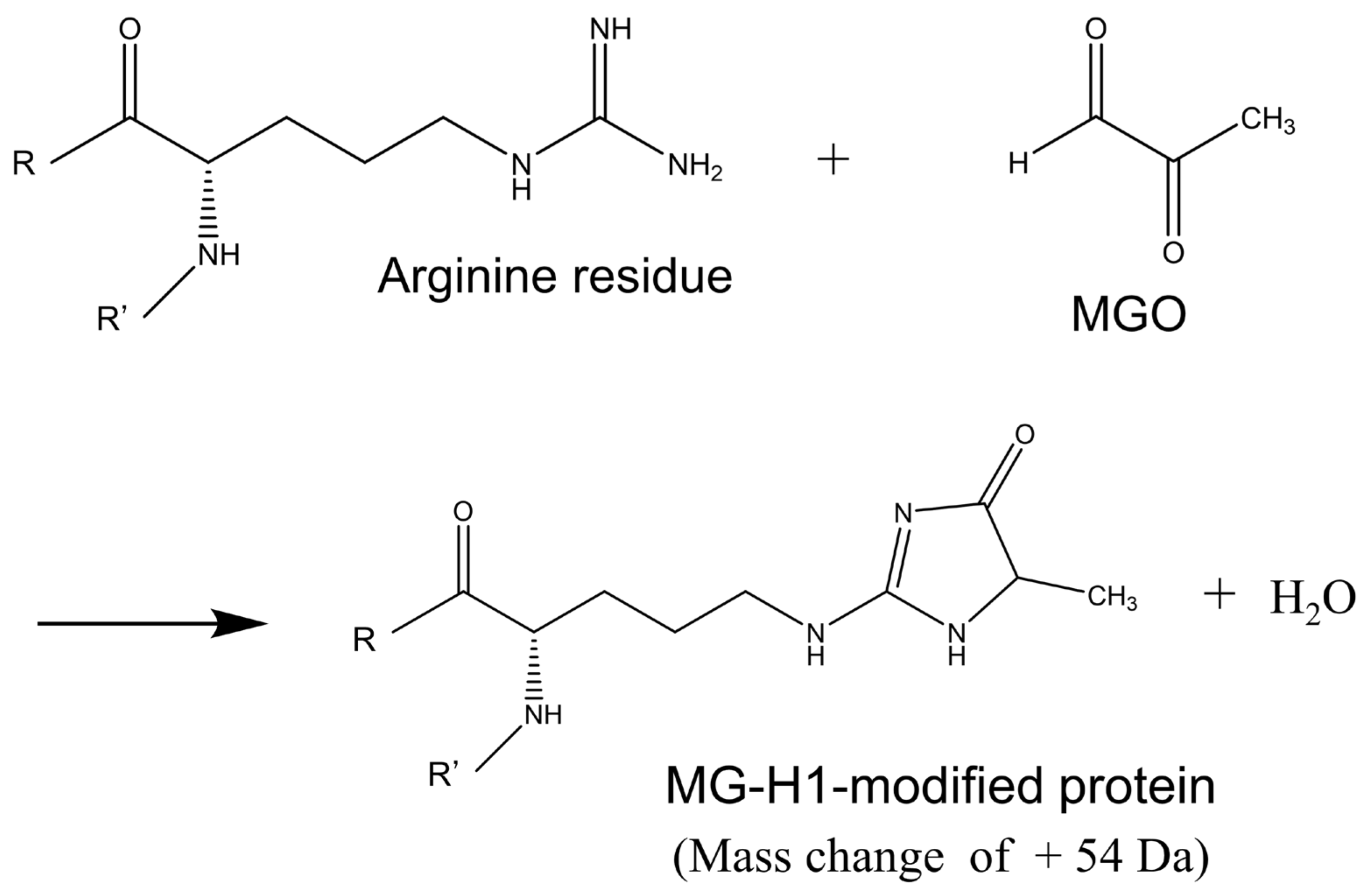
3.2. Identification of Protein Modified with MG-H1 Moiety in the KO/VB6(−) Mice Brain
3.3. Immunoprecipitation Study for Quantitative Estimation of the MG-H1-Modified Proteins
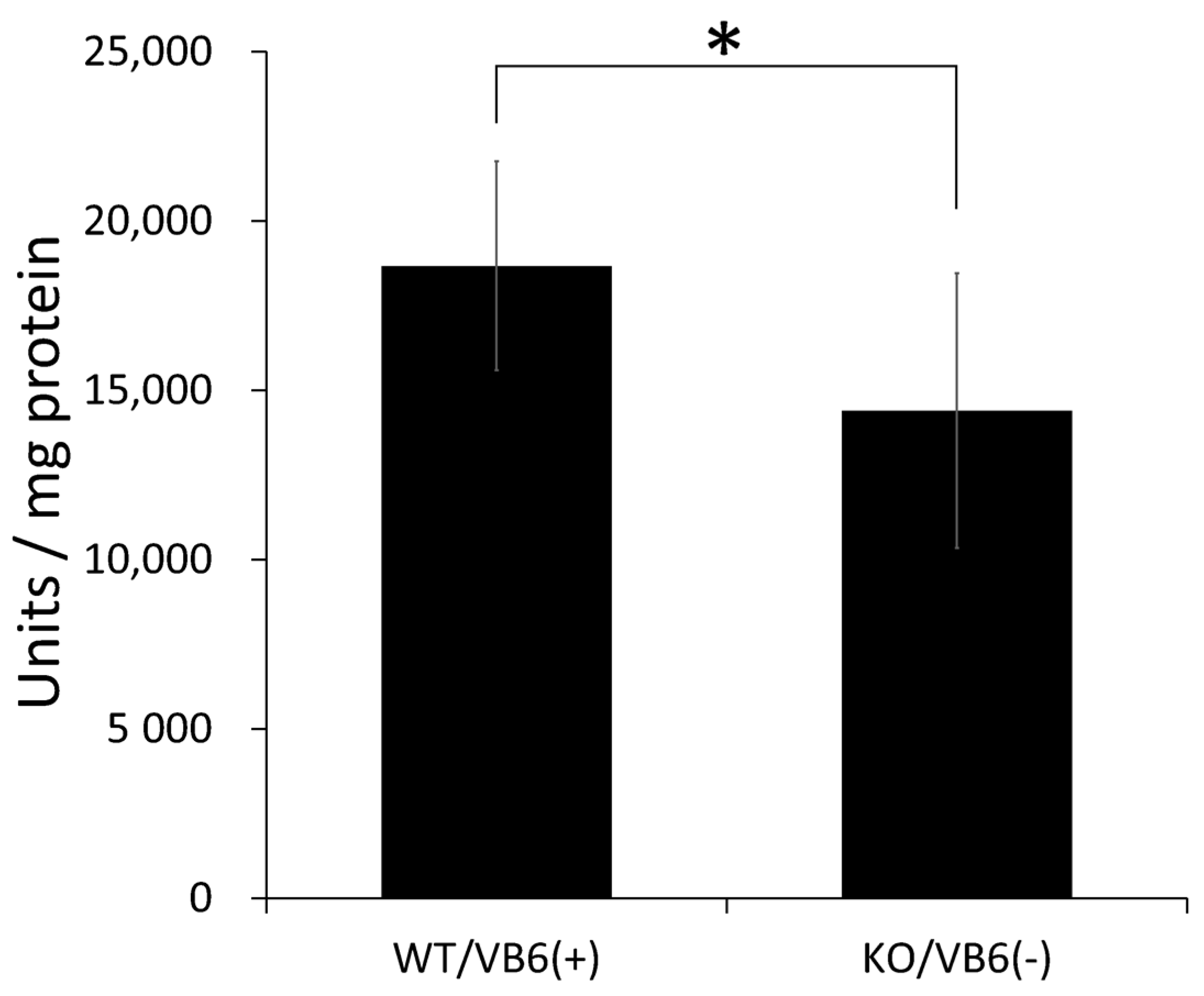
3.4. Intensive Comparison of Mitochondrial Creatine Kinase in Hippocampus between KO/VB6(−) and Wild/VB6(+) Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, N.; Jobanputra, V.; Pal, H. Molecular genetics of schizophrenia: A critical review. J. Psychiatry Neurosci. 2003, 28, 415–429. [Google Scholar]
- Arai, M.; Yuzawa, H.; Nohara, I.; Ohnishi, T.; Obata, N.; Iwayama, Y.; Haga, S.; Toyota, T.; Ujike, H.; Arai, M.; et al. Enhanced Carbonyl Stress in a Subpopulation of Schizophrenia. Arch. Gen. Psychiatry 2010, 67, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Miyashita, M.; Kobori, A.; Toriumi, K.; Horiuchi, Y.; Itokawa, M. Carbonyl stress and schizophrenia. Psychiatry Clin. Neurosci. 2014, 68, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Watanabe, T.; Ichikawa, T.; Toriumi, K.; Horiuchi, Y.; Kobori, A.; Kushima, I.; Hashimoto, R.; Fukumoto, M.; Koike, S.; et al. The regulation of soluble receptor for AGEs contributes to carbonyl stress in schizophrenia. Biochem. Biophys. Res. Commun. 2016, 479, 447–452. [Google Scholar] [CrossRef]
- Ohnuma, T.; Nishimon, S.; Takeda, M.; Sannohe, T.; Katsuta, N.; Arai, H. Carbonyl Stress and Microinflammation-Related Molecules as Potential Biomarkers in Schizophrenia. Front. Psychiatry 2018, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Jiang, X.; Ogawa, T.; Ohnishi, T.; Yoshihara, S.; Balan, S.; Yoshikawa, T.; Hirokawa, N. Enhanced carbonyl stress induces irreversible multimerization of CRMP2 in schizophrenia pathogenesis. Life Sci. Alliance 2019, 2, e201900478. [Google Scholar] [CrossRef]
