Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases
Abstract
1. Introduction
1.1. Absorption and Transport of Lutein
1.2. Bioavailability and Metabolism of Lutein
1.3. Toxicity and Safety of Lutein
2. Lutein in Inflammatory Diseases
2.1. Neurodegenerative Disorders
2.2. Eye Diseases
2.3. Osteoporosis
2.4. Cardiovascular Diseases
2.5. Skin Diseases
2.6. Liver Injury
2.7. Obesity
2.8. Colon Diseases
2.9. Diabetes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arteni, A.A.; Fradot, M.; Galzerano, D.; Mendes-Pinto, M.M.; Sahel, J.A.; Picaud, S.; Robert, B.; Pascal, A.A. Structure and conformation of the carotenoids in human retinal macular pigment. PLoS ONE 2015, 10, e0135779. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Sommerburg, O.; Keunen, J.E.E.; Bird, A.C.; Van Kuijk, F.J.G.M. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; Reig García-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Alves-Rodrigues, A.; Shao, A. The Science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Suzuki, Y.; Hirano, T.; Koyama, Y.; Yoshida, K.; Ilieva, I.; Iseki, K.; Ohno, S. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Izumi-Nagai, K.; Nagai, N.; Ohgami, K.; Satofuka, S.; Ozawa, Y.; Tsubota, K.; Umezawa, K.; Ohno, S.; Oike, Y.; Ishida, S. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2555–2562. [Google Scholar] [CrossRef]
- Horváth, G.; Kemény, Á.; Barthó, L.; Molnár, P.; Deli, J.; Szente, L.; Bozó, T.; Pál, S.; Sándor, K.; Szőke, É.; et al. Effects of some natural carotenoids on TRPA1- and TRPV1-induced neurogenic inflammatory processes in vivo in the mouse skin. J. Mol. Neurosci. 2015, 56, 113–121. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Li, Y.; Hu, L.; Dai, Y.; Chen, J.; Xu, S.; Xu, X.; Jiang, H. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. Int. Immunopharmacol. 2018, 56, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by caco-2 cells: Beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Desmarchelier, C.; Margier, M.; Reboul, E.; Melendez Martinez, A.J.; Borel, P. Phytoene and phytofluene isolated from a tomato extract are readily incorporated in mixed micelles and absorbed by Caco-2 cells, as compared to lycopene, and SR-BI is involved in their cellular uptake. Mol. Nutr. Food. Res. 2018, 62, e1800703. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef]
- Nagao, A. Bioavailability of dietary carotenoids: Intestinal absorption and metabolism. Jpn. Agric. Res. Q. 2014, 48, 385–391. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nut. Food. Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Van het hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Handelman, G.J.; Nightingale, Z.D.; Lichtenstein, A.H.; Schaefer, E.J.; Blumberg, J.B. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 1999, 70, 247–251. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Nagao, A. Absorption and metabolism of xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef]
- Yao, Y.; Qiu, Q.H.; Wu, X.W.; Cai, Z.Y.; Xu, S.; Liang, X.Q. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition 2013, 29, 958–964. [Google Scholar] [CrossRef]
- Wenzel, A.J.; Sheehan, J.P.; Gerweck, C.; Stringham, J.M.; Fuld, K.; Curran-Celentano, J. Macular pigment optical density at four retinal loci during 120 days of lutein supplementation. Ophthalmic Physiol. Opt. 2007, 27, 329–335. [Google Scholar] [CrossRef]
- Dagnelie, G.; Zorge, I.S.; McDonald, T.M. Lutein improves visual function in some patients with retinal degeneration: A pilot study via the Internet. Optometry 2000, 71, 147–164. [Google Scholar] [PubMed]
- Fatani, A.J.; Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Assaf, A.; Parmar, M.Y.; Ahmed, M.M. Lutein dietary supplementation attenuates streptozotocin-induced testicular damage and oxidative stress in diabetic rats. BMC. Complement. Altern. Med. 2015, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Neuringer, M.; Snodderly, D.M.; Schalch, W.; Johnson, E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef]
