UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. UHPLC-HRMS and MS/MS Parameters
2.3. Radical Scavenging Capacity: DPPH and ABTS Tests
2.4. Fe(III) Reducing Power
2.5. Determination of Total Phenols
2.6. Determination of Total Flavonoids
2.7. Cell Culture, Cytotoxicity and Intracellular ROS Assessment
3. Results and Discussion
3.1. Chemical Composition of F. sylvatica Leaf Methanolic Extract
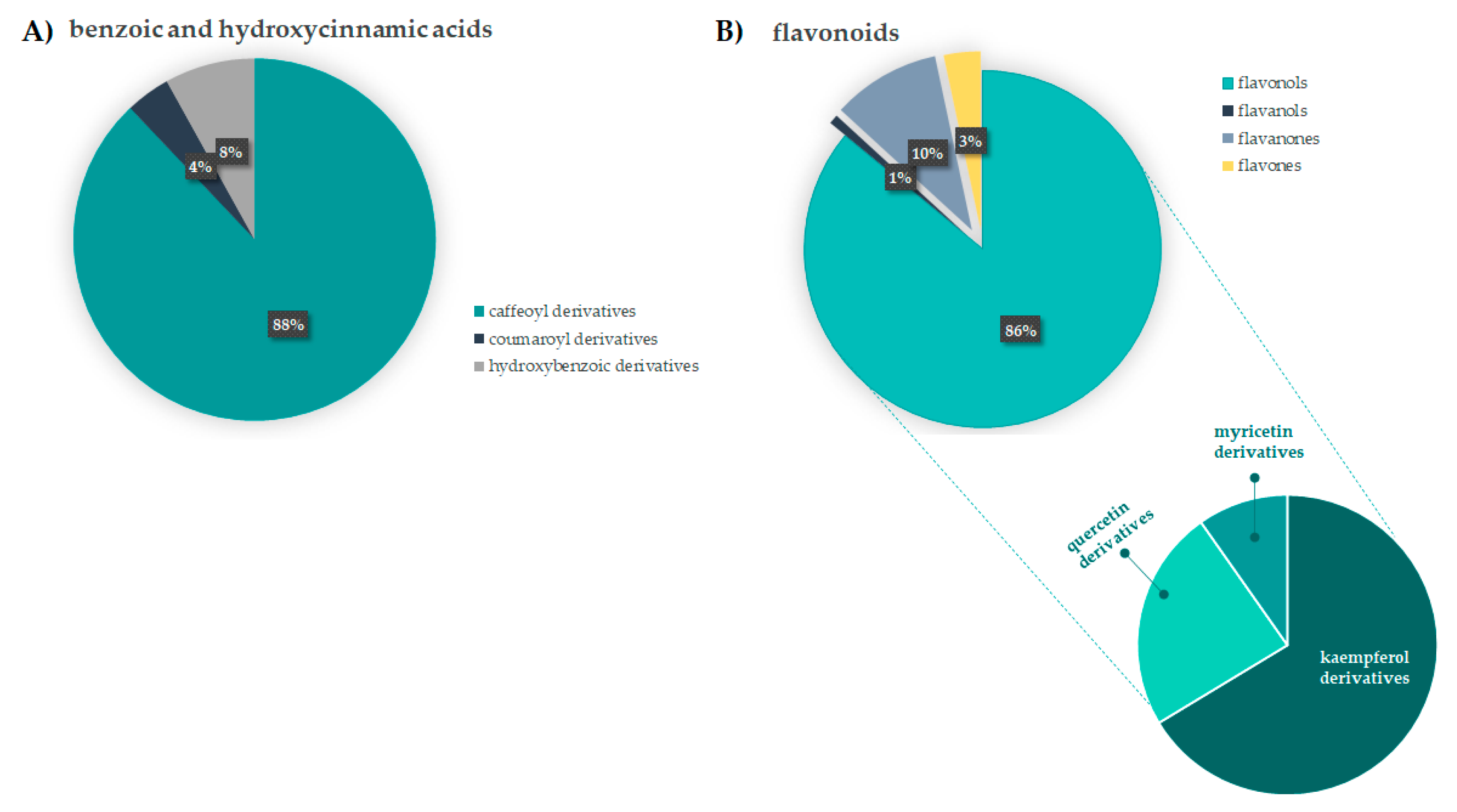
3.1.1. Benzoic and Hydroxycinnamic Acids Derivatives
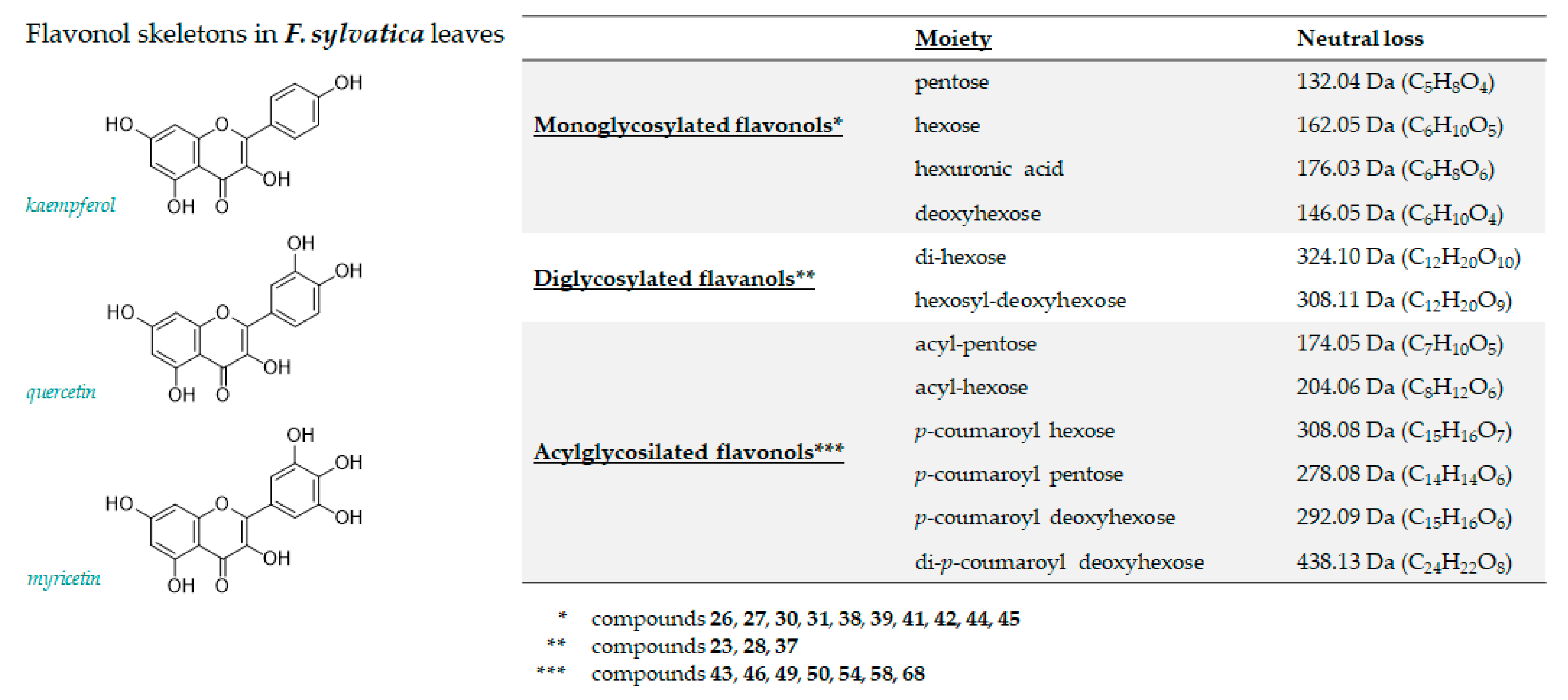
3.1.2. Flavonoids
3.1.3. Lignans
3.1.4. Fatty Acids
3.1.5. Other Minor Compounds
3.2. Relative Quantitation of F. sylvatica Leaf Chemical Constituents
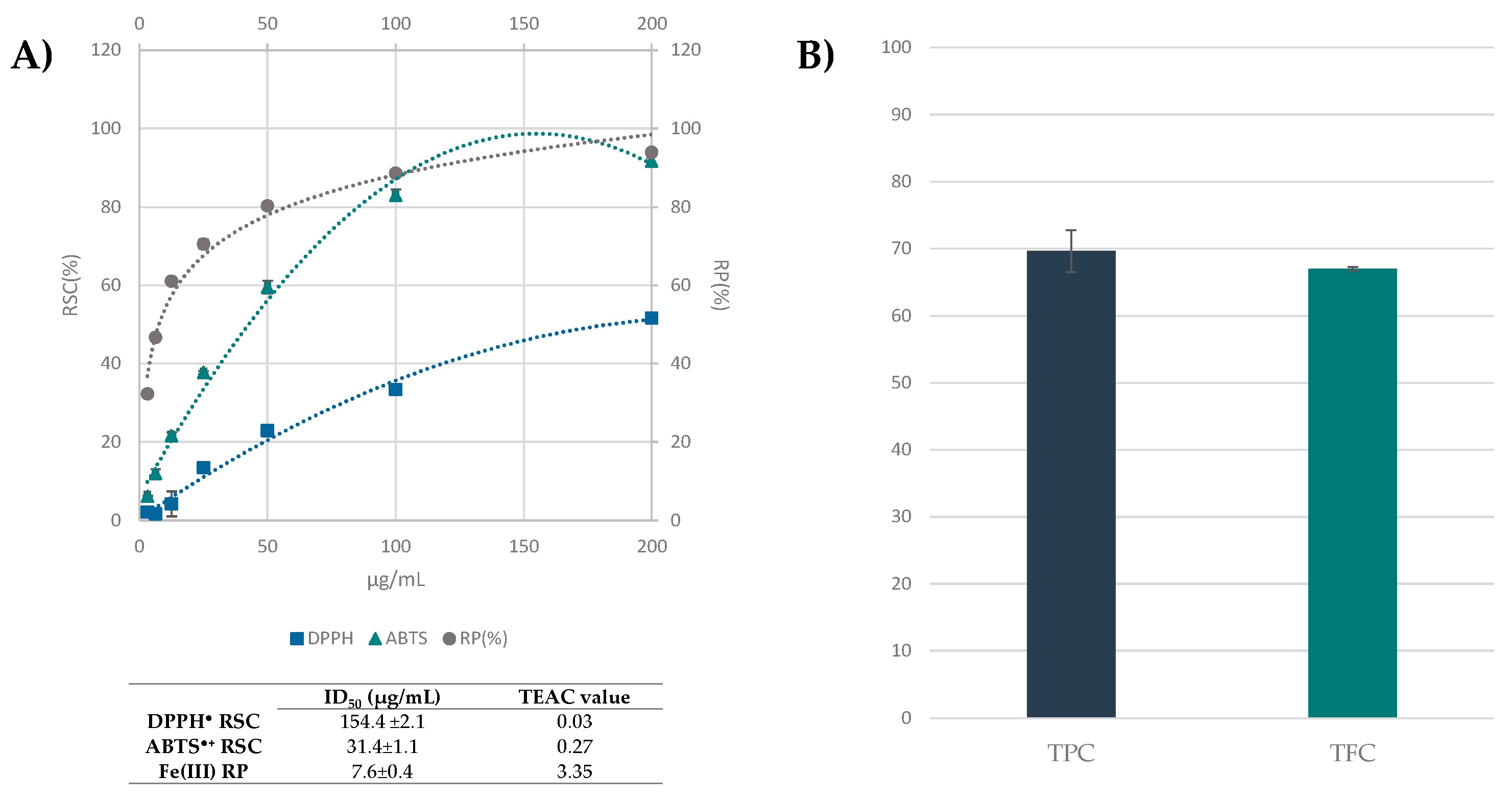
3.3. Beech Leaf Alcoholic Extract Showed Antioxidant Efficacy in Cell-Free Assays
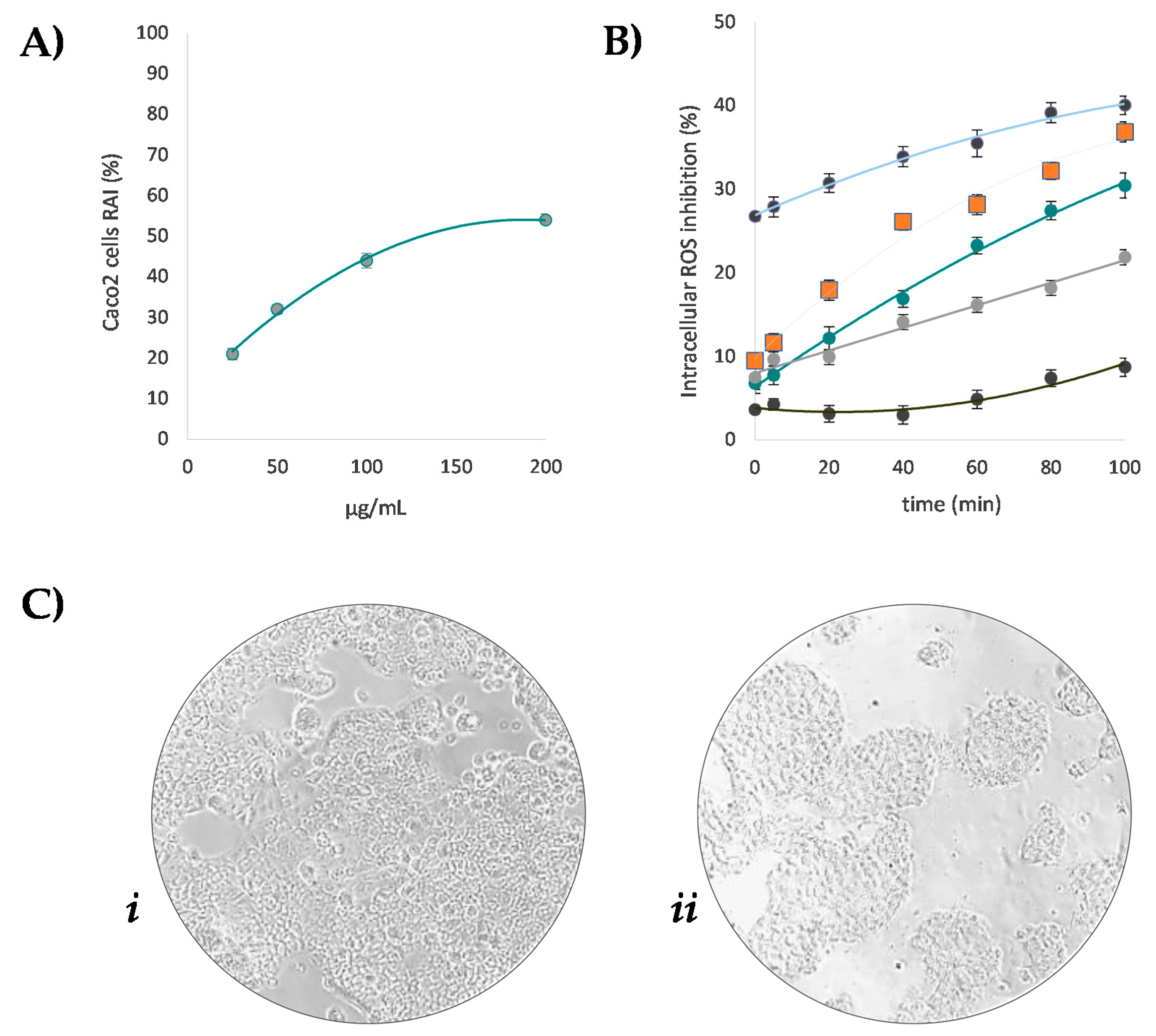
3.4. Beech Leaf Alcoholic Extract Decreased Intracellular ROS in Caco-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-Guided Recovery of Bioactive Flavonol Compounds from Greco di Tufo Vine Leaves. Molecules 2019, 24, 3630. [Google Scholar] [CrossRef] [Green Version]
- Piccolella, S.; Bianco, A.; Crescente, G.; Santillo, A.; Chieffi Baccari, G.; Pacifico, S. Recovering Cucurbita pepo cv. ‘Lungo Fiorentino’ Wastes: UHPLC-HRMS/MS metabolic profile, the basis for establishing their nutra- and cosmeceutical valorisation. Molecules 2019, 24, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujor, O.-C.; Talmaciu, I.A.; Volf, I.; Popa, V.I. Biorefining to recover aromatic compounds with biological properties. Tappi J. 2015, 14, 187–193. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Value-Added Compound Recovery from Invasive Forest for Biofunctional Applications: Eucalyptus Species as a Case Study. Molecules 2020, 25, 4227. [Google Scholar] [CrossRef] [PubMed]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life cycle assessment of polyphenols extraction processes from waste biomass. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Vek, V.; Oven, P.; Poljanšek, I. Review on Lipophilic and Hydrophilic Extractives in Tissues of Common Beech. Drv. Ind. 2016, 67, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Nebehaj, E.; Albert, L. The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J. Chromatogr. A. 2015, 1393, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Dwiecki, K.; Borzyszkowski, W.; Turski, M.; Rudzińska, M.; Nogala-Kalucka, M. Physicochemical characteristics of the cold-pressed oil obtained from seeds of Fagus sylvatica L. Food Chem. 2017, 225, 239–245. [Google Scholar] [CrossRef]
- Cadahía, E.; de Simón, M.B.F.; Aranda, I.; Sanz, M.; Sanchez-Gomez, D.; Pinto, E. Non-targeted Metabolomic Profile of Fagus Sylvatica L. Leaves using Liquid Chromatography with Mass Spectrometry and Gas Chromatography with Mass Spectrometry. Phytochem. Anal. 2014, 26, 171–182. [Google Scholar] [CrossRef]