- Miyata, T.; van Ypersele de Strihou, C.; Kurokawa, K.; Baynes, J.W. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999, 55, 389–399. [Google Scholar] [CrossRef]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J., Jr.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end products. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.A.; Schalkwijk, C.G. Plasma advanced glycation end products are associated with incident cardiovascular events in individuals with type 2 diabetes: A case-cohort study with a median follow-up of10 years (EP-IC-NL). Diabetes 2015, 64, 257–265. [Google Scholar] [CrossRef]
- Makita, Z.; Radoff, S.; Rayfield, E.J.; Yang, Z.; Skolnik, E.; Delaney, V.; Friedman, E.A.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Patients with Diabetic Nephropathy. N. Engl. J. Med. 1991, 325, 836–842. [Google Scholar] [CrossRef]
- Koyama, Y.; Takeishi, Y.; Arimoto, T.; Niizeki, T.; Shishido, T.; Takahashi, H.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; Nitobe, J.; et al. High Serum Level of Pentosidine, an Advanced Glycation End Product (AGE), is a Risk Factor of Patients with Heart Failure. J. Card. Fail. 2007, 13, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Arai, M.; Kobori, A.; Ichikawa, T.; Toriumi, K.; Niizato, K.; Oshima, K.; Okazaki, Y.; Yoshikawa, T.; Amano, N.; et al. Clinical Features of Schizophrenia with Enhanced Carbonyl Stress. Schizophr. Bull. 2014, 40, 1040–1046. [Google Scholar] [CrossRef]
- Itokawa, M.; Miyashita, M.; Arai, M.; Dan, T.; Takahashi, K.; Tokunaga, T.; Ishimoto, K.; Toriumi, K.; Ichikawa, T.; Horiuchi, Y.; et al. Pyridoxamine: A novel treatment for schizophrenia with enhanced carbonyl stress. Psychiatry Clin. Neurosci. 2018, 72, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Stehouwer, C.D.; Schalkwijk, C.G. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem. Soc. Trans. 2014, 42, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.; Stehouwer, C.D.; Schalkwijk, C.G. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 2015, 128, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Krömer, S.A.; Kessler, M.S.; Milfay, D.; Birg, I.N.; Bunck, M.; Czibere, L.; Panhuysen, M.; Pütz, B.; Deussing, J.M.; Holsboer, F.; et al. Identification of Glyoxalase-I as a Protein Marker in a Mouse Model of Extremes in Trait Anxiety. J. Neurosci. 2005, 25, 4375–4384. [Google Scholar] [CrossRef] [PubMed]