- Addo, E.K.; Gorusupudi, A.; Allman, S.; Bernstein, P.S. The lutein and zeaxanthin in pregnancy (L-ZIP) study-carotenoid supplementation during pregnancy: Ocular and systemic effects-study protocol for a randomized controlled trial. Trials 2021, 22, 300. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Terry, D.P.; Renzi-Hammond, L.M.; Hammond, B.R.; Miller, L.S. Relation of retinal and serum lutein and zeaxanthin to white matter integrity in older adults: A diffusion tensor imaging study. Arch. Clin. Neuropsychol. 2018, 33, 861–874. [Google Scholar] [CrossRef]
- Feeney, J.; O’Leary, N.; Moran, R.; O’Halloran, A.M.; Nolan, J.M.; Beatty, S.; Young, I.S.; Kenny, R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: Findings from the Irish longitudinal study on aging. J. Gerontol. A 2017, 72, 1431–1436. [Google Scholar] [CrossRef]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Cherubini, A.; Ingegni, T.; Mattioli, P.; Catani, M.; Rinaldi, P.; Cecchetti, R.; Stahl, W.; Senin, U.; et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 2002, 59, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.L.; Taylor, H.G.; Cassedy, A.; Zhang, N.; Kirkwood, M.W.; Brown, T.M.; Stancin, T. Long-term behavioral outcomes after a randomized, clinical trial of counselor-assisted problem solving for adolescents with complicated mild-to-severe traumatic Brain Injury. J. Neurotrauma 2015, 32, 967–975. [Google Scholar] [CrossRef]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food. Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009, 4, e6588. [Google Scholar] [CrossRef]
- TakafumiSuzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef]
- Shimazu, Y.; Kobayashi, A.; Endo, S.; Takemura, J.; Takeda, M. Effect of lutein on the acute inflammation-induced c-Fos expression of rat trigeminal spinal nucleus caudalis and C1 dorsal horn neurons. Eur. J. Oral. Sci. 2019, 127, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Bohm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with singlet oxygen (1O2) and free radicals: Potential effects for human health. Acta Biochim. Pol. 2012, 59, 27–130. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Fisher, A.I.; Klein, R.; Palta, M.; Block, G.; Millen, A.E.; Wright, J.D. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am. J. Epidemiol. 2001, 153, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Chakravarthy, U.; Nolan, J.M.; Muldrew, K.A.; Woodside, M.A.; Denny, F.; Stevenson, M.R. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Guerra, L.H.; Ruiz, C.A. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J. Nutr. 2003, 133, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Dennison, J. The photobiology of lutein and zeaxanthin in the eye. J Ophthalmol. 2015, 687173. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef]
- Bian, Q.; Qin, T.; Ren, Z.; Wu, D.; Shang, F. Lutein or zeaxanthin supplementation suppresses inflammatory responses in retinal pigment epithelial cells and macrophages. Adv. Exp. Med. Biol. 2012, 723, 43–50. [Google Scholar]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked placebo-controlled randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004, 75, 216–229. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retinal. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, X.M.; Zou, Z.Y.; Xu, X.R.; Li, Y.; Xu, R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br. J. Nutr. 2009, 102, 186–190. [Google Scholar] [CrossRef]
- Moreau, K.L.; King, J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol. Med. 2012, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Koo, E.; Agron, E.; Hallak, J.; Clemons, T.; Azar, D.; Sperduto, R.D.; Ferris, F.L., III; Chew, E.Y. Risk factors associated with incident cataracts and cataract surgery in the age-related eye disease Study (AREDS). AREDS Report Number 32. Ophthalmology 2011, 118, 2113–2119. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Smitha, P.; Maneesh, M.; Magdalena, S.; Liew, J.M.; Marcus, A.G.; Mikhail, D.L. Modulation of advanced glycation endproduct by kynurenines in human lens proteins. Biochim. Biophys. Acta 2010, 1804, 829–838. [Google Scholar] [CrossRef][Green Version]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Lutein, a carotenoid, suppresses osteoclastic bone resorption and stimulates bone formation in cultures. Biosci. Biotechnol. Biochem. 2017, 81, 302–306. [Google Scholar] [CrossRef]
