Attenuation of Polycyclic Aromatic Hydrocarbon (PAH)-Mediated Pulmonary DNA Adducts and Cytochrome P450 (CYP)1B1 by Dietary Antioxidants, Omega-3 Fatty Acids, in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Diets
2.4. Animal Experiments
2.4.1. Animal Experiment 1
2.4.2. Animal Experiment 2
2.5. DNA Adduct Analyses
2.6. qPCR Analysis
2.7. Western Blot Analysis
3. Results
3.1. Animal Experiment 1
3.1.1. Diet and Body Weights
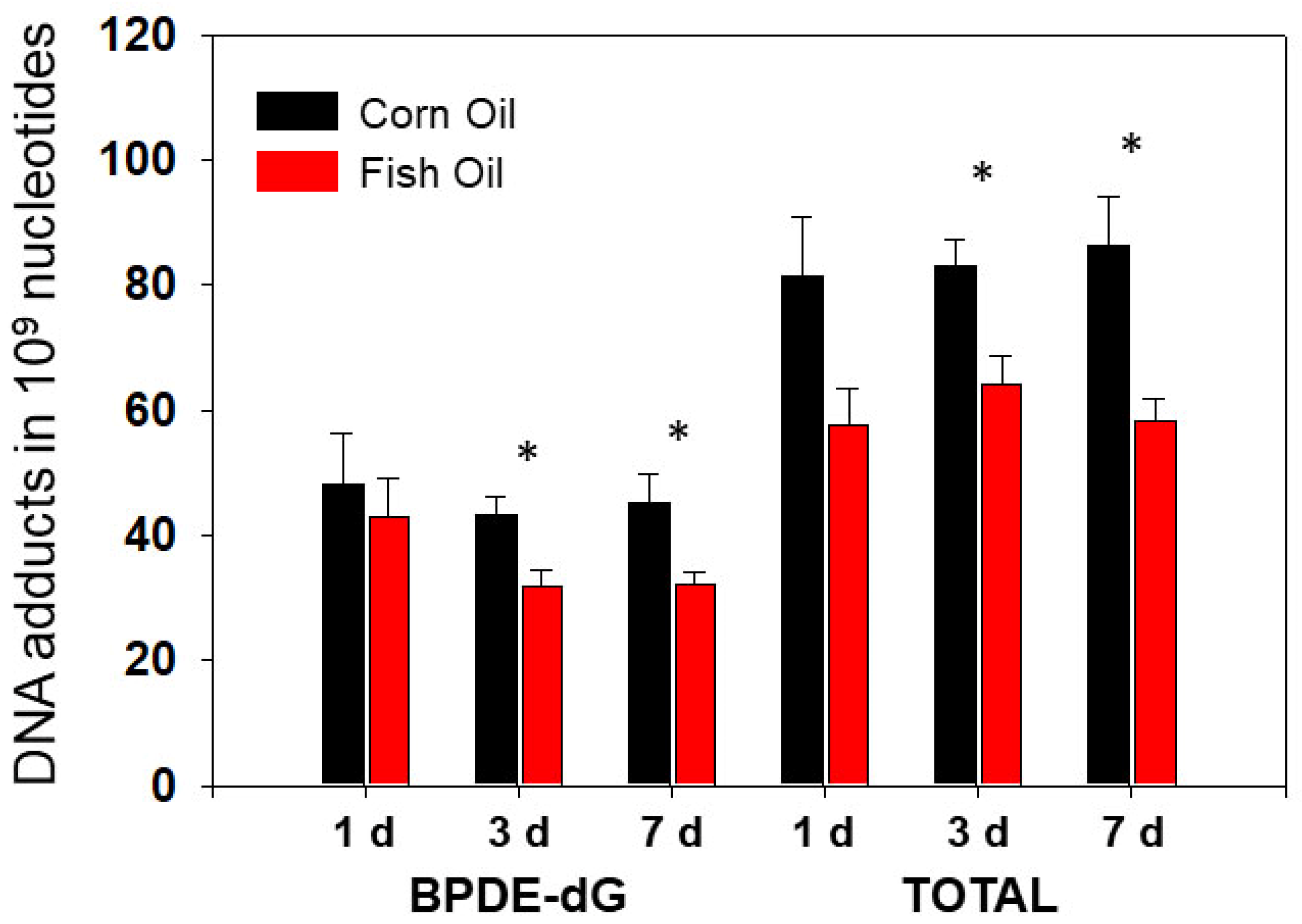
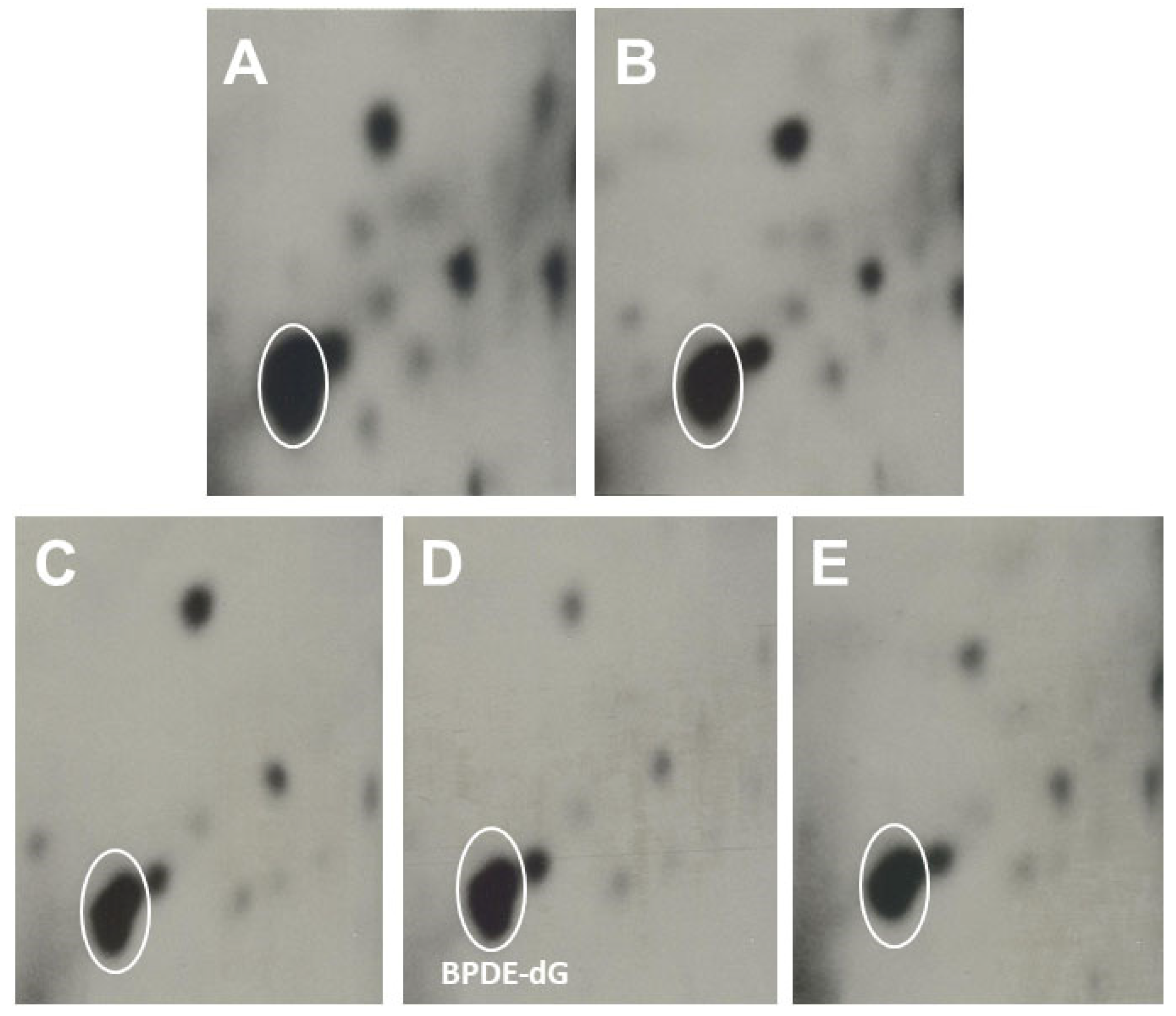
3.1.2. The Effects of Dietary Fish Oil on Pulmonary PAH-DNA Adducts
3.1.3. Histological Examination
3.2. Animal Experiment 2
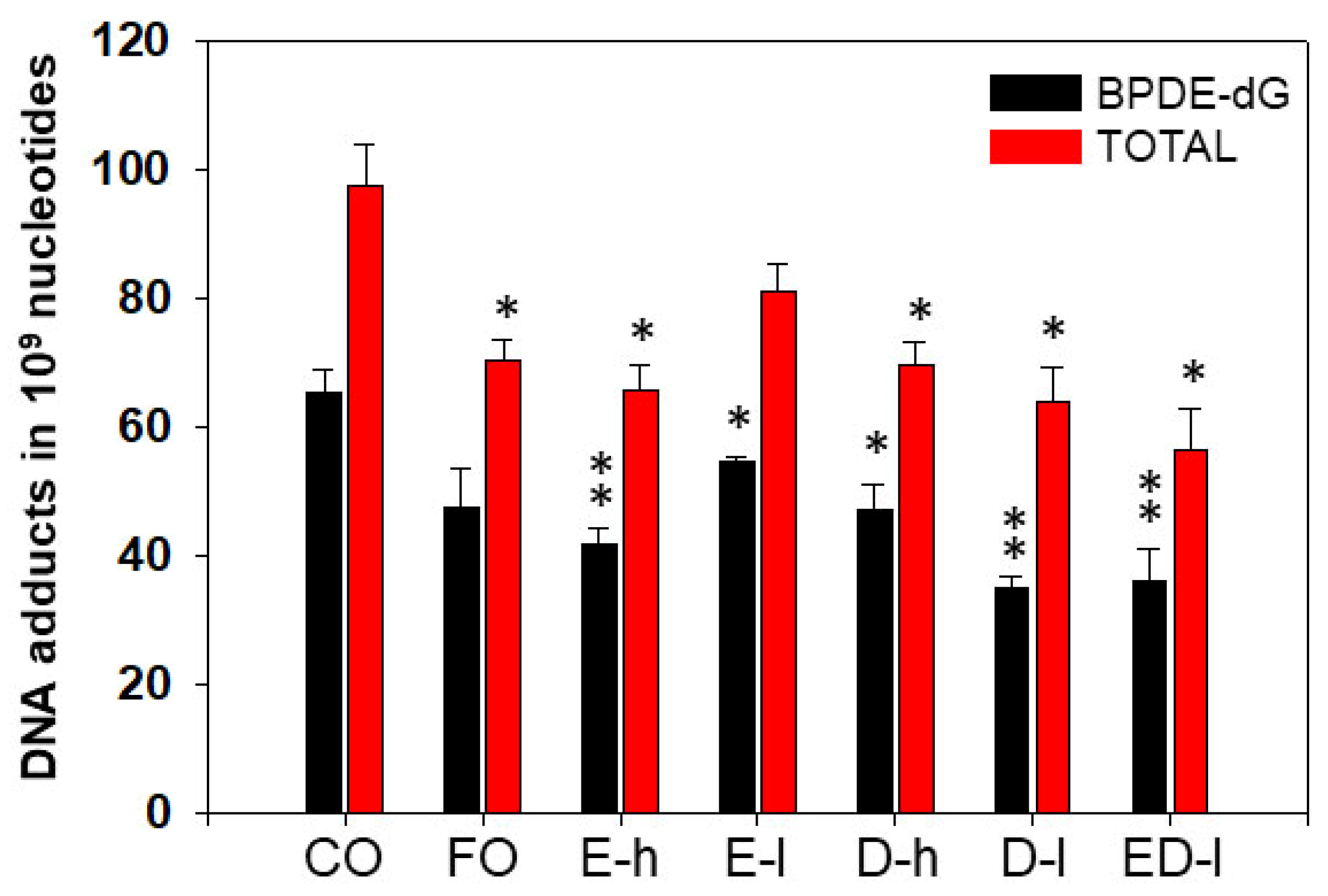
3.2.1. The Effects of Omega-3 Fatty Acids EPA and DHA on Pulmonary PAH-DNA Adducts
3.2.2. The Effects of Omega-3 Fatty Acids EPA and DHA on Hepatic PAH-DNA Adducts
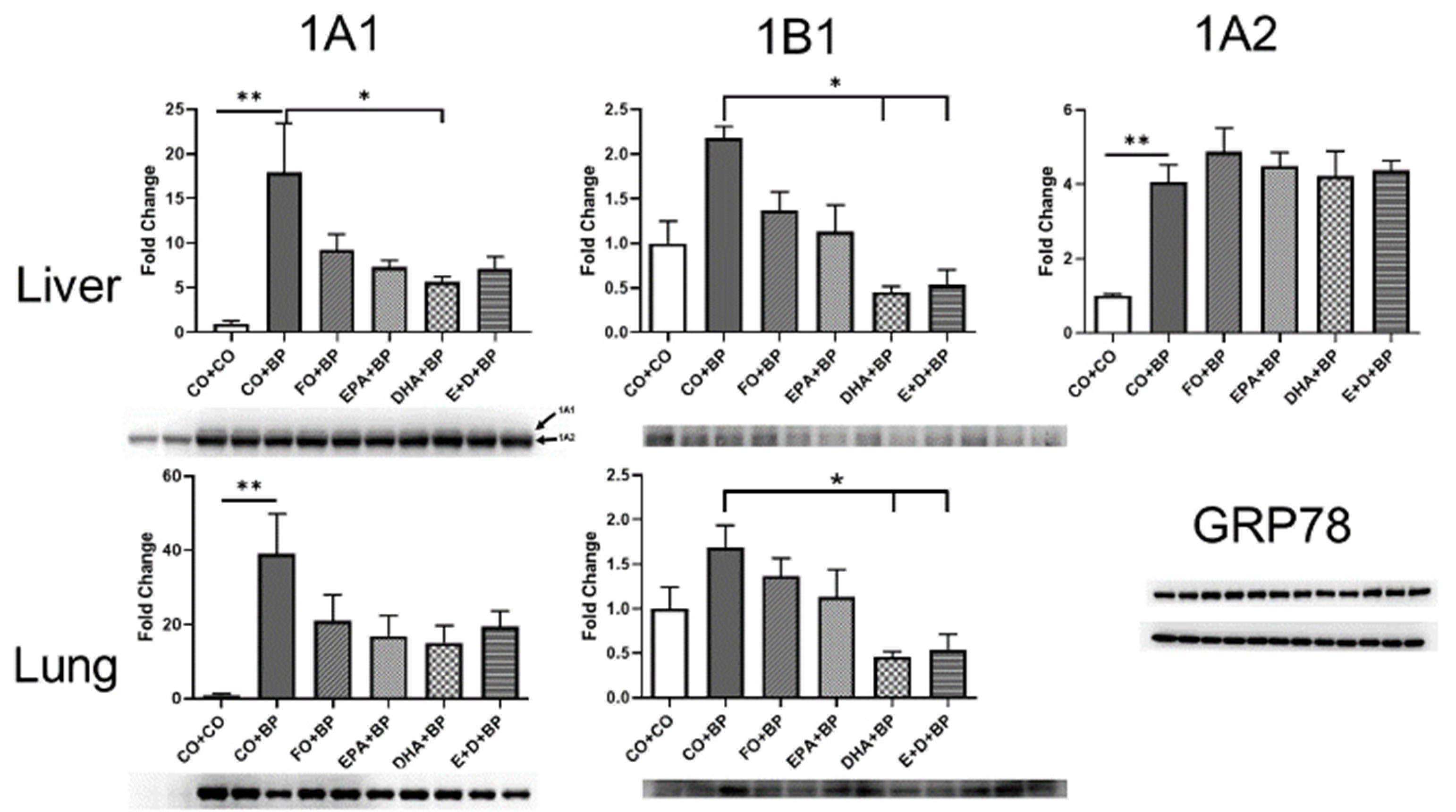
3.2.3. The Modulation of CYP and AHR Genes by BP and Omega-3 Fatty Acids
3.2.4. Western Blot Analysis
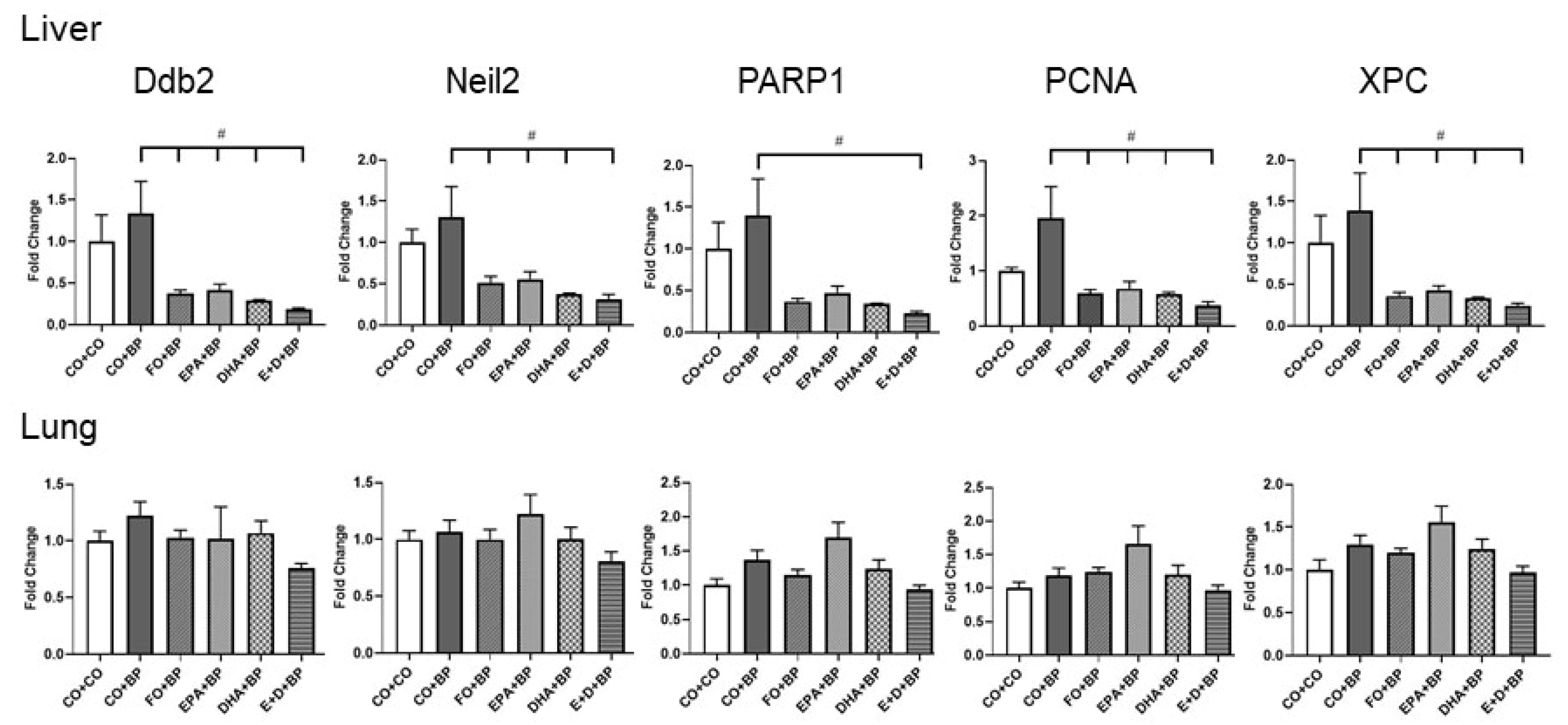
3.2.5. The Modulation of DNA Repair Genes
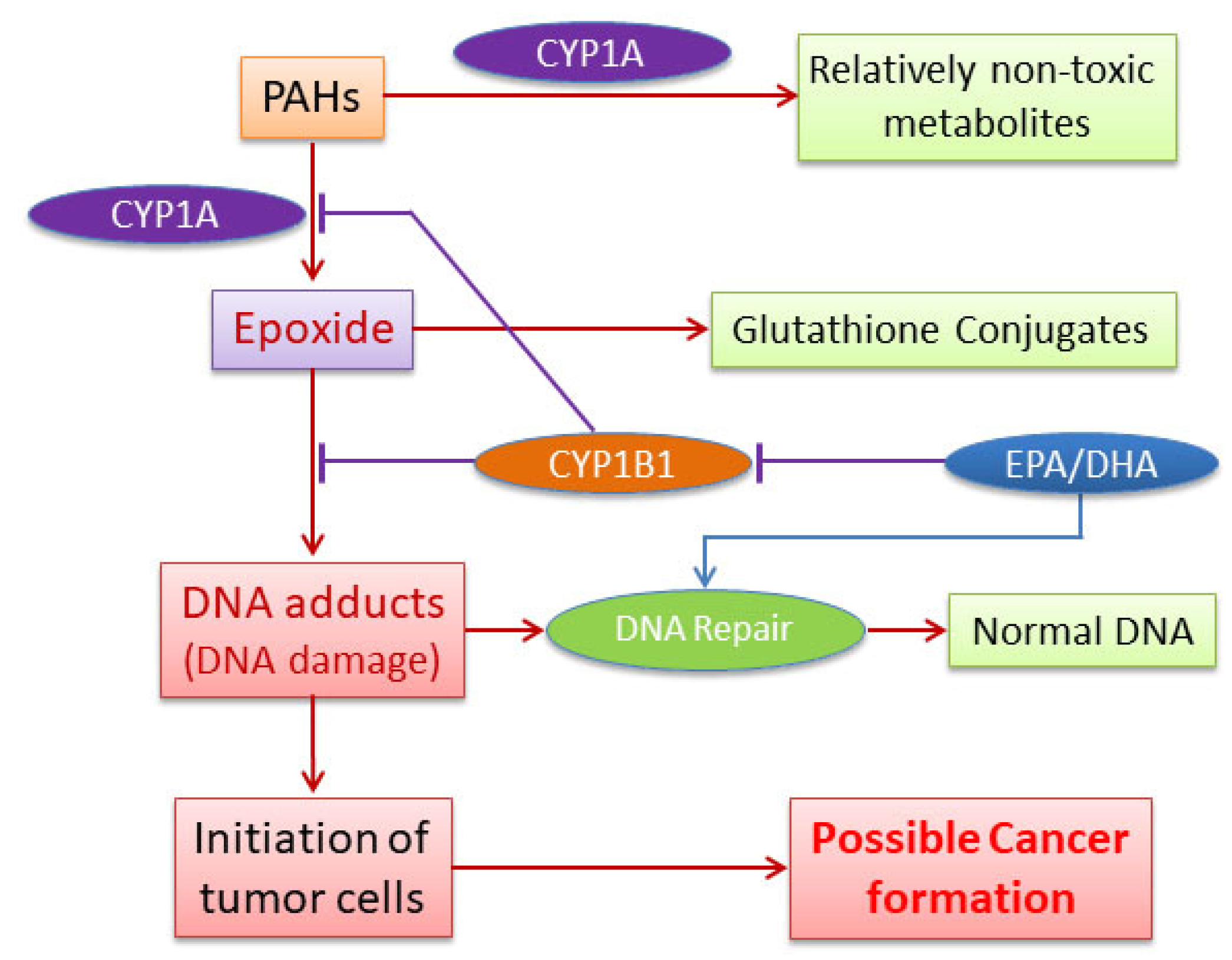
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Air pollution in relation to U.S. cancer mortality rates: An ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res. 2009, 29, 3537–3545. [Google Scholar] [PubMed]
- Petit, P.; Maitre, A.; Persoons, R.; Bicout, D.J. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef]
- Suter, M.A.; Aagaard, K.M.; Coarfa, C.; Robertson, M.; Zhou, G.; Jackson, B.P.; Thompson, D.; Putluri, V.; Putluri, N.; Hagan, J.; et al. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem. Biophys. Res. Commun. 2019, 516, 344–349. [Google Scholar] [CrossRef]
- Phillips, T.D.; Richardson, M.; Cheng, Y.S.; He, L.; McDonald, T.J.; Cizmas, L.H.; Safe, S.H.; Donnelly, K.C.; Wang, F.; Moorthy, B.; et al. Mechanistic relationships between hepatic genotoxicity and carcinogenicity in male B6C3F1 mice treated with polycyclic aromatic hydrocarbon mixtures. Arch. Toxicol. 2015, 89, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [Green Version]