- Hambsch, B.; Chen, B.-G.; Brenndörfer, J.; Meyer, M.; Avrabos, C.; Maccarrone, G.; Liu, R.H.; Eder, M.; Turck, C.W.; Landgraf, R. Methylglyoxal-mediated anxiolysis involves increased protein modification and elevated expression of glyoxalase 1 in the brain. J. Neurochem. 2010, 113, 1240–1251. [Google Scholar] [CrossRef]
- Toriumi, K.; Berto, S.; Koike, S.; Usui, N.; Dan, T.; Suzuki, K.; Miyashita, M.; Horiuchi, Y.; Yoshikawa, A.; Sugaya, Y.; et al. Impairment of methylglyoxal detoxification systems causes mitochondrial dysfunction and schizophrenia-like behavioral deficits. bioRxiv 2020. [Google Scholar] [CrossRef]
- Koike, S.; Ando, C.; Usui, Y.; Kibune, Y.; Nishimoto, S.; Suzuki, T.; Ogasawara, Y. Age-related alteration in the distribution of methylglyoxal and its metabolic enzymes in the mouse brain. Brain Res. Bull. 2019, 144, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and dis-ease. Drug Metabol. Drug Interact. 2009, 23, 1–19. [Google Scholar]
- Wood, T.D.; Guan, Z.; Borders, C.L.; Chen, L.H.; Kenyon, G.L.; McLafferty, F.W. Creatine kinase: Essential arginine residues at the nucleotide binding site identified by chemical modification and high-resolution tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1998, 95, 3362–3365. [Google Scholar] [CrossRef]
- Ishida, Y.; Kayama, T.; Kibune, Y.; Nishimoto, S.; Koike, S.; Suzuki, T.; Horiuchi, Y.; Miyashita, M.; Itokawa, M.; Arai, M.; et al. Identification of an argpyrimidine-modified protein in human red blood cells from schizophrenic patients: A possible biomarker for diseases involving carbonyl stress. Biochem. Biophys. Res. Commun. 2017, 493, 573–577. [Google Scholar] [CrossRef]
- Goto, Y.; Yang, C.R.; Otani, S. Functional and Dysfunctional Synaptic Plasticity in Prefrontal Cortex: Roles in Psychiatric Dis-orders. Biol. Psychiatry 2010, 67, 199–207. [Google Scholar] [CrossRef]
