Alterations of Endogenous Hormones, Antioxidant Metabolism, and Aquaporin Gene Expression in Relation to γ-Aminobutyric Acid-Regulated Thermotolerance in White Clover
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Endogenous GABA and Phytohormones Content
2.3. Measurement of Water Status and Photosynthetic Parameters
2.4. Determination of Antioxidant Metabolism
2.5. Total RNA Extraction and qRT-PCR Analysis
2.6. Statistical Analysis
3. Results
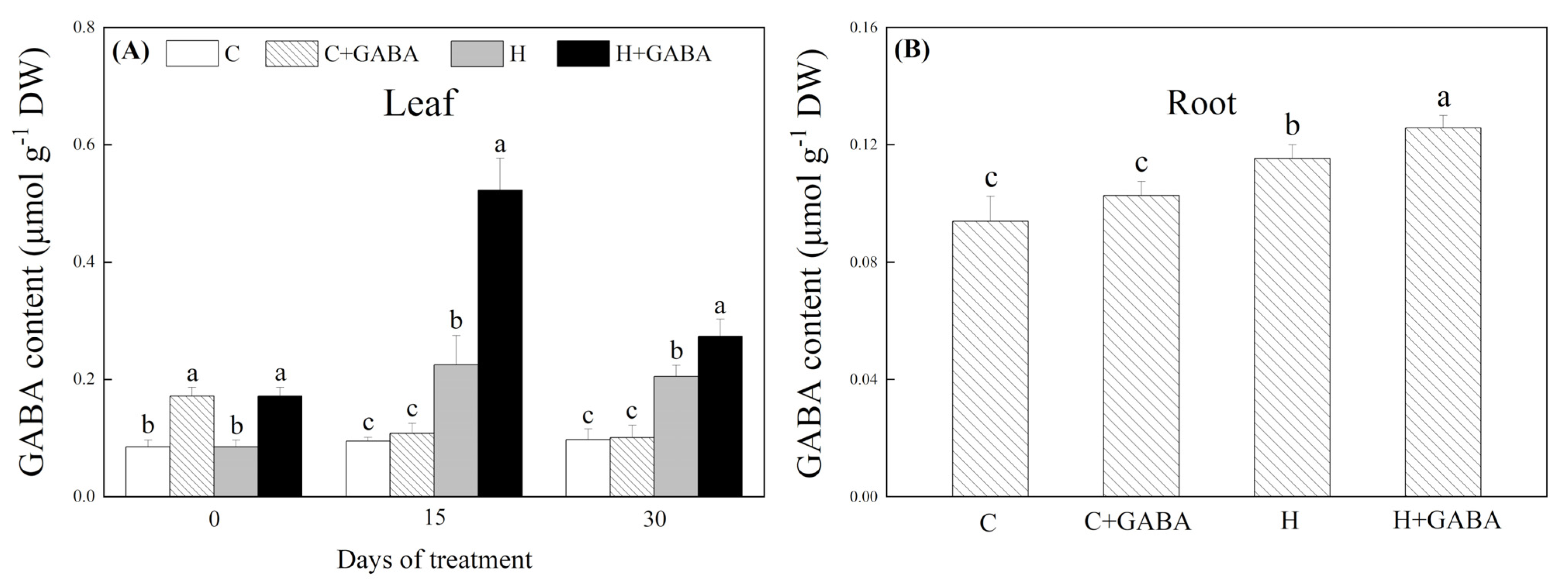
3.1. Changes in Endogenous GABA and Hormones Content
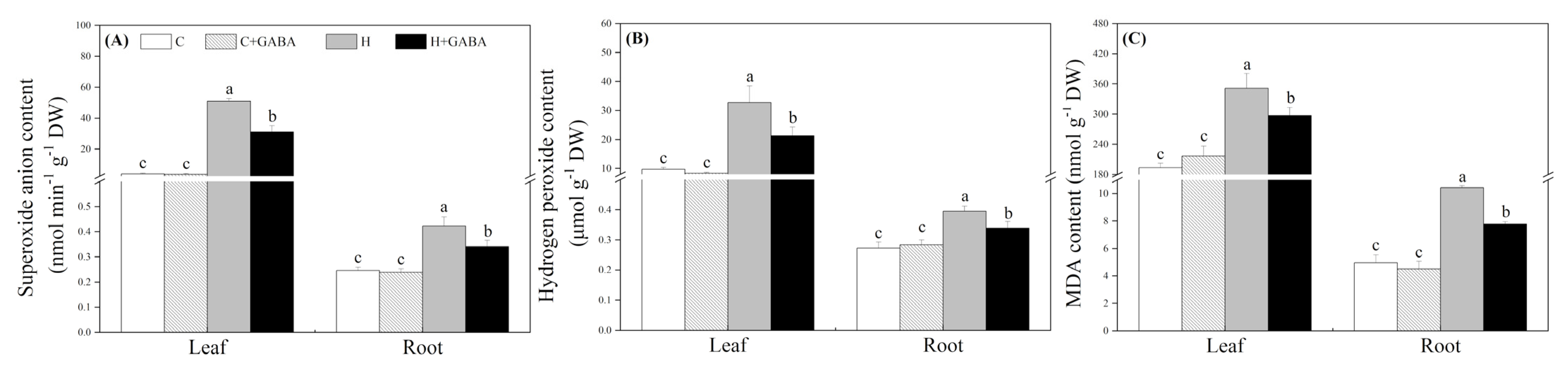
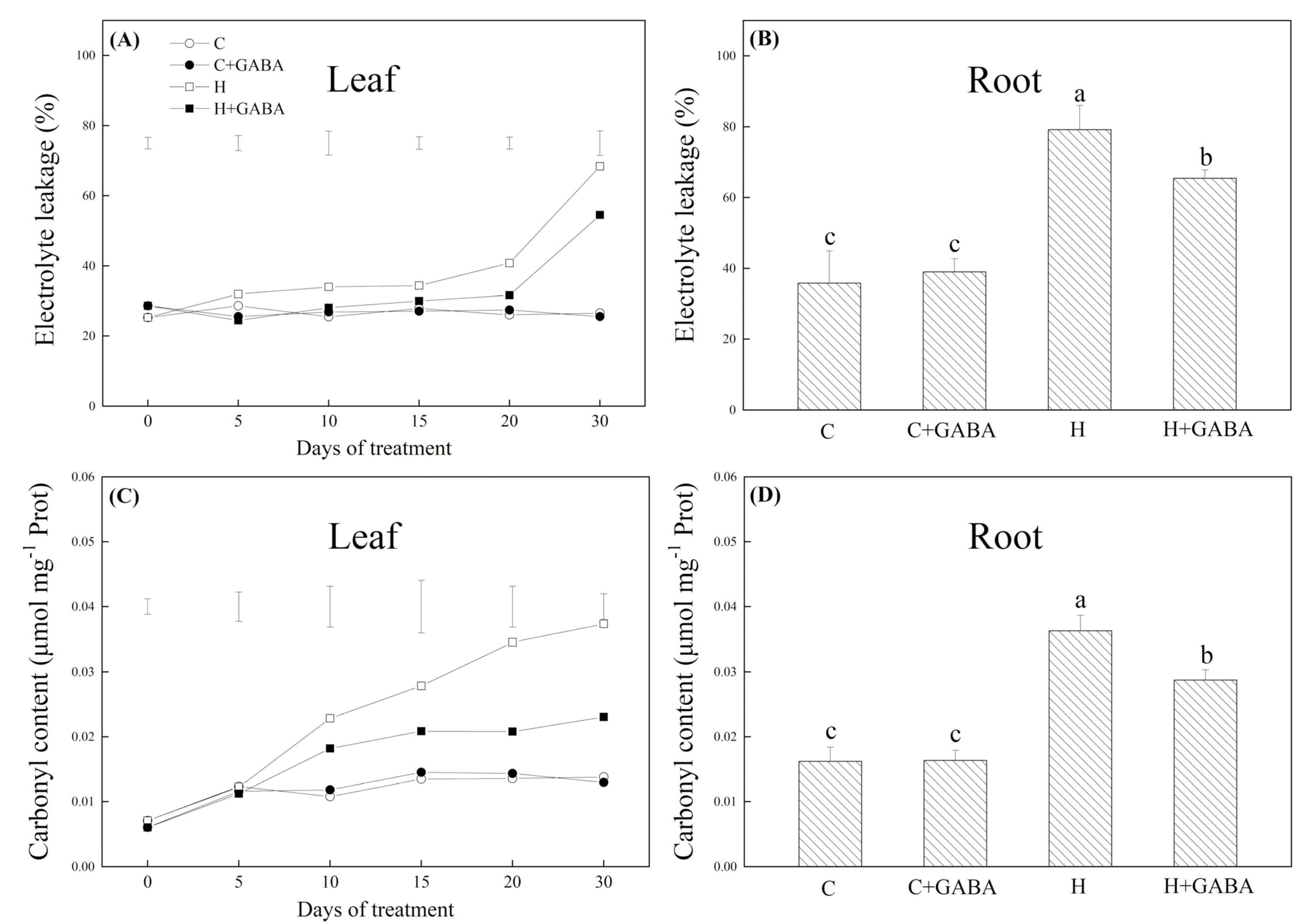
3.2. Effects of GABA on Cell Membrane Stability and Oxidative Damage
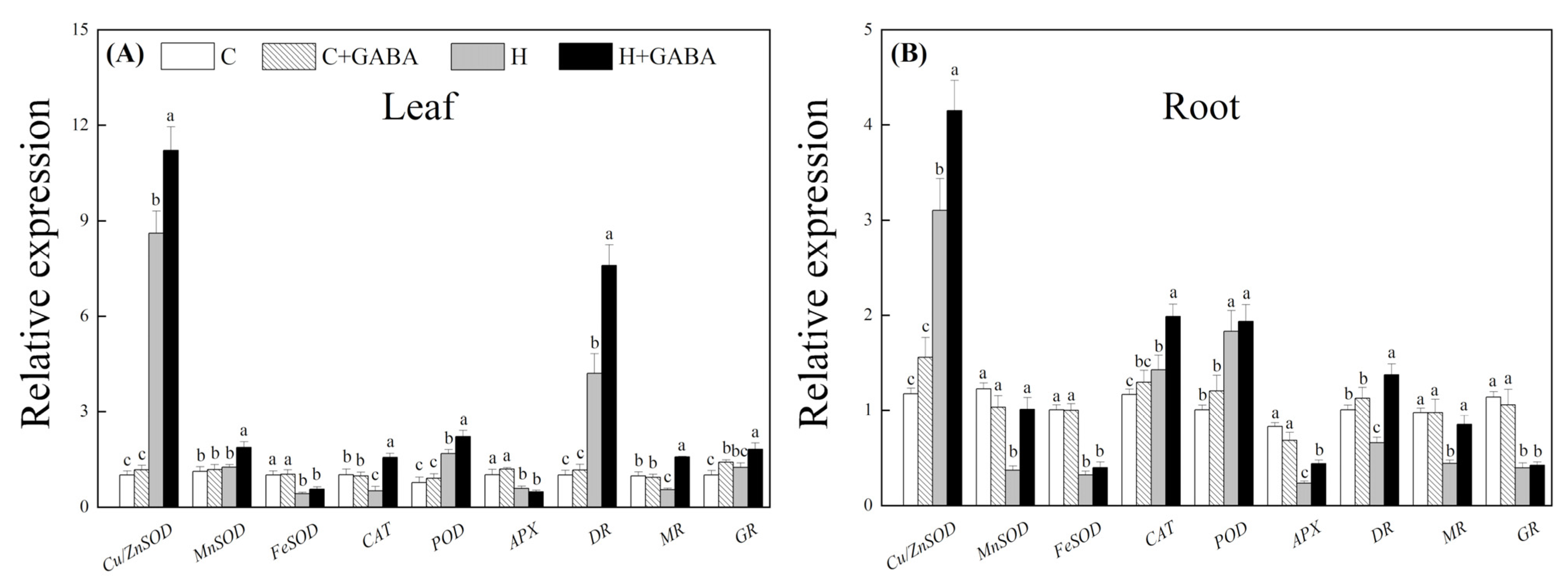
3.3. Effect of GABA on Antioxidant Metabolism
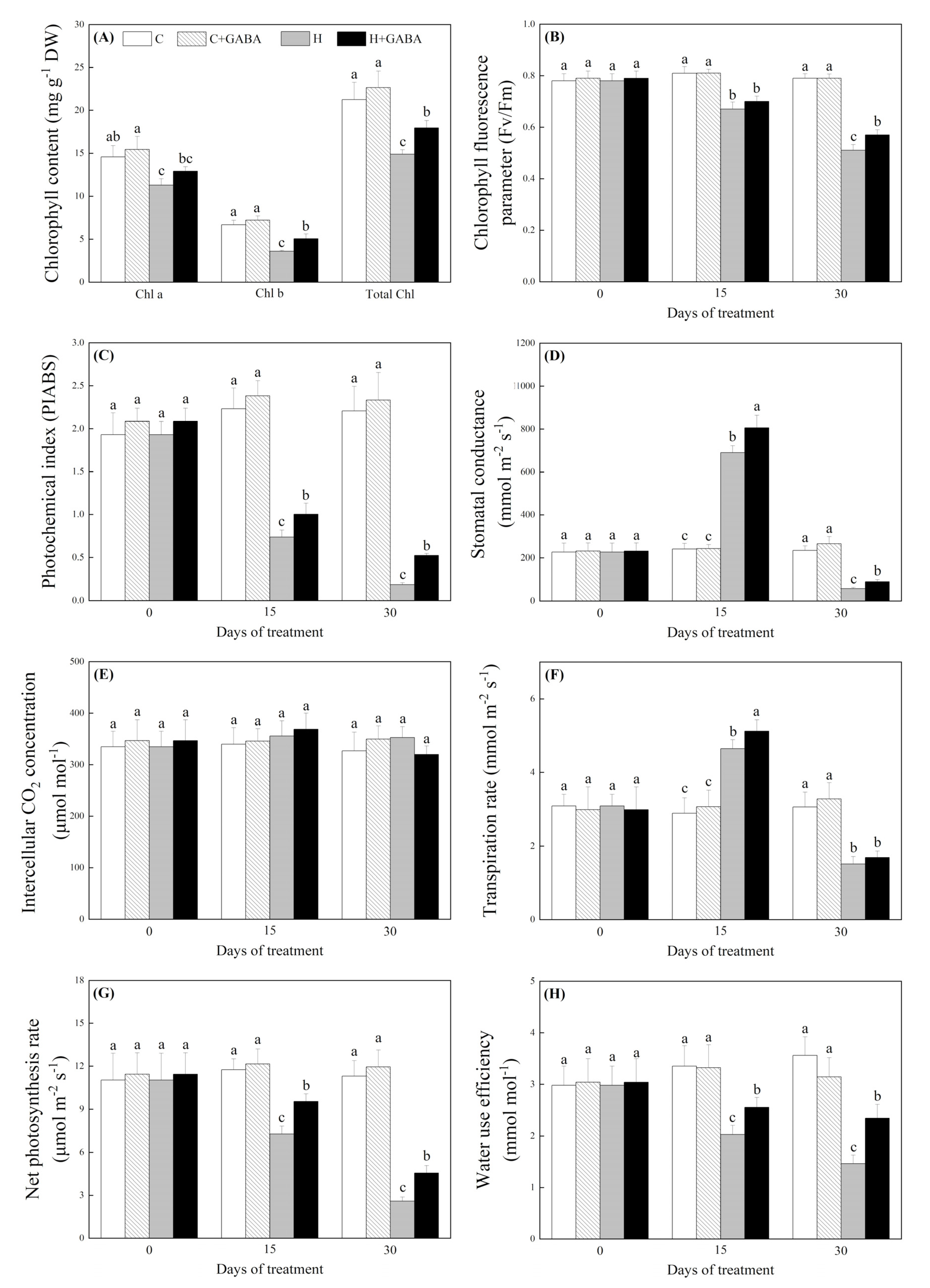
3.4. Effects of GABA on Photosynthesis and Water Status
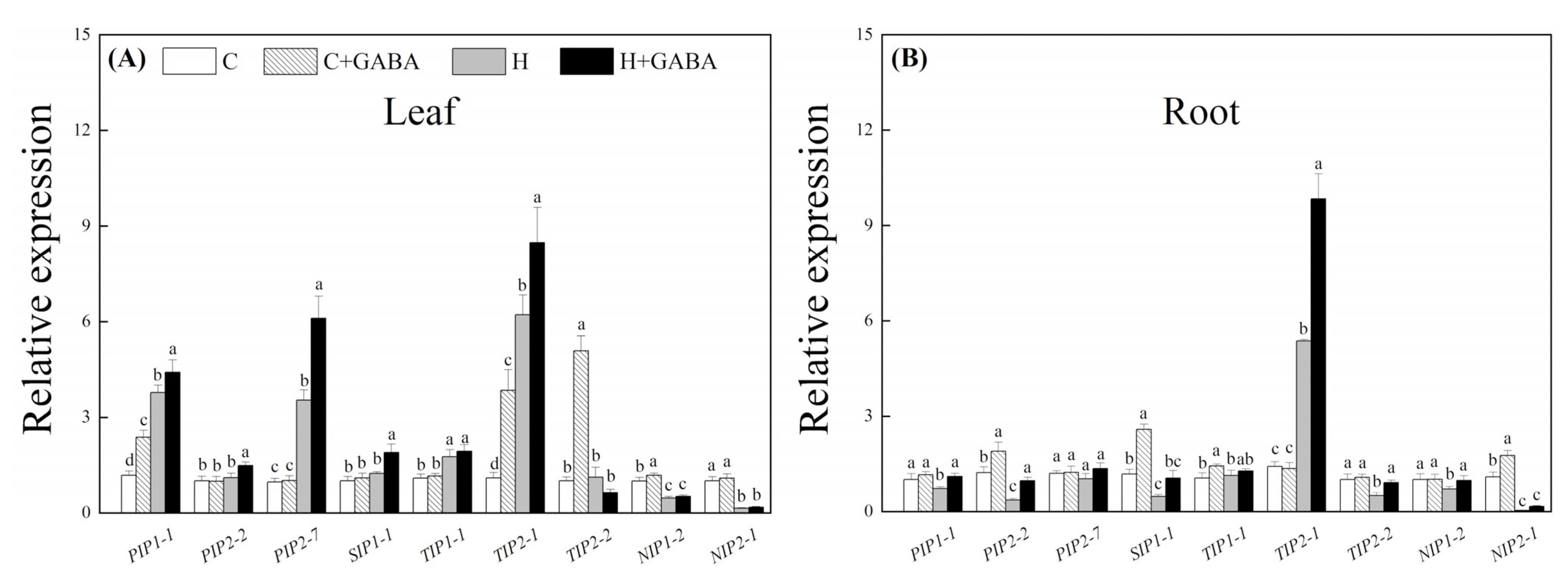
3.5. Effect of GABA on AQP Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweigk, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nat. Cell Biol. 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; (Ted) Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Akter, N.; Islam, M.R. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Huang, B. Alteration of transcripts of stress-protective genes and transcriptional factors by γ-aminobutyric acid (GABA) associated with improved heat and drought tolerance in creeping bentgrass (Agrostis stolonifera). Int. J. Mol. Sci. 2018, 19, 1623. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Li, Z.; Tang, M.Y.; Cheng, B.Z.; Zeng, W.H.; Peng, Y.; Nie, G.; Zhang, X.Q. Metabolic regulation of polyamines and γ-aminobutyric acid in relation to spermidine-induced heat tolerance in white clover. Plant Biol. 2020, 22, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, W.; Cheng, B.; Huang, T.; Peng, Y.; Zhang, X. γ-Aminobutyric acid enhances heat tolerance associated with the change of proteomic profiling in creeping bentgrass. Molecules 2020, 25, 4270. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R.S. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Atique-ur-Rehman; Farooq, O.; Wasaya, A.; Saliq, S.; Mubeen, K. Improved auxin level at panicle initiation stage enhance the heat stress tolerance in rice plants. In Proceedings of the Agronomy Australia Conference, Wagga Wagga, NSW, Australia, 25–29 August 2019. [Google Scholar]
- Valero, D.; Martínez-Romero, D.; Serrano, M.; Riquelme, F. Postharvest gibberellin and heat treatment effects on polyamines, abscisic acid and firmness in lemons. J. Food Sci. 2006, 63, 611–615. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Cytokinin effects on creeping bentgrass response to heat stress. Crop. Sci. 2002, 42, 466–472. [Google Scholar] [CrossRef]
- Bi, A.; Fan, J.; Hu, Z.; Wang, G.; Amombo, E.; Fu, J.; Hu, T. Differential acclimation of enzymatic antioxidant metabolism and photosystem ii photochemistry in tall fescue under drought and heat and the combined stresses. Front. Plant Sci. 2016, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.; Wang, L.; Li, J.; Jiao, Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolás, E.; Torrecillas, A.; Sánchez-Blanco, M. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant. 2003, 46, 203–208. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjo¨vall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [Green Version]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.-J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, F.; Suga, S.; Uemura, T.; Sato, M.H.; Maeshima, M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett. 2005, 579, 5814–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Lin, W.; Cai, W.; Arora, R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 2007, 226, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Feng, Z.J.; Liu, N.; Zhang, G.-W.; Niu, F.-G.; Xu, S.-C.; Gong, Y.-M. Investigation of the AQP family in soybean and the promoter activity of tip2;6 in heat stress and hormone responses. Int. J. Mol. Sci. 2019, 20, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafqat, W.; Jaskani, M.J.; Maqbool, R.; Chattha, W.S.; Ali, Z.; Naqvi, S.A.; Haider, M.S.; Khan, I.A.; Vincent, C.I. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ. Exp. Bot. 2021, 181, 104270. [Google Scholar] [CrossRef]
- Marshall, A.H.; Williams, T.A.; Abberton, M.T.; Michaelson-Yeates, T.P.T.; Olyott, P.; Powell, H.G. Forage quality of white clover (Trifolium repens L.) × Caucasian clover (T. ambiguum M. Bieb.) hybrids and their grass companion when grown over three harvest years. Grass Forage Sci. 2004, 59, 91–99. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Huang, Y.; Li, S.; Zhang, Y.; Wu, P. Optimization of extraction technology of flavonoids from white clover by lonic liquids. Liaoning J. Tradit. Chin. Med. 2018, 45, 1445–1448. [Google Scholar] [CrossRef]
- He, J.; He, S.; Fu, Q.; Lei, H.; Yang, Y. Effects on relative water content, membrane permeability, praline content and chlorophyll content of white clover under high temperature stress. Guizhou Anim. Vet. Med. 2007, 126, 1358–1369. [Google Scholar] [CrossRef] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 357–359. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Rapid and sensitive hormonal profiling of complex plant samples by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Plant Methods 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrs, H.; Weatherley, P. A Re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- McMichael, B.; Burke, J. Metabolic activity of cotton roots in response to temperature. Environ. Exp. Bot. 1994, 34, 201–206. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop. Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat1. Crop. Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence: Correlated with incressed leaves of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Odorant Bind. Chemosens. Proteins 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant to and salt-sensitivity cultivars of cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, B.; Zeng, W.; Liu, Z.; Peng, Y. The transcriptional and post-transcriptional regulation in perennial creeping bentgrass in response to γ-aminobutyric acid (GABA) and heat stress. Environ. Exp. Bot. 2019, 162, 515–524. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Xiang, Z. Effect of γ-aminobutyric acid on photosynthetic characteristics and carbohydrate metabolism under high temperature stress in perennial ryegrass. Acta Prataculturae Sin. 2019, 28, 168–178. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sasanuma, T.; Abe, T. Accumulation of gamma-aminobutyrate (GABA) caused by heat-drying and expression of related genes in immature vegetable soybean (edamame). Breed. Sci. 2013, 63, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Dreher, K.; Callis, J. Ubiquitin, Hormones and biotic stress in plants. Ann. Bot. 2007, 99, 787–822. [Google Scholar] [CrossRef]