- Stading, R.; Gastelum, G.; Chu, C.; Jiang, W.; Moorthy, B. Molecular mechanisms of pulmonary carcinogenesis by polycyclic aromatic hydrocarbons (PAHs): Implications for human lung cancer. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Gastelum, G.; Jiang, W.; Wang, L.; Zhou, G.; Borkar, R.; Putluri, N.; Moorthy, B. Polycyclic Aromatic Hydrocarbon-induced Pulmonary Carcinogenesis in Cytochrome P450 (CYP)1A1- and 1A2-Null Mice: Roles of CYP1A1 and CYP1A2. Toxicol. Sci. 2020, 177, 347–361. [Google Scholar] [CrossRef]
- Tang, D.; Phillips, D.H.; Stampfer, M.; Mooney, L.A.; Hsu, Y.; Cho, S.; Tsai, W.Y.; Ma, J.; Cole, K.J.; She, M.N.; et al. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Cancer Res. 2001, 61, 6708–6712. [Google Scholar]
- Ceppi, M.; Munnia, A.; Cellai, F.; Bruzzone, M.; Peluso, M.E.M. Linking the generation of DNA adducts to lung cancer. Toxicology 2017, 390, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Munnia, A.; Giese, R.W.; Polvani, S.; Galli, A.; Cellai, F.; Peluso, M.E.M. Bulky DNA Adducts, Tobacco Smoking, Genetic Susceptibility, and Lung Cancer Risk. Adv. Clin. Chem. 2017, 81, 231–277. [Google Scholar] [CrossRef]
- Gilberson, T.; Peluso, M.E.; Munia, A.; Lujan-Barroso, L.; Sanchez, M.J.; Navarro, C.; Amiano, P.; Barricarte, A.; Quiros, J.R.; Molina-Montes, E.; et al. Aromatic adducts and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Spanish cohort. Carcinogenesis 2014, 35, 2047–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, H.; Autrup, H.; Thomsen, B.L.; Tjonneland, A.; Overvad, K.; Vogel, U.; Raaschou-Nielsen, O.; Loft, S. Bulky DNA adducts as risk indicator of lung cancer in a Danish case-cohort study. Int. J. Cancer 2006, 118, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Xing, G.; Ren, J.; Gu, J. Role of hepatic cytochrome P450 enzymes in the detoxication of aristolochic acid I; effects on DNA adduct, mutation, and tumor formation. Genes Environ. 2015, 37, 11. [Google Scholar] [CrossRef] [Green Version]