- Rimol, L.M.; Hartberg, C.; Nesvåg, R.; Fennema-Notestine, C.; Hagler, D.; Pung, C.J.; Jennings, R.G.; Haukvik, U.K.; Lange, E.; Nakstad, P.H.; et al. Cortical Thickness and Subcortical Volumes in Schizophrenia and Bipolar Disorder. Biol. Psychiatry 2010, 68, 41–50. [Google Scholar] [CrossRef]
- Howes, O.D.; Montgomery, A.J.; Asselin, M.-C.; Murray, R.M.; Valli, I.; Tabraham, P.; Bramon-Bosch, E.; Valmaggia, L.; Johns, L.; Broome, M.; et al. Elevated Striatal Dopamine Function Linked to Prodromal Signs of Schizophrenia. Arch. Gen. Psychiatry 2009, 66, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kegeles, L.S.; Abi-Dargham, A.; Frankle, W.G.; Gil, R.; Cooper, T.B.; Slifstein, M.; Hwang, D.-R.; Huang, Y.; Haber, S.N.; Laruelle, M. Increased Synaptic Dopamine Function in Associative Regions of the Striatum in Schizophrenia. Arch. Gen. Psychiatry 2010, 67, 231–239. [Google Scholar] [CrossRef]
- Adriano, F.; Caltagirone, C.; Spalletta, G. Hippocampal volume reduction in first-episode and chronic schizophrenia: A re-view and meta-analysis. Neuroscientist 2012, 18, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Grac, A.A. Dopamine system dysregulation by the Hippocampus: Implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 2012, 62, 1342–1348. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Girgis, R.R.; Brucato, G.; Moore, H.; Provenzano, F.; Kegeles, L.; Javitt, D.; Kantrowitz, J.; Wall, M.M.; Corcoran, C.M.; et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Mol. Psychiatry 2018, 23, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.M.; Law, A.S.; Ford, J.M.; Mathalon, D.H.; Ahmadi, A.; Damaraju, E.; Bustillo, J.; Belger, A.; Lee, H.J.; Mueller, B.A.; et al. Turner, Disrupted network cross talk, hippo-campal dysfunction and hallucinations in schizophrenia. Schizophr. Res. 2018, 199, 226–234. [Google Scholar] [CrossRef]