- Takeda, H.; Tominari, T.; Hirata, M.; Watanabe, K.; Matsumoto, C.; Grundler, F.; Inada, M.; Miyaura, C. Lutein enhances bone mass by stimulating bone formation and suppressing bone resorption in growing mice. Biol. Pharm. Bull. 2017, 40, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Boucher, B.A.; Cheung, A.M.; Beyene, J.; Shah, P.S. Fruit and vegetable intake and bone health in women aged 45 years and over: A systematic review. Osteoporos. Int. 2011, 22, 1681–1693. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, R.; Ang, L.W.; Low, Y.L.; Yuan, J.M.; Koh, W.P. Protective effects of dietary carotenoids on risk of hip fracture in men: The Singapore Chinese Health Study. J. Bone. Miner. Res. 2014, 29, 408–417. [Google Scholar] [CrossRef]
- Altindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2008, 28, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T.; et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Xu, X.; Huang, Y.; Xiao, X.; Ma, L.; Sun, T.; Dong, P.; Wang, X.; Lin, X. High serum level of lutein may be protective against early atherosclerosis: The Beijing atherosclerosis study. Atherosclerosis 2011, 219, 789–793. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Leducq transatlantic network on atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Mauriello, A.; Sangiorgi, G.M.; Virmani, R.; Trimarchi, S.; Holmes, D.R., Jr.; Kolodgie, F.D.; Piepgras, D.G.; Piperno, G.; Liotti, D.; Narula, J.; et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis 2010, 208, 572–580. [Google Scholar] [CrossRef]
- Xu, X.R.; Zou, Z.Y.; Xiao, X.; Huang, Y.M.; Wang, X.; Lin, X.M. Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. J. Atheroscler. Thromb. 2013, 20, 170–177. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Zhang, S.; Zhao, L. Oxidative stress in rats with hyperhomo-cysteinemia and intervention effect of lutein. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 359–364. [Google Scholar]
- Guéant-Rodriguez, R.M.; Spada, R.; Moreno-Garcia, M.; Anello, G.; Bosco, P.; Lagrost, L.; Romano, A.; Elia, M.; Guéant, J.L. Homocysteine is a determinant of ApoA-I and both are associated with ankle brachial index, in an Ambulatory Elderly Population. Atherosclerosis 2011, 214, 480–485. [Google Scholar] [CrossRef]
- Lentz, S.R.; Sobey, C.G.; Piegors, D.J.; Bhopatkar, M.Y.; Faraci, F.M.; Malinow, M.R.; Heistad, D.D. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J. Clin. Investig. 1996, 98, 24–29. [Google Scholar] [CrossRef]
- Armoza, A.; Haim, Y.; Bashiri, A.; Wolak, T.; Paran, E. Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-kB signaling in endothelial cells. J. Hypertens. 2013, 31, 521–529. [Google Scholar] [CrossRef]
- Sung, J.H.; Jo, Y.S.; Kim, S.J.; Ryu, J.S.; Kim, M.C.; Ko, H.J.; Sim, S.S. Effect of lutein on L-NAME-induced hypertensive rats. Korean J. Physiol. Pharmacol. 2013, 17, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef]
- Howard, A.N.; Thurnham, D.I. Lutein and atherosclerosis: Belfast versus Toulouse revisited. Med. Hypotheses 2017, 98, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Faulhaber, D.; Hanson, K.M.; Ding, W.; Peters, S.; Kodali, S.; Granstein, R.D. Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J. Investig. Dermatol. 2004, 122, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef]
- Fuchs, J. Potentials and limitations of the natural antioxidants RRR-alpha- tocopherol, l-ascorbic acid and beta-carotene in cutaneous photoprotection. Free Radic. Biol. Med. 1998, 25, 848–873. [Google Scholar] [CrossRef]
- Fuchs, J.; Kern, H. Modulation of UV-light-induced skin inflammation by D-alpha- tocopherol and l-ascorbic acid: A clinical study using solar simulated radiation. Free Radic. Biol. Med. 1998, 25, 1006–1012. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; Wu, A.; Goukassian, D.; Pathak, M. Dietary lutein/ zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Mokos, M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Beier, J.I.; McClain, C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol. Chem. 2010, 391, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Firdous, A.P.; Preethi, K.C.; Kuttan, R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride- and ethanol-induced hepatic damage. J. Pharm. Pharmacol. 2010, 62, 1054–1060. [Google Scholar] [CrossRef]