- Pirvu, L.; Grigore, A.; Bubueanu, C.; Draghici, E.M. Comparative analytical and antioxidant activity studies on a series of Fagus sylvatica L. leaves extracts. J. Planar Chromatogr. Mod. TLC 2013, 26, 237–242. [Google Scholar] [CrossRef]
- Romussi, G.; Bignardi, G.; Falsone, G.; Wendisch, D. Triterpene saponins from Fagus sylvatica L. Arch. Pharm. 1987, 320, 153–158. [Google Scholar] [CrossRef]
- Nicu, A.I.; Pirvu, L.; Stoian, G.; Vamanu, A. Antibacterial activity of ethanolic extracts from Fagus sylvatica L. and Juglans regia L. leaves. Farmacia 2018, 66, 483–486. [Google Scholar] [CrossRef]
- Pirvu, L.; Nicu, A.I.; Schiopu, S.; Coprean, D. Gastroprotective potential of Fagus sylvatica leaves extracts on stress-induced ulcer model on rats. Sci. Bull. Series F. Biotechnol. 2016, 20, 293–299. [Google Scholar]
- Frédérich, M.; Marcowycz, A.; Cieckiewicz, E.; Mégalizzi, V.; Angenot, L.; Kiss, R. In Vitro Anticancer Potential of Tree Extracts from the Walloon Region Forest. Planta Med. 2009, 75, 1634–1637. [Google Scholar] [CrossRef]
- Jehnes, S.; Betz, G.; Bahnweg, G.; Haberer, K.; Sandermann, H.; Rennenberg, H. Tree internal signalling and defence reactions under ozone exposure in sun and shade leaves of European beech (Fagus sylvatica L.) trees. Plant Biol. 2007, 9, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal variation in phenolic composition, antioxidant and anti-inflammatory activities of Calamintha nepeta (L.). Savi. Int. J. Food Res. 2014, 69, 121–132. [Google Scholar] [CrossRef]
- Ferrara, L.; Dosi, R.; Di Maro, A.; Guida, V.; Cefarelli, G.; Pacifico, S.; Mastellone, C.; Fiorentino, A.; Rosati, A.; Parente, A. Nutritional values, metabolic profile and radical scavenging capacities of wild asparagus (A. acutifolius L.). J. Food Compos. Anal. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant Properties of Sour Cherries (Prunus cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, D.C.; Muntean, D.-L.; Crișan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Tálos-Nebehaj, E.; Albert, L. Leaf polyphenols as indicators of climatic adaptation of Beech (Fagus sylvatica L.)—An HPLC-MS/MS via MRM approach. Int. Labmate 2017, 42, 12–14. [Google Scholar]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Potter, C.; Jones, D. Polyphenolic Profiling of Forestry Waste by UPLC-HDMSE. Processes 2020, 8, 1411. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Freire, M.S.; Ramírez-López, C.; Fernández-Moya, J.; González-Álvarez, J. Valorization of residual walnut biomass from forest management and wood processing for the production of bioactive compounds. Biomass Convers. Biorefinery 2021, 11, 609–618. [Google Scholar] [CrossRef]
- Gascón, S.; Jiménez-Moreno, N.; Jiménez, S.; Quero, J.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Nutraceutical composition of three pine bark extracts and their antiproliferative effect on Caco-2 cells. J. Funct. Food. 2018, 48, 420–429. [Google Scholar] [CrossRef]