- Koike, S.; Kayama, T.; Yamamoto, S.; Komine, D.; Tanaka, R.; Nishimoto, S.; Suzuki, T.; Kishida, A.; Ogasawara, Y. Polysulfides protect SH-SY5Y cells from methylglyoxal-induced toxicity by suppressing protein carbonylation: A possible physiological scavenger for carbonyl stress in the brain. Neurotoxicology 2016, 55, 13–19. [Google Scholar] [CrossRef]
- Heier, M.; Margeirsdottir, H.D.; Torjesen, P.A.; Seljeflot, I.; Stensæth, K.H.; Gaarder, M.; Brunborg, C.; Hanssen, K.F.; Dahl-Jørgensen, K. The advanced glycation end product methylglyoxal-derived hydroimidazolone-1 and early signs of athero-sclerosis in childhood diabetes. Diab. Vasc. Dis. Res. 2015, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Thornalley, P.J.; Rabbani, N. Possible role of methylglyoxal and glyoxalase in arthritis. Biochem. Soc. Trans. 2014, 42, 538–542. [Google Scholar] [CrossRef]
- Ito, K.; Sakata, N.; Nagai, R.; Shirakawa, J.I.; Watanabe, M.; Mimata, A.; Abe, Y.; Yasuno, T.; Sasatomi, Y.; Miyake, K.; et al. High serum level of methylglyoxal-derived AGE, Nδ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine, inde-pendently relates to renal dysfunction. Clin. Exp. Nephrol. 2017, 21, 398–406. [Google Scholar] [CrossRef]
- Wetzels, S.; Vanmierlo, T.; Scheijen, J.L.J.M.; Van Horssen, J.; Amor, S.; Somers, V.; Schalkwijk, C.G.; Hendriks, J.J.A.; Wouters, K. Methylglyoxal-Derived Advanced Glycation Endproducts Accumulate in Multiple Sclerosis Lesions. Front. Immunol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kayama, T.; Arai, M.; Horiuchi, Y.; Kobori, A.; Miyashita, M.; Itokawa, M.; Ogasawara, Y. Characterization of modified proteins in plasma from a subtype of schizophrenia based on carbonyl stress: Protein carbonyl is a possible biomarker of psychiatric disorders. Biochem. Biophys. Res. Commun. 2015, 467, 361–366. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef]
- Belousova, L.V.; Muizhnek, E.L. Kinetics of chemical modification of arginine residues in mitochondrial creatine kinase from bovine heart: Evidence for negative cooperativity. Biochemistry 2004, 69, 455–461. [Google Scholar] [CrossRef]