- Hang, B. Repair of exocyclic DNA adducts: Rings of complexity. Bioessays 2004, 26, 1195–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.E.; Hu, Z.; Sturgis, E.M.; Spitz, M.R.; Strom, S.S.; Amos, C.I.; Guo, Z.; Qiao, Y.; Gillenwater, A.M.; Myers, J.N.; et al. Reduced DNA repair capacity for removing tobacco carcinogen-induced DNA adducts contributes to risk of head and neck cancer but not tumor characteristics. Clin. Cancer Res. 2010, 16, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Foresta, M.; Izzotti, A.; La Maestra, S.; Micale, R.; Poggi, A.; Vecchio, D.; Frosina, G. Accelerated repair and reduced mutagenicity of DNA damage induced by cigarette smoke in human bronchial cells transfected with E. coli formamidopyrimidine DNA glycosylase. PLoS ONE 2014, 9, e87984. [Google Scholar] [CrossRef]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Manson, M.M.; Farmer, P.B.; Gescher, A.; Steward, W.P. Innovative agents in cancer prevention. Recent Results Cancer Res. Fortschr. Krebsforsch. 2005, 166, 257–275. [Google Scholar]
- Barhoumi, R.; Mouneimne, Y.; Chapkin, R.S.; Burghardt, R.C. Effects of Fatty Acids on Benzo[a]pyrene Uptake and Metabolism in Human Lung Adenocarcinoma A549 Cells. PLoS ONE 2014, 9, e90908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.D.; Richardson, M.; Fazili, I.S.; Wang, J.; Donnelly, K.C.; Wang, F.; Amendt, B.; Moorthy, B. Role of retinoic acid in the modulation of benzo(a)pyrene-DNA adducts in human hepatoma cells: Implications for cancer prevention. Toxicol. Appl. Pharmacol. 2010, 249, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Piazzi, G.; D’Argenio, G.; Prossomariti, A.; Lembo, V.; Mazzone, G.; Candela, M.; Biagi, E.; Brigidi, P.; Vitaglione, P.; Fogliano, V.; et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int. J. Cancer 2014, 135, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Song, K.S.; Shin, S.; Kim, N.; Jeong, S.; Oh, H.R.; Park, J.H.; Seo, K.S.; Heo, J.Y.; Han, J.; et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy 2011, 7, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.; Han, C.; Xu, L.; Isse, K.; Demetris, A.J.; Wu, T. Cyclooxygenase-2-derived prostaglandin E2 activates beta-catenin in human cholangiocarcinoma cells: Evidence for inhibition of these signaling pathways by omega 3 polyunsaturated fatty acids. Cancer Res. 2008, 68, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.Y. Chemopreventive agent-induced modulation of death receptors. Apoptosis 2005, 10, 1203–1210. [Google Scholar] [CrossRef]