- Du, S.Y.; Zhang, Y.L.; Bai, R.X.; Ai, Z.L.; Xie, B.S.; Yang, H.Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785–8793. [Google Scholar]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef]
- Mai, J.; Shen, X.; Shi, D.; Wei, Y.; Shen, H.; Wu, M. Effect of lutein on relieving oxidative stress in mice induced by D-galactose. J. Hyg. Res. 2010, 39, 430–432. [Google Scholar]
- Li, S.; Ding, Y.; Niu, Q.; Xu, S.; Pang, L.; Ma, R.; Jing, M.; Feng, G.; Tang, J.X.; Zhang, Q.; et al. Lutein has a protective effect on hepatotoxicity induced by arsenic via Nrf2 Signaling. BioMed Res. Int. 2015, 2015, 315205. [Google Scholar] [CrossRef]
- Hinnouho, G.M.; Czernichow, S.; Dugravot, A.; Nabi, H.; Brunner, E.J.; Kivimaki, M.; Singh-Manoux, A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur. Heart J. 2015, 36, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer–Viewpoint of the IARC working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F., Jr.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Indulekha, K.; Anjana, R.M.; Surendar, J.; Mohan, V. Association of visceral and subcutaneous fat with glucose intolerance, insulin resistance, adipocytokines and inflammatory markers in Asian Indians (CURES-113). Clin. Biochem. 2011, 44, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Gillespie, C.; Ballew, C.; Sowell, A.; Mannino, D. Serum carotenoid concentrations in US children and adolescents. Am. J. Clin. Nutr. 2002, 76, 818–827. [Google Scholar] [CrossRef]
- Gopal, S.S.; Eligar, S.M.; Vallikannan, B.; Ponesakki, G. Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2021, 1866, 158812. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef]
- Johnson, E.J. Obesity, lutein metabolism, and age-related macular degeneration: A web of connections. Nutr. Rev. 2005, 63, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC. Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Rana, S.V.; Sharma, S.; Prasad, K.K.; Sinha, S.K.; Singh, K. Role of oxidative stress and antioxidant defence in ulcerative colitis patients from north India. Indian. J. Med. Res. 2014, 139, 568–571. [Google Scholar]
- Meurer, M.C.; Mees, M.; Mariano, L.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.; Dos Santos, A.C.; Longo, B.; Santos França, T.C.; et al. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr. Res. 2019, 66, 95–106. [Google Scholar] [CrossRef]
- Rumi, G., Jr.; Szabo, I.; Vincze, A.; Matus, Z.; Toth, G.; Mozsik, G. Decrease of serum carotenoids in Crohn’s disease. J. Physiol. Paris 2000, 94, 159–161. [Google Scholar] [CrossRef]
- Sahli, M.W.; Mares, J.A.; Meyers, K.J.; Klein, R.; Brady, W.E.; Klein, B.E.K.; Ochs-Balcom, H.M.; Donahue, R.P.; Millen, A.E. Dietary Intake of Lutein and Diabetic Retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol. 2016, 23, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tam, K.C.; Ng, T.C.; Goit, R.K.; Chan, K.L.S.; Lo, A.C.Y. Long-term lutein administration attenuates retinal inflammation and functional deficits in early diabetic retinopathy using the Ins2Akita/+ mice. BMJ Open. Diabetes. Res. Care 2020, 8, e001519. [Google Scholar] [CrossRef] [PubMed]
- Shivarudrappa, A.H.; Ponesakki, G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J. Cell Commun. Signal. 2019, 14, 207–221. [Google Scholar] [CrossRef]
- Leermakers, E.T.M.; Darweesh, S.K.L. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481. [Google Scholar] [CrossRef]

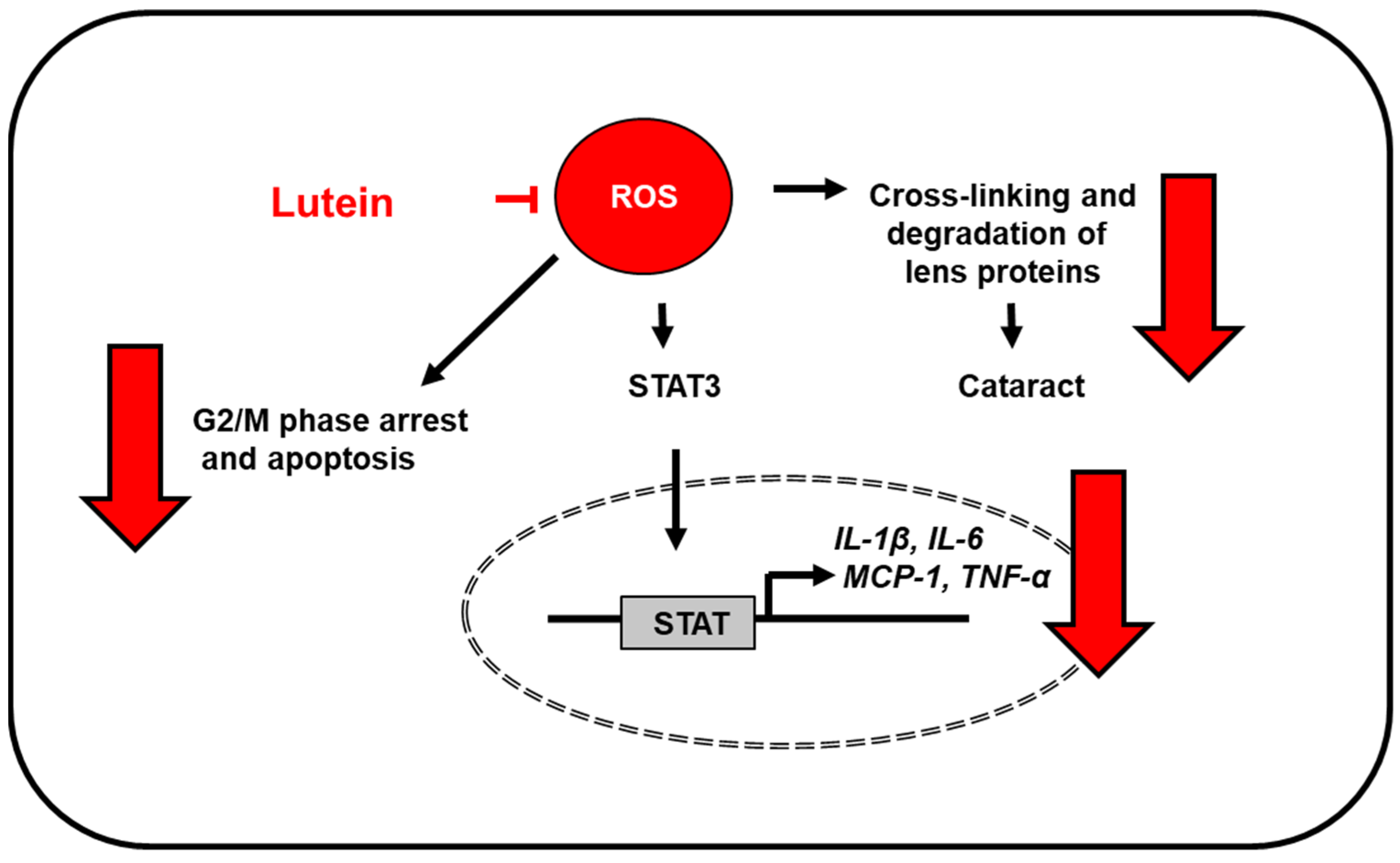


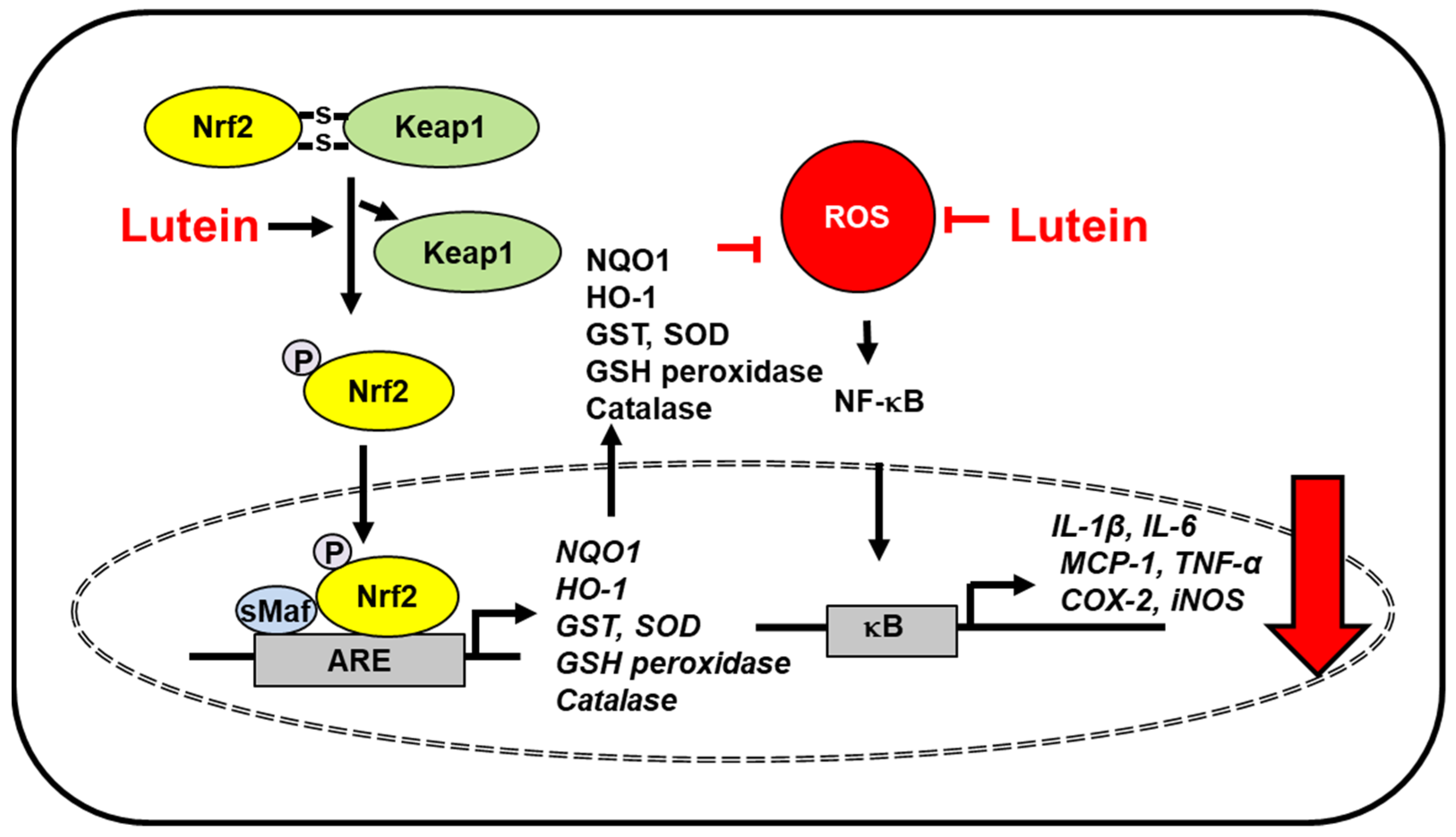
| Diseases | Experimental Model | Lutein Dose | Key Findings | Ref. |
|---|---|---|---|---|