- Lancien, M.; Roberts, M.R. Regulation of arabidopsis thaliana 14-3-3 gene expression by gamma-aminobutyric acid. Plant Cell Environ. 2006, 29, 1430–1436. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, B.; Gu, W.; Li, Z.; Mao, J.; Guo, J.; Shao, R.; Yang, Q. γ-Aminobutyric acid on oxidative damage and endogenous hormones in maize seedling roots under salt stress. Chin. J. Pestic. Sci. 2018, 20, 607–617. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Maruyama, K.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Tran, L.-S.P.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 2004, 39, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Zhang, J.; Katerji, N.; Bethenod, O.; Palmer, S.; Davies, W.J. Xylem ABA controls the stomatal conductance of field-grown maize subjected to soil compaction or soil drying. Plant Cell Environ. 1992, 15, 193–197. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, Q.; Lin, J.; Deng, K.; Zhao, X.; Tang, N.; Liu, X. Arabidopsis cryptochrome-1 restrains lateral roots growth by inhibiting auxin transport. J. Plant Physiol. 2010, 167, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Zeng, H.; Cai, Q.; Zhou, X.; Yin, C. Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J. Plant Growth Regul. 2016, 35, 366–376. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Mohapatra, P.K.; Peng, S. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front. Plant Sci. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.; Nijo, N.; Pospíšil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality control of photosystem II: Reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cheng, B.; Zeng, W.; Zhang, X.; Peng, Y. Proteomic and metabolomic profilings reveal crucial functions of γ-aminobutyric acid in regulating ionic, water, and metabolic homeostasis in creeping bentgrass under salt stress. J. Proteome Res. 2020, 19, 769–780. [Google Scholar] [CrossRef]