- Ma, J.; Peng, X.; Zhang, X.; Chen, F.; Wang, M. Dual effects of phloretin and phloridzin on the glycation induced by methyl-glyoxal in model systems. Chem. Res. Toxicol. 2011, 24, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Kaldis, P.; Hemmer, W.; Zanolla, E.; Holtzman, D.; Wallimann, T. ‘Hot Spots’ of creatine kinase localization in brain: Cerebel-lum, Hippocampus and choroid plexus. Dev. Neurosci. 1996, 18, 542–554. [Google Scholar] [CrossRef]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, M.; Aksenova, M.; Butterfield, D.A.; Markesbery, W.R. Oxidative Modification of Creatine Kinase BB in Alzheimer’s Disease Brain. J. Neurochem. 2000, 74, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Becker, I.R.; Scaini, G.; Petronilho, F.; Oses, J.P.; Kaddurah-Daouk, R.; Ceretta, L.B.; Zugno, A.I.; Dal-Pizzol, F.; Quevedo, J.; et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 81, 55–63. [Google Scholar] [CrossRef]
- Zugno, A.I.; Pacheco, F.D.; Budni, J.; de Oliveira, M.B.; Canever, L.; Heylmann, A.S.; Wessler, P.G.; da Rosa Silveira, F.; Mastella, G.A.; Gonçalves, C.L.; et al. Maternal deprivation disrupts mitochondrial energy homeostasis in the brain of rats subjected to keta-mine-induced schizophrenia. Metab. Brain Dis. 2015, 30, 1043–1053. [Google Scholar] [CrossRef]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef]
- Ferreira, N.; Saraiva, M.J.; Almeida, M.R. Uncovering the Neuroprotective Mechanisms of Curcumin on Transthyretin Amy-loidosis. Int. J. Mol. Sci. 2019, 20, 1287. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Cardoso, I.; Domingues, M.R.; Vitorino, R.; Bastos, M.; Bai, G.; Saraiva, M.J.; Almeida, M.R. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009, 583, 3569–3576. [Google Scholar] [CrossRef]

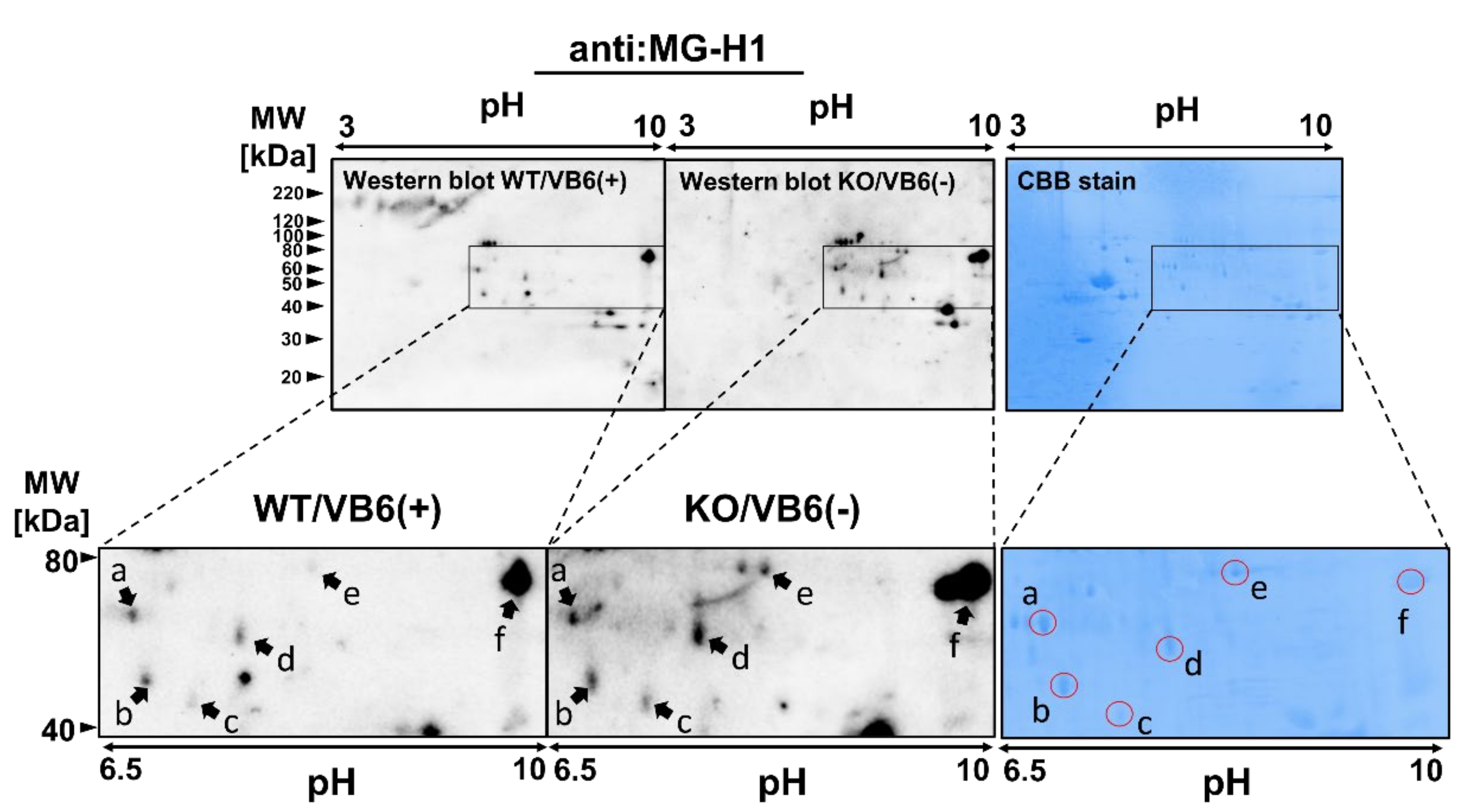

| Spot | Accession No. | Protein Name | Coverage | Peptide Identification | Modified Residue |
|---|---|---|---|---|---|