- Trivelato, P.; Mayer-Laigle, C.; Barakat, A.; Fulcrand, H.; Aouf, C. Douglas bark dry fractionation for polyphenols isolation: From forestry waste to added value products. Ind. Crop. Prod. 2016, 86, 12–15. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Poggetto, F.D.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef]
- Gohari, A.; Saeidnia, S.; Mollazadeh, K.; Yassa, N.; Malmir, M.; Shahverdi, A. Isolation of a new quinic acid derivative and its antibacterial modulating activity. DARU J. Pharm. Sci. 2010, 18, 69–73. [Google Scholar]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Ferreres, F.; Silva, B.M.; Andrade, P.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Rawat, P.; Kumar, M.; Sharan, K.; Chattopadhyay, N.; Maurya, R. Ulmosides A and B: Flavonoid 6-C-glycosides from Ulmuswallichiana, stimulating osteoblast differentiation assessed by alkaline phosphatase. Bioorganic Med. Chem. Lett. 2009, 19, 4684–4687. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, N.; Zhang, H.-F.; Wang, Q.-Q.; Yu, Q.; Wang, F.; Dai, Y.-H.; Wang, D.; Liu, D.-C. Simultaneous quantitative analysis of 11 flavonoid derivatives with a single marker in persimmon leaf extraction and evaluation of their myocardium protection activity. J. Nat. Med. 2019, 73, 404–418. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-C.; Chen, C.-K.; Tsai, S.-F.; Lee, S.-S. Triterpenes as α-glucosidase inhibitors from Fagus hayatae. Phytochemistry 2012, 74, 206–211. [Google Scholar] [CrossRef]
- Hong, S.S.; Jeong, W.; Kim, J.K.; Kwon, J.G.; Lee, J.Y.; Ahn, E.-K.; Oh, J.; Seo, D.W.; Oh, J.S. Neolignan inhibitors of anti-gen-induced degranulation in RBL-2H3 cells from the needles of Pinus thunbergii. Fitoterapia 2014, 99, 347–351. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.-D.; Chen, J.-G.; Li, C.-S. Two new rhamnopyranosides of neolignans from Sanguisorba officinalis. J. Asian Nat. Prod. Res. 2012, 14, 171–175. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Yang, M.; Huang, X.; Zhong, G.; Sang, Z.; Dong, J.; Shu, J.; Liu, J.; Zhang, R.; et al. Structures and biological evaluation of phenylpropanoid derivatives from Murraya koenigii. Bioorganic Chem. 2019, 86, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Matzke, K.; Riederer, M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst, Quercus robur L., and Fagus sylvatica L. Planta 1991, 185, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-Y.; Yang, Y.-F.; Li, K. Analysis of Hydroxy Fatty Acids from the Pollen of Brassica campestris L. var. oleifera DC. by UPLC-MS/MS. J. Pharm. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Li, W.; Koike, K.; Liu, L.; Fu, X.; Lin, L.; Chen, Y.; Nikaido, T. Two New Galloylglucosides from the Leaves of Mallotus furetianus. Chem. Pharm. Bull. 2004, 35, 776–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.J.; Wei, H.L.; Qi, L.W.; Chen, J.; Ren, M.T.; Li, P. Characterization and identification of saponins in Achyranthes bidentata by rapid-resolution liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 2010, 24, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Hofmann, T. Structure and Antioxidant Efficiency of Beech (Fagus sylvatica L.) Bark Polyphenols Unraveled by High-Performance Liquid Chromatography/Photodiode Array Detection/Multistage Electrospray Mass Spectrometry and Chemometrics. In Polyphenols in Plants; Watson, R., Ed.; Elsevier: London, UK, 2019; pp. 83–109. [Google Scholar]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, D.; Sak, K.; Tuli, H.S.; Sharma, A. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef]