- Tang, M.; Li, Z.; Luo, L.; Cheng, B.; Zhang, Y.; Zeng, W.; Peng, Y. Nitric oxide signal, nitrogen metabolism, and water balance affected by γ-aminobutyric acid (GABA) in relation to enhanced tolerance to water stress in creeping bentgrass. Int. J. Mol. Sci. 2020, 21, 7460. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Du, H. Effectsof heat stress on differential expression of proteins in leaves and roots of hybrid bermudagrass (Cynodon transvaalensis × Cynodon dactylon). J. Shanghai Jiaotong Univ. (Agric.) 2016, 34, 62–68. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Al-Huqail, A.A.; Alqahtani, M.A.; Ahmad, P. Silicon is dependent on hydrogen sulphide to improve boron toxicity tolerance in pepper plants by regulating the ASA-GSH cycle and glyoxalase system. Chemosphere 2020, 257, 127241. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, X.; Xu, X.; Gao, Y.; Wen, G.; Zhang, R.; Wang, Y. Responses of anti-oxidant enzymes and the ascorbate-glutathione cycle to heat, drought, and synergistic stress in Phyllostachys edulis seedlings. J. Zhejiang AF Univ. 2018, 35, 268–276. [Google Scholar] [CrossRef]
- Dong, L.; He, Y.; Wang, Y.; Dong, Z. Research progress on application of superoxide dismutase (SOD). J. Agric. Sci. Technol. 2013, 15, 53–58. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul. 2005, 47, 17–28. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, A.K.; Upadhyay, A.K.; Gautam, A.; Ranjan, R.; Srikishna, S.; Sahu, N.; Behera, S.K.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zheng, G.; Guan, J.; Li, G. Changes of root activity and membrane permeability under drought stress in maize. Acta Agric. Boreall—Sin. 2002, 2, 21–23. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Peng, Y.; Zhang, Y. Adaptability to abiotic stress regulated by γ-aminobutyric acid in relation to alterations of endogenous polyamines and organic metabolites in creeping bentgrass. Plant Physiol. Biochem. 2020, 157, 185–194. [Google Scholar] [CrossRef]