- Zhou, G.D.; Zhu, H.; Phillips, T.D.; Wang, J.; Wang, S.Z.; Wang, F.; Amendt, B.A.; Couroucli, X.I.; Donnelly, K.C.; Moorthy, B. Effects of dietary fish oil on the depletion of carcinogenic PAH-DNA adduct levels in the liver of B6C3F1 mouse. PLoS ONE 2011, 6, e26589. [Google Scholar] [CrossRef]
- Mernitz, H.; Lian, F.; Smith, D.E.; Meydani, S.N.; Wang, X.D. Fish oil supplementation inhibits NNK-induced lung carcinogenesis in the A/J mouse. Nutr. Cancer 2009, 61, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Song, E.A.; Kim, H. Docosahexaenoic Acid Induces Oxidative DNA Damage and Apoptosis, and Enhances the Chemosensitivity of Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.V.; Randerath, K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis 1986, 7, 1543–1551. [Google Scholar] [CrossRef]
- Zhou, G.D.; Hernandez, N.S.; Randerath, E.; Randerath, K. Acute elevation by short-term dietary restriction or food deprivation of type I I-compound levels in rat liver DNA. Nutr. Cancer 1999, 35, 87–95. [Google Scholar] [CrossRef]
- Gupta, R.C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc. Natl. Acad. Sci. USA 1984, 81, 6943–6947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randerath, K.; Reddy, M.V.; Gupta, R.C. 32P-labeling test for DNA damage. Proc. Natl. Acad. Sci. USA 1981, 78, 6126–6129. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Cabrera, R.M.; Wlodarczyk, B.J.; Bozinov, D.; Wang, D.; Schwartz, R.J.; Finnell, R.H. Differentially expressed genes in embryonic cardiac tissues of mice lacking Folr1 gene activity. BMC Dev. Biol. 2007, 7, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.D.; Randerath, K.; Donnelly, K.C.; Jaiswal, A.K. Effects of NQO1 deficiency on levels of cyclopurines and other oxidative DNA lesions in liver and kidney of young mice. Int. J. Cancer 2004, 112, 877–883. [Google Scholar] [CrossRef]