- Hofmann, T.; Nebehaj, E.; Stefanovits-Bányai, É.; Albert, L. Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Ind. Crop. Prod. 2015, 77, 375–381. [Google Scholar] [CrossRef]
- Pirvu, L.; Armatu, A.; Bubueanu, C.; Pintilie, G.; Nita, S. Obtaining and chemical characterization of some vegetal extracts with corrosion-scaling inhibition properties. Part I. Fagus sylvatica L. and Alii cepae bulbus extracts. Rom. Biotechnol. Lett. 2010, 15, 5683–5689. [Google Scholar]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Han, M.; Zhang, X. Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn. Mag. 2016, 12, S237–S244. [Google Scholar] [CrossRef] [Green Version]
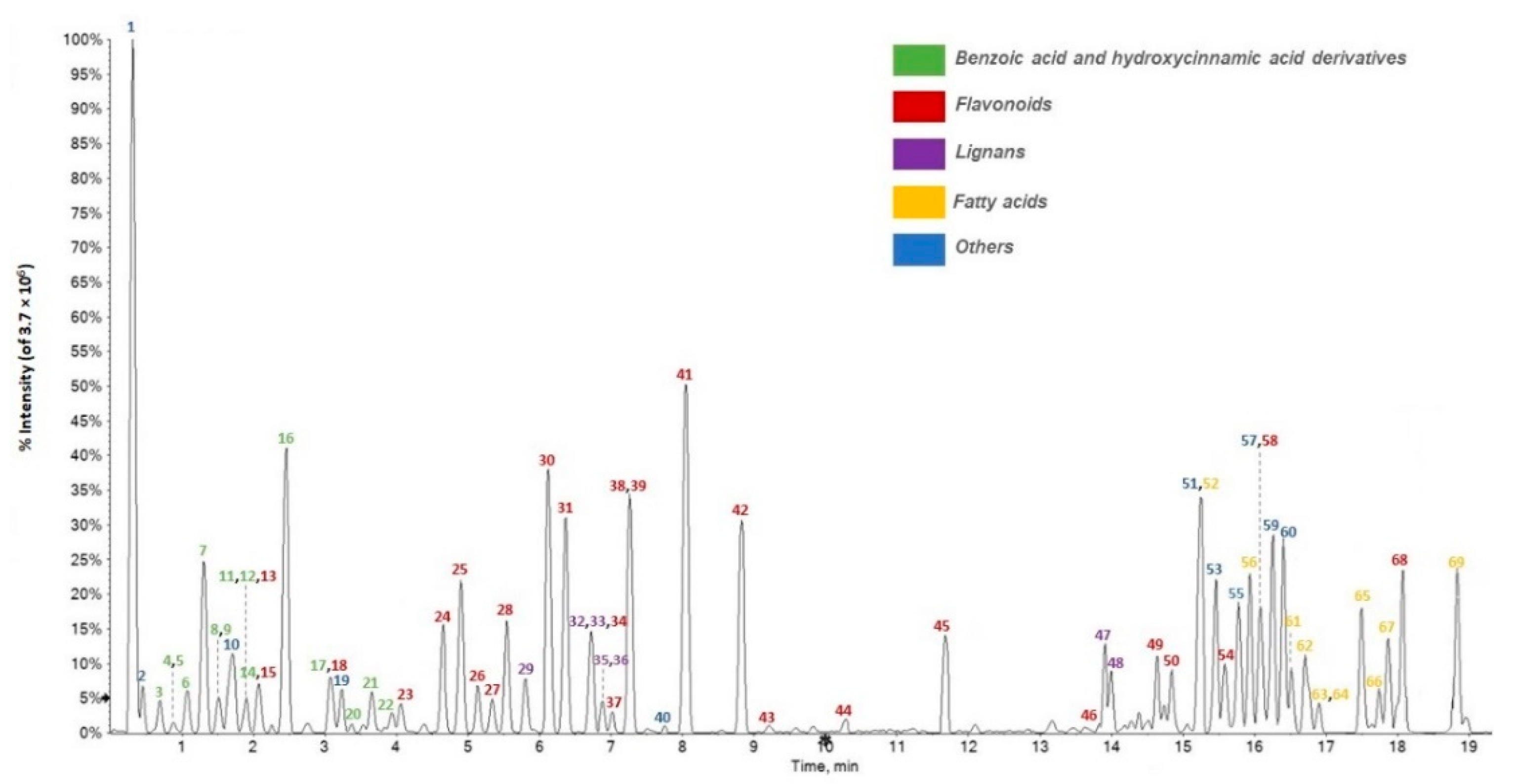
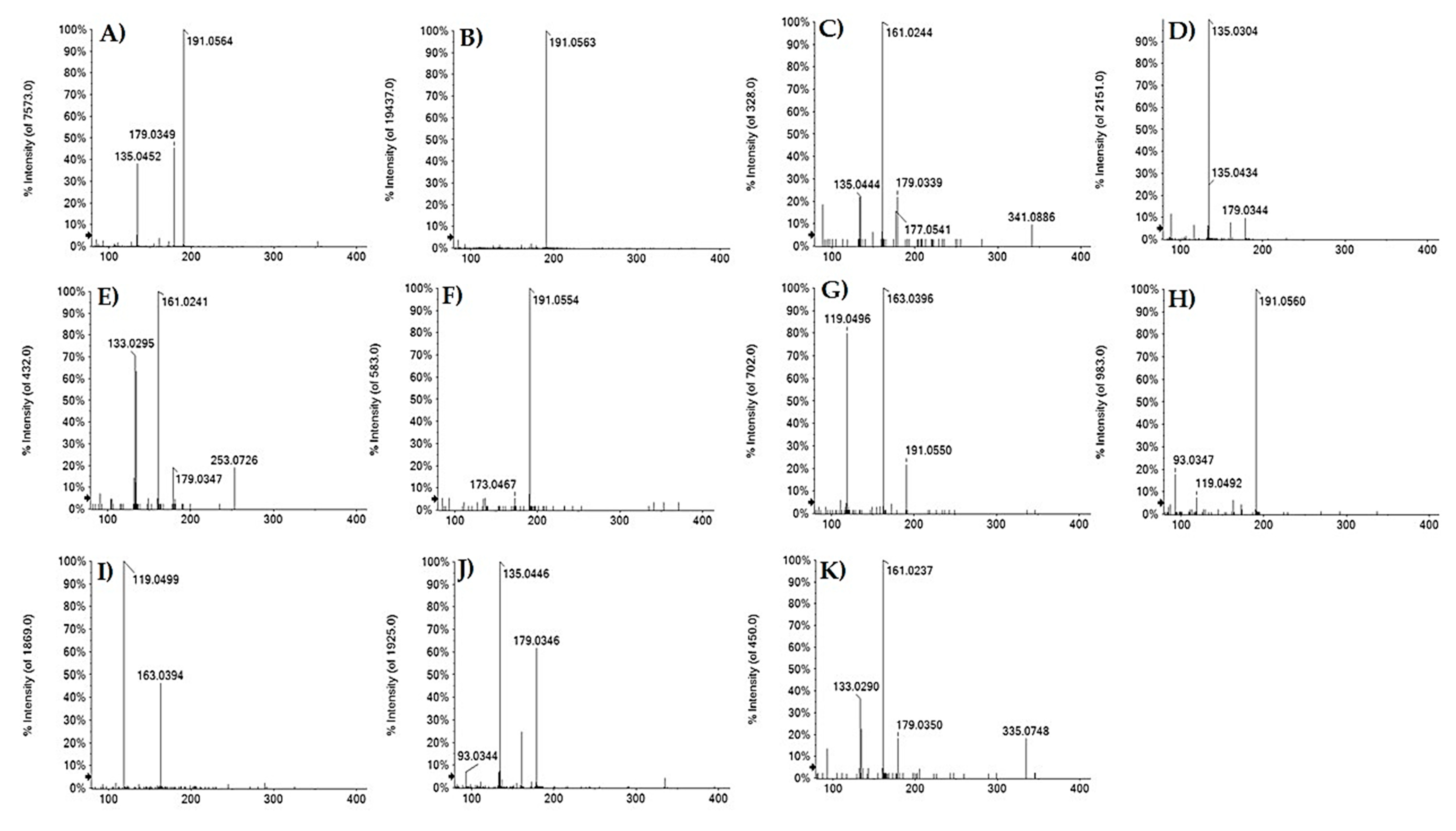
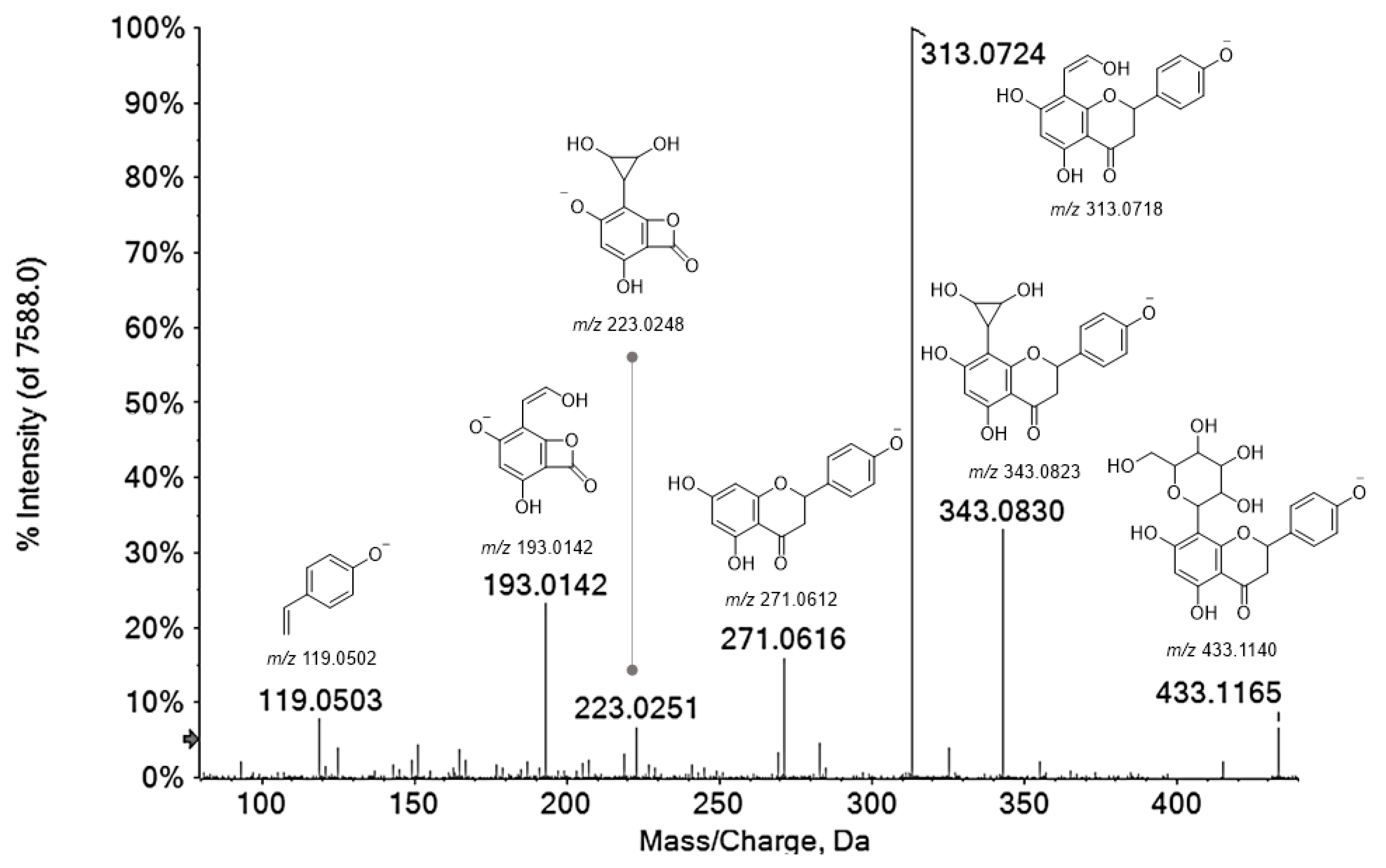
| Peak | RT (Min) | Tentative Assignment | Formula | [M−H]− Calc. (m/z) | [M−H]− Found (m/z) | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) and Relative Intensity |
|---|---|---|---|---|---|---|---|---|
| Benzoic and hydroxycinnamic acid derivatives | ||||||||
| 3 | 0.699 | Hydroxycaffeoyl quinic acid | C16H20O10 | 371.0984 | 371.0970 | −3.7 | 7 | 371.1052(3.4); 353.0872(3.4); 341.0900(3.4); 191.0554(100); 173.0467(5.3); 135.0447(5.3) |
| 4 | 0.875 | Dihydroxybenzoic acid hexoside | C13H16O9 | 315.0719 | 315.0722 | −0.8 | 6.0 | 315.0714(18.8); 153.0191(16.0); 152.0112(60.5); 109.0294(34.8); 108.0217(100) |
| 5 | 1.034 | Hydroxybenzoic acid hexoside | C13H1608 | 299.0772 | 299.0769 | −1.1 | 6 | 299.0780(29.3); 239.0572(17.8); 179.0341(41.4); 137.0242(58.6); 136.0159(17.8); 121.0290(100); 93.0345 (41.4) |
| 6 | 1.073 | Dihydroxybenzoic acid | C7H16O4 | 153.0193 | 153.0198 | 3.1 | 5 | 109.0291(100); 108.0215(87.5); 91.0185(12.4); 81.0341(8.7) |
| 7 | 1.226 | 3-O-Caffeoyl quinic acid | C16H18O9 | 353.0878 | 353.0893 | 0.8 | 8 | 191.0565(100); 179.0354(48.1); 135.0453(44.5); 134.0370(5.8) |
| 8 | 1.495 | Caffeoyl acid hexoside | C15H18O9 | 341.0874 | 341.0878 | −1.2 | 7 | 341.0886(9.4); 179.0339(22.0); 161.0244(100); 135.0444(9.4); 89.0243(18.6) |
| 9 | 1.514 | Caffeoyl threonic acid | C13H14O8 | 297.0616 | 297.0613 | −0.1 | 7 | 179.0344(9.6); 135.0304 (100); 117.0192 (6.6); 89.0246(11.6) |
| 11 | 1.921 | 3-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0929 | 337.0921 | −2.3 | 8 | 191.0550(61.4);173.0493(4.5);163.0396(100); 119.0946(74.6) |
| 12 | 1.921 | Caffeoyl quinic acid dimer | C32H36O18 | 707.1829 | 707.1831 | 0.3 | 15 | 707.1845(100); 533.1307(2.3); 515.1192(3.8); 463.1085(3.5); 353.0870(8.2); 323.0546(3.2); 243.0651(2.6); 191.0557(32.1) |