| Neurodegenerative disorders | rats with severe traumatic brain injury | 40, 80, 160 mg/kg body weight (BW) | -suppressed IL-1β, IL-6, and monocyte chemoattractant protein (MCP)-1 expression -reduced serum reactive oxygen species (ROS) levels -downregulated the expression of nuclear factor-κΒ (NF-κB) p65, and cyclooxygenase (COX) -2 -upregulated nuclear factor erythroid 2–related factor 2 (Nrf2) and endothelin-1 protein levels | [37] |
| LPS-induced neuroinflammation in mouse microglial cells | 50 μM | -inhibited inducible nitric oxide synthase (iNOS) and COX-2 expression -inhibited TNF-α, IL-1β, and nitric oxide (NO) production -suppressed lipopolysaccharide-induced NF-κB activation -decrease of Keap1 and activation of Nrf2, and subsequent upregulation of heme oxygenase(HO)-1 and NAD(P)H: quinone oxidoreductase 1 (NQO1) in the presence or absence of LPS -induced activation of extracellular signal-regulated kinase (ERK), which was linked to Nrf2 activation | [38] | |
| acute inflammation-induced sensitization of nociceptive processing in rats | 10 mg/kg BW | -decreased in the inflammation-induced mean times of face grooming and the thickness of inflammation-induced edema in whisker pads -decreased numbers of c-Fos-positive neurons in both spinal trigeminal nucleus caudalis and upper cervical dorsal horn neurons | [42] | |
| Eye diseases | H2O2 stress-induced acute retinal pigment epithelial cells | 0, 1, 5, 10 and 15 µM | -increased cell viability, and decreased apoptosis and ROS levels -reversed the increased proportion of cells in the G2/M phase in a concentration-dependent manner -attenuated cell cycle arrest in G2/M phase by activating cyclin-dependent kinase 1 and cell division cycle 25C, and decreasing cyclin B1 | [48] |
| Lipopolysaccharide-stimulated human retinal pigment epithelial cells | 1, 10 μM | -reduced expression of IL-6 and IL-8 dose-dependently | [49] | |
| sodium selenite-induced cataract in male Wistar rat pups | 1.3 μmol/kg BW | -lutein + eicosahexaenoic acid (EPA) + docosahexaenoic acid (DHA) exhibited the highest cataract prevention -pups had the highest amount of lutein in the lens with lutein + EPA + DHA | [57] | |
| Osteoporosis | monosodium iodoacetate-induced osteoarthritis in primary chondrocyte cells | 0.5, 1, 5 and 10 μM | -increased the cell viability of chondrocytes -downregulated inflammatory proteins (NF-κB, COX-2) and pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) -reduced monosodium iodoacetate-induced apoptosis through downregulating the caspase-3 activity | [58] |
| ovariectomized rats (osteoporosis model) | 50 mg/kg BW | -decreased the lipid peroxidation and ROS levels -activated Nrf2-driven antioxidant gene expression (HO-1, NQO1) -downregulated osteoclast-specific marker [nuclear factor of activated T cells 1 (NFATc1)] expression | [59] | |
| mouse bone marrow cells and osteoblastic cells | 3, 10, 30 μM | -inhibited IL-1-induced osteoclast differentiation and bone resorption -enhanced the formation of mineralized bone nodules by increasing bone morphogenetic protein 2 expression and inhibiting sclerostin expression | [60] | |
| newborn and 5- and 6-week-old ddy mice | 66 mg/kg BW | -enhanced the femoral bone mass in growing male mice in vivo | [61] | |
| bone marrow cells isolated from 6-week-old mice and co-cultured with primary osteoblast cells | 3, 10 µM | -stimulated bone formation and suppressed bone resorption in vitro -regulated Receptor activator of NF-kΒ ligand (RANKL)-dependent osteoclast formation in vitro | [61] | |
| Cardiovascular diseases | a co-culture model of the artery wall formed from endothelial and smooth muscle cells from human aortas | 0.1, 1.0, 10, and 100 nmol/L | -inhibited low-density lipoprotein-induced migration in a dose-dependent manner in vitro | [65] |
| apoE-null mice | 0.2% by chow weight | -reduced atherosclerotic lesion size in the aortic arch in apoE-null mice in vivo -reduced plasma very low-density lipoprotein+ intermediate-density lipoprotein in vivo | [65] | |
| hyperhomocysteinemia rat | 20 mg/kg BW | -increased serum levels of superoxide dismutase (SOD) and glutathione peroxidase -downregulated the expression of NF-kΒ and intercellular adhesion molecule-1 -increased the serum NO level and decreased endothelin-1 content | [71] | |