- Regon, P.; Panda, P.; Kshetrimayum, E.; Panda, S.K. Genome-wide comparative analysis of tonoplast intrinsic protein (TIP) genes in plants. Funct. Integr. Genom. 2014, 14, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.L.; Bhave, M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Funct. Integr. Genom. 2007, 8, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hou, J.; Zhang, Y.; Zeng, W.; Cheng, B.; Hassan, M.J.; Zhang, Y.; Pu, Q.; Peng, Y. Spermine regulates water balance associated with Ca2+-dependent aquaporin (TrTIP2-1, TrTIP2-2 and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol. 2020, 61, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Grondin, A.; Mauleon, R.; Vadez, V.; Henry, A. Root aquaporins contribute to whole plant water fluxes under drought stress in rice (Oryza sativa L.). Plant Cell Environ. 2016, 39, 347–365. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Li, Z.; Peng, D.; Zhang, X.; Peng, Y.; Chen, M.; Ma, X.; Huang, L.; Yan, Y. Na+ induces the tolerance to water stress in white clover associated with osmotic adjustment and aquaporins-mediated water transport and balance in root and leaf. Environ. Exp. Bot. 2017, 144, 11–24. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant Aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwiazek, J.J.; Xu, H.; Tan, X.; Rodenas, A.N.; Morte, A. Significance of oxygen transport through aquaporins. Sci. Rep. 2017, 7, 40411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1574–1582. [Google Scholar] [CrossRef]