| 14 | 2.058 | p-Coumaroyl acid hexoside | C15H18O8 | 325.0929 | 325.0921 | −2.4 | 7 | 163.0394(46.2); 119.0499(100) |
| 16 | 2.370 | 5-O-Caffeoyl quinic acid | C16H1809 | 353.0878 | 353.0878 | 0 | 8 | 191.0562(100); 85.0296(3.4) |
| 17 | 3.048 | Caffeoyl propionic acid | C12H14O6 | 253.0718 | 253.0718 | 0.1 | 6 | 253.0726(19.0); 179.0347(19.0); 161.0241(100); 135.0454(63.4); 133.0295(70.8) |
| 20 | 3.576 | 5-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0929 | 337.0918 | −3.2 | 8 | 191.0560(100); 173.0442(4.0); 163.0405(6.0); 119.0491(6.7); 93.0346(16.9); 87.0073(3.7); 85.0290(2.7) |
| 21 | 3.929 | 5-O-Caffeoyl shikimic acid | C16H16O8 | 335.0772 | 335.0773 | −0.4 | 9 | 335.0755(3.9); 179.0346(61.5); 173.0454(2.3); 161.0239(24.5); 135.0446(100); 134.0372(6.9); 93.0344(6.9) |
| 22 | 4.047 | 4-O-Caffeoyl shikimic acid | C16H16O8 | 335.0772 | 355.0768 | −1.3 | 9 | 335.0748(3.9); 179.0350(21.5); 161.0237 (100); 135.0449 (22.7) |
| Flavonoids | ||||||||
| 13 | 1.921 | Procyanidin (B type) | C30H26O12 | 577.1352 | 577.1355 | 0.6 | 18 | 577.1366(22.0); 451.1030(14.9); 425.0863(27.3); 407.0772(100); 381.0998(12.6); 299.0523(11.1); 289.0710(79.4); 245.0444(18.5); 125.0240(79.4) |
| 15 | 2.136 | Catechin | C15H14O6 | 289.0718 | 289.0708 | −3.3 | 9 | 289.0696(38.3); 245.0818(30.8); 221.0797(30.8); 205.0505(15.0); 203.0709(54.1); 187.0381(30.8); 179.0365(23.3); 151.0403(40.6); 137.0246(30.8); 125.0238(30.8); 123.0447(84.2); 109.0290(100) |
| 18 | 3.048 | Eriodictyol 7-O-hexoside | C21H22O11 | 449.1089 | 449.1097 | 1.7 | 11 | 449.1097(2.5); 421.1142(8.5); 313.0717(2.5); 301.0709(9.4); 287.0553(33.9); 259.0609(100); 243.0661(8.2) |
| 23 | 4.125 | Kaempferol 3,7 di-O-hexoside | C27H30O16 | 609.1461 | 609.1466 | 0.8 | 13 | 609.1517(100); 489.1064(25.2); 447.0941(82.9); 446.0886(49.6); 285.0390(82.9); 284.0300(25.2); 283.0241(28.1) |
| 24 | 4.637 | Naringenin 8-C-hexoside (1) | C21H22O10 | 433.1140 | 433.1145 | 1.1 | 11 | 433.1165(7.1); 343.0830(34.3); 313.0724(100); 271.0616(15.9); 223.0251(6.7); 193.0142(24.0); 119.0503(5.4) |
| 25 | 4.917 | Naringenin 8-C-hexoside (2) | C21H22O10 | 433.1140 | 433.1145 | 1.1 | 11 | 433.1146(6.2); 343.0819(30.6); 313.0714(100); 271.0603(14.8); 223.0243(5.6); 193.0136(24.3); 165.0817(4.8); 119.0501(9.1) |
| 26 | 5.149 | Myricetin 3-O-hexoside | C21H20O13 | 479.0831 | 479.0844 | 12 | 2.7 | 479.0855(32.8); 317.0300(14.7); 316.0226(100); 287.0193(6.1); 271.0239(11.1) |
| 27 | 5.772 | Myricetin 3-O-pentoside | C20H18O12 | 449.0725 | 449.0746 | 4.6 | 12 | 449.0728(26.4); 317.0287(7.5); 316.0216(100); 287.0183(10.5); 271.0241(18.4); |
| 28 | 5.812 | Kaempferol 3-O-dihexoside | C27H30O16 | 609.1461 | 609.1481 | 3.3 | 13 | 609.1481(28.6); 285.0408(69.3); 284.0320(100); 255.0289(7.3) |
| 30 | 6.046 | Quercetin 3-O-hexoside | C21H20O12 | 463.0882 | 463.0893 | 2.4 | 12 | 463.0902(21.1); 301.0352(60.3); 300.0274(100); 271.0246(28.6); 255.0292(15.9) |
| 31 | 6.223 | Quercetin 3-O-hexuronide | C21H18O13 | 477.0675 | 477.0692 | 3.6 | 13 | 477.0694(5.5); 301.0354(100); 283.0237(2.5); 255.0295(2.3); 178.9974(6.3); 151.0029(6.2) |