| two cultured endothelial cell models (EA.hy926 and human umbilical vein endothelial cells) | 0.3 µmol/L | -decreased TNF-α -induced leukocytes adhesion, expression of intercellular adhesion molecule-1, and vascular cell adhesion molecule 1 -attenuated leukocytes adhesion to endothelial cells | [74] | |
| Skin diseases | female C3H/HeJ mice | 0.04, 0.4% lutein of 100 g diet | - inhibited UVB-induced skin swelling and reversed the inhibition of contact hypersensitivity) -reduced local UVB-radiation-induced immune suppression -reduced ROS generation in murine skin | [79] |
| female hairless SKh-1 mice | 0.4%, 0.04% lutein and zeaxanthin of 100 g diet | -suppressed UVB-induced skin inflammation -decreased the number of UVB-induced apoptotic keratinocytes -decreased the number of UVB-induced proliferating cell nuclear antigen-positive cells in murine skin -reduced UVB-induced increase in bromodeoxyuridine incorporation into basal epidermal keratinocytes | [84] | |
| Liver Injury | rats with alcohol-induced liver injury | 40 mg/kg BW | - reduced hepatic tissue levels of oxidative stress markers (ROS levels, lipid peroxidation, protein carbonyls, and sulfhydryls content), inflammatory cytokines (TNF-α, MCP-1, IL-1β, IL-6), and inflammatory proteins (NF-κB, COX-2, iNOS) -reduced serum levels of liver markers (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and alkaline phosphatase -increased tissues levels of Nrf2 and activities of antioxidant enzymes (catalase, glutathione peroxidase, glutathione, glutathione-s-transferase) | [92] |
| hypercholesterolemic diet-fed guinea pigs | 0.1 g lutein/100 g high cholesterol diets (0.25% cholesterol) | -reduced hepatic free cholesterol -reduced hepatic malondialdehyde level -reduced hepatic TNF-α and NF-κB DNA binding activity | [93] | |
| Kunming strain mice received D-galactose-induced oxidative stress | 10, 40 mg/kg BW | -decreased ROS contents in liver tissues -increased the activity of Na(+)- K(+)-ATPase and the activity of Ca(2+)-ATPase in liver tissues -decreased the activities of total NO synthase and iNOS and the content of NO in liver tissues -increased HO-1 mRNA, but decreased Toll-like receptor 4 mRNA expression in liver tissues | [94] | |
| arsenic trioxide-induced liver damage in Kunming mice | 40 mg/kg BW | -reduced ROS and malondialdehyde in liver tissues -increased mRNA and protein expression of Nrf2 signaling related genes [Nrf2, HO-1, NQO1, and glutathion-s-transferase] -improved hepatic function comparing with arsenic trioxide group | [95] | |
| Obesity | 3T3-L1 adipocyte model | 1, 5, 10 μM | -decreased the numbers and sizes of the lipid droplets -reduced triglyceride content in a concentration-dependent manner -suppressed the expression of transcription factors [CCAAT-enhancer-binding protein (CEBP)-α and peroxisome proliferator-activated receptor (PPAR)-γ] and associated adipogenic markers (fatty acid synthase, fatty acid-binding protein 4, and stearoyl-CoA desaturase 1) -blocked the process of adipogenesis at the early stage of adipocyte differentiation -delayed cell cycle progression with increased cell count at G0/G1 phase through regulating the levels of cyclin D and E, cyclin-dependent kinase 4, and cyclin-dependent kinase 2 | [102] |
| high fat-diet induced obesity rats | 100 mg/kg | -decreased free fatty acid levels and oxidative damage by reducing MDA levels -improved activities of SOD, catalase, and glutathione peroxidase -decreased levels of vascular endothelial growth factor, intracellular adhesion molecule-1, iNOS, and NF-κB -increased Nrf-2 and HO-1 levels | [107] | |
| Colon diseases | LPS-induced murine intestinal epithelial cells | 100 μg/mL | -reduced ROS and NO production in lipopolysaccharide-stimulated IEC-6 cells | [110] |
| dextran sulfate sodium-treated mice | 30, 100, 300 mg/kg | -attenuated weight loss, disease activity index, colon shortening, and histopathological changes in dextran sulfate sodium-treated mice -decreased myeloperoxidase activity as well as TNF and IL-6 levels -increased glutathione levels and catalase activity -normalized SOD and glutathione-s-transferase activities | [110] | |