- Randerath, K.; Randerath, E.; Zhou, G.D.; Supunpong, N.; He, L.Y.; McDonald, T.J.; Donnelly, K.C. Genotoxicity of complex PAH mixtures recovered from contaminated lake sediments as assessed by three different methods. Environ. Mol. Mutagenesis 1999, 33, 303–312. [Google Scholar] [CrossRef]
- Chang, W.C.; Chapkin, R.S.; Lupton, J.R. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis 1997, 18, 721–730. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.D.; Popovic, N.; Lupton, J.R.; Turner, N.D.; Chapkin, R.S.; Donnelly, K.C. Tissue-specific attenuation of endogenous DNA I-compounds in rats by carcinogen azoxymethane: Possible role of dietary fish oil in colon cancer prevention. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Mabon, N.; Moorthy, B.; Randerath, E.; Randerath, K. Monophosphate 32P-postlabeling assay of DNA adducts from 1,2:3,4-diepoxybutane, the most genotoxic metabolite of 1,3-butadiene: In vitro methodological studies and in vivo dosimetry. Mutat. Res. 1996, 371, 87–104. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall: Hoboken, NJ, USA, 2009. [Google Scholar]
- Randerath, E.; Zhou, G.D.; Donnelly, K.C.; Safe, S.H.; Randerath, K. DNA damage induced in mouse tissues by organic wood preserving waste extracts as assayed by 32P-postlabeling. Arch. Toxicol. 1996, 70, 683–695. [Google Scholar] [CrossRef]
- Miller, B.G.; Doust, E.; Cherrie, J.W.; Hurley, J.F. Lung cancer mortality and exposure to polycyclic aromatic hydrocarbons in British coke oven workers. BMC Public Health 2013, 13, 962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 2002, 23, 1979–2004. [Google Scholar] [CrossRef] [Green Version]
- Poirier, M.C.; Beland, F.A. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in rodents. Environ. Health Perspect. 1994, 102 (Suppl. 6), 161–165. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Takezaki, T.; Inoue, M.; Kataoka, H.; Ikeda, S.; Yoshida, M.; Ohashi, Y.; Tajima, K.; Tominaga, S. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: With special reference to fish consumption. Nutr. Cancer 2003, 45, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.M.; Abkenari, S.; Tong, Z.; Tedman, A.; Huerta-Yepez, S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer 2021, 73, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Seong, H.J.; Kim, G.; Jeong, G.H.; Kim, J.Y.; Park, H.; Jung, E.; Kronbichler, A.; Eisenhut, M.; Stubbs, B.; et al. Consumption of Fish and omega-3 Fatty Acids and Cancer Risk: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2020, 11, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Connolly, J.M. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol. Ther. 1999, 83, 217–244. [Google Scholar] [CrossRef]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids--current state and future perspectives. Anti-Cancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
- Zhang, G.; Panigrahy, D.; Mahakian, L.M.; Yang, J.; Liu, J.Y.; Stephen Lee, K.S.; Wettersten, H.I.; Ulu, A.; Hu, X.; Tam, S.; et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 6530–6535. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.Y.; Lupton, J.R.; Morris, J.S.; Wang, N.; Carroll, R.J.; Davidson, L.A.; Elder, R.H.; Chapkin, R.S. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol. Biomark. Prev. 2000, 9, 819–826. [Google Scholar]
- Hong, M.Y.; Bancroft, L.K.; Turner, N.D.; Davidson, L.A.; Murphy, M.E.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr. Cancer 2005, 52, 166–175. [Google Scholar] [CrossRef]
- Jayathilake, A.G.; Senior, P.V.; Su, X.Q. Krill oil extract suppresses cell growth and induces apoptosis of human colorectal cancer cells. BMC Complement. Altern. Med. 2016, 16, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Dituri, F.; Caruso, M.G.; Giannelli, G. Dietary omega-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation. Int. J. Mol. Sci. 2017, 18, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, P.H.; Bolt, H.M. Cytochrome P450 interactions in human cancers: New aspects considering CYP1B1. Expert Opin. Drug Metab. Toxicol. 2005, 1, 187–202. [Google Scholar] [CrossRef]