| 34 | 6.722 | Naringenin 8-C-hexoside (3) | C21H22O10 | 433.1140 | 433.1154 | 3.2 | 11 | 433.1144(3.1); 343.0822(34.7); 313.0715(100); 271.0600(19.4); 269.0818(3.9); 223.0235(7.0); 205.0127(3.0); 193.0141(31.8); 151.0039(7.1); 119.0501(10.8) |
| 37 | 7.024 | Isorhamnetin hexosyl deoxyhexoside | C28H32O16 | 623.1618 | 623.1642 | 3.9 | 13 | 623.1636(100); 315.0501(11.1); 314.0423(70.7); 299.0176(15.2); 285.0423(5.4); 271.0256(5.4) |
| 38 | 7.196 | Quercetin 3-O-pentoside | C20H18O11 | 433.0776 | 433.0795 | 4.3 | 12 | 433.0796(16.5); 301.0355(19.2); 300.0281(100); 271.0247(21.7); 255.0295(10.4) |
| 39 | 7.240 | Kaempferol 3-O-galactopyranoside | C21H20O11 | 447.0933 | 447.0946 | 2.9 | 12 | 447.0944(39.4); 327.0500(2.6); 285.0397(27.2); 284.0322(100); 255.0293(40.7); 227.0342(23.5) |
| 41 | 8.003 | Kaempferol 3-O-glucopyranoside | C21H20O11 | 447.0933 | 447.0943 | 2.3 | 12 | 447.0943(35.5); 285.0398(55.2); 284.0322(100); 255.0295(47.0); 227.0345(27.7) |
| 42 | 8.897 | Kaempferol 3-O-pentoside | C20H18010 | 417.0827 | 417.0844 | 4.0 | 12 | 417.0858(31.8); 285.0410(22.1); 284.0337(100); 255.0306(53.1); 227.0355(31.5) |
| 43 | 9.203 | Kaempferol (acetyl)-hexoside | C23H22O12 | 489.1039 | 489.1054 | 3.2 | 13 | 489.1071(36.3); 285.0383(37.7);284.0313(100); 255.0307(28.4); 227.0329(12.3) |
| 44 | 10.260 | Kaempferol 7-O-pentoside | C20H18010 | 417.0827 | 417.0833 | 3.3 | 12 | 417.0833(38.1); 285.0398(100); 284.0330(85.6); 255.0301(52.1); 227.0355(38.1) |
| 45 | 11.672 | Kaempferol 7-O-deoxyhexoside | C21H20O10 | 431.0984 | 431.0999 | 3.5 | 12 | 431.1006(24.2); 285.0407(100); 284.0328(87.5); 255.0299(44.6); 227.0349(20.6) |
| 46 | 13.891 | Kaempferol (acetyl)-pentoside | C22H20O11 | 459.0933 | 459.0938 | 1.1 | 13 | 459.0970(60.6); 285.0395(10.4); 284.0331(100); 255.0304(36.7); 227.0350(26.1) |
| 49 | 14.626 | Kaempferol p-coumaroyl-hexoside (1) | C30H26O13 | 593.1301 | 593.1326 | 4.3 | 18 | 593.1359(81.4); 447.0956(6.9); 285.0408(100); 284.0332(52.3); 255.0297(10.0); 227.0346(4.1) |
| 50 | 14.747 | Kaempferol p-coumaroyl-hexoside (2) | C30H26O13 | 593.1301 | 593.1331 | 4.3 | 18 | 593.1349(100); 447.0958(9.1); 307.0820(6.2); 285.0404(97.7); 284.0313(57.1); 255.0288(9.3) |
| 54 | 15.612 | Kaempferol p-coumaroyl Pentoside | C29H24O12 | 563.1195 | 563.1219 | 4.3 | 18 | 563.1230(69.8); 285.0406(100); 284.0316(52.3) |
| 58 | 16.202 | Luteolin p-coumaroyl-deoxyhexoside | C30H26O12 | 577.1352 | 577.1378 | 4.6 | 18 | 577.1384(11.9); 431.1034(1.8); 284.0333(6.1); 285.0409(100); 283.0223(1.8); 257.0469(2.7); 229.0514(2.3) |
| 68 | 18.075 | Kaempferol di-p-coumaroyl deoxyhexoside | C39H32O14 | 723.1719 | 723.1752 | 4.5 | 24 | 723.1763(2.5); 577.1391(14.5); 559.1232(8.1); 437.1261(48.6); 397.1358(4.8); 285.0404(100); 284.0322(19.1);273.0759(5.7); 187.0395(8.6); 163.0400(19); 145.0295(4.9) |
| Lignans | ||||||||