| Diabetes | mice (model of early diabetic retinopathy) | 2.1, 4.2, 8.4 mg/kg in drinking water | -suppressed microglial reactivity -reduced the upregulation of vascular endothelial growth factor -attenuated retinal vascular leakage -protected retinas from functional impairment | [113] |
| retinal pigment epithelial cells | 0.5, 1 μM | -blocked high glucose-mediated elevation of intracellular ROS, protein carbonyl, and malondialdehyde content -reversed down-regulation of a redox-sensitive transcription factor, Nrf2, and antioxidant enzymes, SOD2, HO-1, and catalase | [114] |
| Diseases | Epidemiological Study | Study Design | Key Findings | Ref. |
|---|---|---|---|---|
| Neurodegenerative disorders | cross-sectional study, the Irish longitudinal study on aging | 4076 individuals, aged 50 and older | -higher plasma lutein and zeaxanthin were associated with better composite scores across the domains of global cognition, memory, and executive function | [31] |
| case-control study | 25 patients with mild cognitive impairment, 63 Alzheimer’s disease patients, and 53 controls, mean age of 75.8 | -plasma antioxidants (vitamin C, uric acid, vitamin A, vitamin E, carotenoids; lutein, zeaxanthin, β-cryptoxanthin, lycopene, β-carotene, α-carotene) are depleted in patients with mild cognitive impairment and Alzheimer’s disease -activities of plasma SOD and plasma glutathione peroxidase decreased in patients with mild cognitive impairment and Alzheimer’s disease | [33] | |
| cross-sectional study in the NHANES III database and the NHANES III linked mortality file | total of 6958 participants, aged 50 and older | -high serum levels of lutein + zeaxanthin at baseline were associated with a lower risk of Alzheimer’s disease mortality | [35] | |
| Eye diseases | cross-sectional study in the NHANES III (third national health and nutrition examination survey) | 8222 persons, aged 40 and older | -higher dietary intakes of lutein and zeaxanthin were related to lower odds for pigmentary abnormalities and one sign of early age-related maculopathy (determined by serum carotenoids levels, food frequency questionnaire, and retinal photographs) | [44] |
| randomized double-masked placebo-controlled clinical trial-oral treatment for 12 months | oral lutein supplementation | -treatment of oral preparation (lutein, zeaxanthin, vitamin C, vitamin E, copper, and zinc) for 12 months | [45] | |
| randomized double-masked placebo-controlled clinical trial-oral treatment for 12 months | 433 adults, aged 55 and older with early age-related macular degeneration in at least one eye, 12 months follow up | -treatment group improved retinal function and increased macular pigment, as well as visual activity | [45] | |
| cross-sectional study in the age-related eye disease study (AREDS). | 4519 AREDS participants, aged 60–80 years | -dietary lutein/zeaxanthin intake was inversely associated with neovascular age-related macular degeneration and large or extensive intermediate drusen | [55] | |
| a prospective, 12-month, randomized, double-masked, placebo-controlled trial | oral lutein supplementation 90 patients with atrophic age-related macular degeneration, mean age of 74.4 in lutein group, mean age of 76.1 in placebo group | -lutein (10 mg/d) for 12 months -lutein supplementation improved visual function -lutein supplementation increased eye macular pigment optical density | [56] | |
| Osteoporosis | systematically review | women, aged 45 and older | -two cross-sectional analyses reported positive associations between food and vegetable intake and bone mineral density of the forearm, lumbar spine, or total hip | [62] |
| cross-sectional study in the Singapore Chinese health study | 63,257 men and women, aged 45–74 years, mean follow-up of 9.9 years | -consumption of lutein/zeaxanthin had a low risk of hip fracture risk among men (interviewed on lifestyle factors and medical history) | [63] | |
| Cardiovascular diseases | The Los Angeles atherosclerosis study | 269 women (aged 45–60) and 304 men (aged 40–60) | -an inverse association between plasma lutein and progression of carotid intima-media thickness | [65] |