- Thakur, V.S.; Liang, Y.W.; Lingappan, K.; Jiang, W.; Wang, L.; Barrios, R.; Zhou, G.; Guntupalli, B.; Shivanna, B.; Maturu, P.; et al. Increased susceptibility to hyperoxic lung injury and alveolar simplification in newborn rats by prenatal administration of benzo[a]pyrene. Toxicol. Lett. 2014, 230, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Miller, K.P.; Jiang, W.; Williams, E.S.; Kondraganti, S.R.; Ramos, K.S. Role of cytochrome P4501B1 in benzo[a]pyrene bioactivation to DNA-binding metabolites in mouse vascular smooth muscle cells: Evidence from 32P-postlabeling for formation of 3-hydroxybenzo[a]pyrene and benzo[a]pyrene-3,6-quinone as major proximate genotoxic intermediates. J. Pharmacol. Exp. Ther. 2003, 305, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.C.; Kim, M.; Kim, K.S.; Kim, H.S.; Sung, J.S. Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2’-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef]
- Ali, F.F.; Rifaai, R.A. Preventive effect of omega-3 fatty acids in a rat model of stress-induced liver injury. J. Cell Physiol. 2019, 234, 11960–11968. [Google Scholar] [CrossRef]
- Wiest, E.F.; Walsh-Wilcox, M.T.; Walker, M.K. Omega-3 Polyunsaturated Fatty Acids Protect Against Cigarette Smoke-Induced Oxidative Stress and Vascular Dysfunction. Toxicol. Sci. 2017, 156, 300–310. [Google Scholar] [CrossRef]
- Li, Z.; Pearlman, A.H.; Hsieh, P. DNA mismatch repair and the DNA damage response. DNA Repair 2016, 38, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudek, A.; Czerwinska, J.; Kosicki, K.; Zdzalik-Bielecka, D.; Shahmoradi Ghahe, S.; Bazlekowa-Karaban, M.; Borsuk, E.M.; Speina, E. DNA damage, repair and the improvement of cancer therapy—A tribute to the life and research of Barbara Tudek. Mutat. Res. 2020, 852, 503160. [Google Scholar] [CrossRef] [PubMed]
- Abbotts, R.; Wilson, D.M., 3rd. Coordination of DNA single strand break repair. Free. Radic. Biol. Med. 2017, 107, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Dalton, T.P.; Derkenne, S.; Curran, C.P.; Miller, M.L.; Shertzer, H.G.; Nebert, D.W. Oral exposure to benzo[a]pyrene in the mouse: Detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004, 65, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Uno, S.; Dalton, T.P.; Dragin, N.; Curran, C.P.; Derkenne, S.; Miller, M.L.; Shertzer, H.G.; Gonzalez, F.J.; Nebert, D.W. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol. Pharmacol. 2006, 69, 1103–1114. [Google Scholar] [CrossRef]