| 29 | 5.812 | Isolariciresinol hexoside | C26H34O11 | 521.2028 | 521.2046 | 3.4 | 10 | 359.1504(2.6); 329.1396(100); 192.0791(3.9); 193.0833(2.8); 175.0760(5.8); 160.0519(3.0) |
| 32 | 6.722 | Neolignan-9′-O- rhamnoside isomer 1 | C25H34O11 | 509.2028 | 509.2053 | 4.1 | 9 | 509.2044(16.9); 491.1926(13.3); 473.1824(27.9); 461.1813(25.9); 367.1395(53.6); 339.1450(6.2); 313.1290(98.3); 179.0712(100); 167.0711(7.2); 161.0611(12.4); 149.0608(27.6); 147.0445(13.3); 134.0373(8.9); 103.0405(8.0) |
| 33 | 6.722 | Cinchonain-I isomer 1 | C42H20O9 | 451.1035 | 451.1046 | 2.5 | 15 | 451.1083(9.6); 341.0669(100); 299.0551(27.9); 297.0762 (11.7); 281.0460(11.7);217.0131(7.5); 189.0186(15.0); 177.0185(18.2); 161.0246(6.4) |
| 35 | 6.848 | Cinchonain-I isomer 2 | C42H20O9 | 451.1035 | 451.1047 | 2.5 | 15 | 451.1056(6.3); 341.0674(100); 299.0593(4.7); 297.0762(6.4); 281.0451(12.5); 279.0652(4.7); 231.0288(6.3); 217.0136(9.3); 189.0178(12.5); 177.0193(12.5); 161.0246 (4.7) |
| 36 | 6.888 | Neolignan-9′-O- rhamnoside isomer 2 | C25H34O11 | 509.2028 | 509.2051 | 4.4 | 9 | 509.2071(4.1); 491.1961(18.1); 473.1830(29.1); 461.1830(25.6); 458.1611(6.6); 367.1398(25.2); 313.1299(66.2); 179.0713(100); 167.0704(6.6); 163.0607(14.0); 147.0443(3.4); 149.0609(26.8); 146.0374(18.9); 103.0374(6.6) |
| 47 | 13.891 | 4,9,9′-Trihydroxy-3,3′,5′ -trimethoxy-8-O-4′ -neolignan-7-O-deoxyhexoside | C27H38O12 | 553.2304 | 553.2321 | 5.5 | 9 | 553.2291(6.1); 343.1382(100); 328.1140(19.2); 211.0595(2.1); 183.0647(1.5) |
| 48 | 14.017 | 9′-Hydroxy-7′-propen-3′,5′-dimethoxyphenyl-3-methoxyphenyl-7,9-propanediol-4-O-hexoside | C27H38O12 | 551.2134 | 551.2167 | 6.0 | 10 | 551.2184(4.7); 533.2083(4.7); 343.1394(14.1); 328.1161(4.7); 209.0915(100); 194.0579(31.1); 176.0481(8.1) |
| Fatty acids | ||||||||
| 52 | 15.278 | Trihydroxy-octadecadienoic acid | C18H32O5 | 327.2177 | 327.2185 | 2.5 | 3 | 327.2187(88.3); 309.2087(3.0); 291.1962(16.4); 229.1448(51.1); 221.1183(15.4); 211.1341(100); 209.1186(7.9); 183.1385(27.6); 171.1026(33.2) |
| 56 | 16.067 | Hydroxyhexadecanoic acid | C16H32O4 | 287.2228 | 287.2233 | 1.8 | 1 | 287.2236(100); 285.2077(7.2); 269.2114(8.8) |
| 61 | 16.512 | Dihydroxyoctadecedienoic quinic acid | C25H42O9 | 485.2756 | 485.2780 | 4.9 | 5 | 485.2746(4.3); 311.2230(100); 293.2117(5.6); 275.2012(3.7); 223.1703(16.6); 191.0559 (87.9) |
| 62 | 16.708 | Linolenic acid derivative | C31H38O6 | 505.2596 | 505.2589 | −1.3 | 13 | 505.2605(37.2); 277.2176(100); 227.0323(5.2); 152.9955(9.2) |
| 63 | 16.864 | Hydroxyoctadecatrienoic acid | C18H30O3 | 293.2122 | 293.2129 | 2.3 | 4 | 293.2111(65.6); 275.2014(82.9); 221.1539(59.0); 211.1338(25.2); 183.1385(100); 171.1016(50.3) |
| 64 | 16.883 | Linoleic acid derivative | C34H44O9 | 595.2913 | 595.2915 | 0.4 | 13 | 595.2940(100); 415.2266(6.3); 315.0492(9.5); 279.2332(43.1); 241.0109(17.2); 152.9952(11.5) |
| 65 | 17.484 | 15,16-Dihydroxy-9,12-octadienoic acid | C18H32O4 | 311.2228 | 311.2232 | 1.3 | 3 | 311.2238(39.4); 293.2125(12.6); 275.2017(9.9); 235.1710(11.1); 253.1804 (3.4); 223.1707(100); 183.0116(2.8) |