| a | P80318 | T-complex protein 1 | 55.23% | GASKEILSEVER* | R389 |
| P68368 | Tubulin alpha-4A chain | 32.14% | CDPR*HGKYMACCLLYR* | R308, R320 | |
| b | P17183 | Gamma enolase | 30.41% | QR*YLGKGVLK | R56 |
| c | P30275 | Creatine kinase U-type, mitochondrial | 39.23% | R*LER*GQDIR403 | R400, R403 |
| d | O35098 | CRMP4 | 32.69% | VVLEDGNLLVTPGAGR* | R467 |
| e | Q99MK8 | Beta-adrenergic receptor kinase 1 | 27.00% | YFYLFPNRLEWR* | R591 |
| f | O88935 | Synapsin-1 | 69.69% | QR*QAGAPQATR* | R556, R565 |
| Accession No. | Protein Name | Coverage | Peptide Identification | Modified Residue |
|---|---|---|---|---|
| P30275 | Creatin kinase U-type, mitochondrial | 69.38% | 1MAGPFSRLLSAR*PGLR*LLALAGAGSLT AGILLR*PESVGAAAAER44 | R12, R16, R33 |
| 46R*LYPPSAEYPDLR*K59 | R46, R58 | |||
| 45RRLYPPSAEYPDLRKHNNCMASHLTPAV YAR*LCDKTTPTGWTLDQCIQTGVDNPGHPFIK104 | R75 | |||
| 152SGYFDERYVLSSR*VR*TGR*169 | R164, R166, R169 | |||
| 400R*LER*GQDIR408 | R400, R403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, S.; Toriumi, K.; Kasahara, S.; Kibune, Y.; Ishida, Y.-i.; Dan, T.; Miyata, T.; Arai, M.; Ogasawara, Y. Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits. Antioxidants 2021, 10, 574. https://doi.org/10.3390/antiox10040574
Koike S, Toriumi K, Kasahara S, Kibune Y, Ishida Y-i, Dan T, Miyata T, Arai M, Ogasawara Y. Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits. Antioxidants. 2021; 10(4):574. https://doi.org/10.3390/antiox10040574
Chicago/Turabian StyleKoike, Shin, Kazuya Toriumi, Sakura Kasahara, Yosuke Kibune, Yo-ichi Ishida, Takashi Dan, Toshio Miyata, Makoto Arai, and Yuki Ogasawara. 2021. "Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits" Antioxidants 10, no. 4: 574. https://doi.org/10.3390/antiox10040574
APA StyleKoike, S., Toriumi, K., Kasahara, S., Kibune, Y., Ishida, Y.-i., Dan, T., Miyata, T., Arai, M., & Ogasawara, Y. (2021). Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits. Antioxidants, 10(4), 574. https://doi.org/10.3390/antiox10040574