| case-control study from the Beijing atherosclerosis study | 125 subjects with early atherosclerosis and 107 controls, aged 45–68 years | -serum lutein level was negatively correlated with carotid intima-media thickness | [66] | |
| cross-sectional study | 134 patients with stable angina, aged 60–72 | -plasma levels of lutein + zeaxanthin were inversely correlated with plasma levels of IL-6 in stable angina patients | [76] | |
| case-control study | 39 patients with acute coronary syndrome and 50 patients with stable coronary artery disease, mean age of 59.3 50 controls, mean age of 60.8 | -both patient groups had lower plasma levels of lutein + zeaxanthin -plasma levels of lutein+zeaxanthin were associated with the proportions of natural killer cells, but not with other lymphocytes, in blood (lutein and zeaxanthin may have a particular role in the immunological scenario of coronary artery disease) | [77] | |
| mini-review of revisit data in Toulouse and Belfast in 1992–1993 | 89 men and 82 women, aged 45–65 in 1992 102 salaried men and 109 women, aged 45–64 in 1993 | -serum level of lutein was twice as high in Toulouse in Southern France as in Belfast in Northern Ireland in both men and women (incidence of coronary heart disease in Southern France was among the lowest in Europe and was higher in Northern Ireland) -high dietary intake of lutein reduced plasma concentrations of complement factors C3 and C3a as well as the membrane attack complex, the atherosclerosis indices | [78] | |
| Skin diseases | a randomized, double-blind, placebo-controlled clinical trial | oral supplementation 50 healthy people, aged 18–45 with mild-to-moderate dry skin | -oral dietary supplement containing lutein (10 mg /d) and zeaxanthin isomers (2 mg /d) for 12 weeks- -treatment group improved overall skin tone and luminance values -treatment group increased mean minimal erythemal dose and the individual typological angle | [80] |
| a randomized, double-Blind, placebo-controlled, 12-week clinical study | either oral, topical, or combined oral and topical administration 40 healthy women, aged 25–50 | -either oral (lutein 10 mg/d, zeaxanthin 0.6 mg/d), topical (lutein 10 ppm/d, zeaxanthin 6 ppm/d), or mixed oral and topical administration of lutein and zeaxanthin -combined administration showed the highest effect on decreasing skin lipid peroxidation (reduced skin malondialdehyde level) -all three groups showed photoprotection against UV light irradiation-induced skin damage -all three groups improved skin elasticity and skin hydration | [85] | |
| Obesity | cross-sectional study in NHANES III | total of 4231 males and nonpregnant females, aged 6–16 | -serum level of high-density lipoprotein was directly related to serum levels of lutein + zeaxanthin -serum levels of C-reactive protein, an inflammation marker, were inversely related to serum levels of lutein + zeaxanthin | [101] |
| cross-sectional study in NHANES III | 8808 U.S. adults, aged 20 and older with and without the metabolic syndrome | -the age-adjusted concentration of lutein + zeaxanthin was lower in participants with metabolic syndrome than that of healthy control without the metabolic syndrome | [103] | |
| a population-based, cross-sectional study | 374 men, aged 40–80 | -higher lutein+zeaxanthin intakes were associated with lower subcutaneous fat mass | [104] | |
| Diabetes | systemic review with meta analysis | 71 relevant articles (including 387,569 participants) | -there was an inconsistent association with higher dietary lutein intake and insulin resistance | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. https://doi.org/10.3390/antiox10091448
Ahn YJ, Kim H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants. 2021; 10(9):1448. https://doi.org/10.3390/antiox10091448
Chicago/Turabian StyleAhn, Yu Jin, and Hyeyoung Kim. 2021. "Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases" Antioxidants 10, no. 9: 1448. https://doi.org/10.3390/antiox10091448
APA StyleAhn, Y. J., & Kim, H. (2021). Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants, 10(9), 1448. https://doi.org/10.3390/antiox10091448