| 66 | 17.738 | Linolenic acid glyceryl-tetrahexoside | C45H76O24 | 999.4654 | 999.4685 | 3.1 | 8 | 999.4751(100); 837.4302(2.0); 739.2582(12.1); 721.2452(29.1); 559.1919(3.4); 397.1370(4.0); 221.0682(1.3); 119.0353(1.3) |
| 67 | 17.885 | Linolenic acid derivative | C32H41NO4 | 502.2963 | 502.2963 | 0.0 | 13 | 502.2958(17.3); 456.1546(6.3); 277.2175(100); 224.0689(6.3) |
| 69 | 18.826 | Linolenic acid glyceryl-dihexoside | C33H56O4 | 675.3597 | 675.3629 | 4.7 | 6 | 675.3622(13.0); 415.1454(43.5); 397.1344(100); 379.1210(2.7); 305.0848(7.8); 277.2158(68.9); 253.0904(5.2); 235.0804(18.3), 179.0557(5.2); 161.0451(3.5); 119.0342(7.8); 89.0247(7.8) |
| Other compounds | ||||||||
| 1 | 0.334 | Quinic acid | C7H12O6 | 191.0561 | 191.0561 | −0.1 | 2 | 191.0565(6.7); 173.0455(5.3); 127.0404(15.0); 111.0459(7.9); 93.0356(60.7); 85.0305(100) |
| 2 | 0.468 | Tyrosine hexoside | C15H21NO8 | 342.1194 | 342.1183 | −3.3 | 6 | 252.0855(2.5); 222.0758(2.5); 191.0567(5.0); 180.0664(100); 119.0507(10.25) |
| 10 | 1.670 | Unknown | C28H26O16 | 617.1148 | 617.1128 | −3.3 | 16 | 617.1133(23.8); 455.0806(11.5); 319.0426(100); 297.0605(12.9); 259.0210(4.4); 215.0321(10.2); 201.0160(27.1); 179.0345(10.2); 135.0295(17.7) |
| 19 | 3.204 | Tuberonic acid | C18H28O9 | 387.1661 | 387.1666 | 1.4 | 5 | 387.1649(100); 207.1027(32.7);163.1125(8.6); 119.0347(9.7); 101.0252(8.4); 89.0251(14.1) |
| 40 | 7.742 | Hexenyl-3-hydroxy-3-methyl-glutaryl hexoside | C18H30O10 | 405.1766 | 405.1775 | 2.2 | 4 | 405.1775(11.4); 343.1799(5.6); 303.1548(5.6); 261.1311(8.6); 179.0545(17.0); 161.0461(14.2); 125.0237(45.7); 101.0240(65.5); 99.0451(100); 89.0246 (34.3) |
| 51 | 15.240 | Unknown | C20H34O9 | 417.2130 | 417.2135 | 1.2 | 4 | 417.2147(51.9); 261.1345(44.5); 247.1190(36.1); 243.1268(3.6); 187.0977(100); 173.0821(94.2); 169.0865(11.1); 125.0973(46.1); 111.0818(46.1) |
| 53 | 15.482 | Unknown | C38H52O16 | 763.3183 | 763.3207 | 3.2 | 13 | 763.3236(16.7); 343.1403(100); 328.1156(5.8) |
| 55 | 15.912 | Unknown | C21H36O9 | 431.2287 | 431.2298 | 2.7 | 4 | 431.2307(26.5); 261.1346(36.6); 187.0976(100); 169.0868(10.3); 125.0972(40.8) |
| 57 | 16.086 | Unknown | C21H36O9 | 431.2287 | 431.2299 | 2.9 | 4 | 431.2309(30.4); 261.1338(31.9); 187.0975(100); 169.0865(8.5); 125.0969(41.5) |
| 59 | 16.261 | Saponin isomer 1 | C48H76O19 | 955.4908 | 955.4948 | 4.2 | 11 | 955.4990(100); 793.4449 (6.8) |
| 60 | 16.397 | Saponin isomer 2 | C48H76O19 | 955.4908 | 955.4941 | 4.1 | 11 | 955.4990(100); 793.4449 (6.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols. Antioxidants 2021, 10, 1140. https://doi.org/10.3390/antiox10071140
Formato M, Piccolella S, Zidorn C, Pacifico S. UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols. Antioxidants. 2021; 10(7):1140. https://doi.org/10.3390/antiox10071140
Chicago/Turabian StyleFormato, Marialuisa, Simona Piccolella, Christian Zidorn, and Severina Pacifico. 2021. "UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols" Antioxidants 10, no. 7: 1140. https://doi.org/10.3390/antiox10071140