- Lin, W.; Peng, Y.; Li, G.; Arora, R.; Tang, Z.; Su, W.; Cai, W. Isolation and functional characterization of PgTIP1, a hormone-autotrophic cells-specific tonoplast aquaporin in ginseng. J. Exp. Bot. 2007, 58, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt Stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving plant stress tolerance and yield production: Is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009, 181, 651–661. [Google Scholar] [CrossRef]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Ribas-Carbo, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.G.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef]
- Sade, N.; Gallé, A.; Flexas, J.; Lerner, S.; Peleg, G.; Yaaran, A.; Moshelion, M. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 2014, 239, 357–366. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Kang, D.; Zeng, W.; Jawad Hassan, M.; Peng, Y.; Zhang, X.; Zhang, Y.; Feng, G.; Li, Z. Alterations of Endogenous Hormones, Antioxidant Metabolism, and Aquaporin Gene Expression in Relation to γ-Aminobutyric Acid-Regulated Thermotolerance in White Clover. Antioxidants 2021, 10, 1099. https://doi.org/10.3390/antiox10071099
Qi H, Kang D, Zeng W, Jawad Hassan M, Peng Y, Zhang X, Zhang Y, Feng G, Li Z. Alterations of Endogenous Hormones, Antioxidant Metabolism, and Aquaporin Gene Expression in Relation to γ-Aminobutyric Acid-Regulated Thermotolerance in White Clover. Antioxidants. 2021; 10(7):1099. https://doi.org/10.3390/antiox10071099
Chicago/Turabian StyleQi, Hongyin, Dingfan Kang, Weihang Zeng, Muhammad Jawad Hassan, Yan Peng, Xinquan Zhang, Yan Zhang, Guangyan Feng, and Zhou Li. 2021. "Alterations of Endogenous Hormones, Antioxidant Metabolism, and Aquaporin Gene Expression in Relation to γ-Aminobutyric Acid-Regulated Thermotolerance in White Clover" Antioxidants 10, no. 7: 1099. https://doi.org/10.3390/antiox10071099