- Shi, Z.; Dragin, N.; Galvez-Peralta, M.; Jorge-Nebert, L.F.; Miller, M.L.; Wang, B.; Nebert, D.W. Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Mol. Pharmacol. 2010, 78, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, M.D.; Sohn, K.H.; Rhee, S.H.; Lee, S.W.; Hunt, J.D.; Hwang, D.H. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: Mediation through cyclooxygenase-independent pathways. Cancer Res. 2001, 61, 1386–1391. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Keffer, J.; Emami, A.; Ma, X.; Lan, R.; Goldman, R.; Chung, F.L. Acrolein-derived DNA adduct formation in human colon cancer cells: Its role in apoptosis induction by docosahexaenoic acid. Chem. Res. Toxicol. 2009, 22, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Investig. 2007, 117, 1866–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strouch, M.J.; Ding, Y.; Salabat, M.R.; Melstrom, L.G.; Adrian, K.; Quinn, C.; Pelham, C.; Rao, S.; Adrian, T.E.; Bentrem, D.J.; et al. A High Omega-3 Fatty Acid Diet Mitigates Murine Pancreatic Precancer Development. J. Surg. Res. 2009, 165, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jude, S.; Roger, S.; Martel, E.; Besson, P.; Richard, S.; Bougnoux, P.; Champeroux, P.; Le Guennec, J.Y. Dietary long-chain omega-3 fatty acids of marine origin: A comparison of their protective effects on coronary heart disease and breast cancers. Prog. Biophys. Mol. Biol. 2006, 90, 299–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Jiang, W.; Xia, G.; Wang, L.; Richardson, M.; Chu, C.; Moorthy, B. Attenuation of Polycyclic Aromatic Hydrocarbon (PAH)-Mediated Pulmonary DNA Adducts and Cytochrome P450 (CYP)1B1 by Dietary Antioxidants, Omega-3 Fatty Acids, in Mice. Antioxidants 2022, 11, 119. https://doi.org/10.3390/antiox11010119
Zhou G, Jiang W, Xia G, Wang L, Richardson M, Chu C, Moorthy B. Attenuation of Polycyclic Aromatic Hydrocarbon (PAH)-Mediated Pulmonary DNA Adducts and Cytochrome P450 (CYP)1B1 by Dietary Antioxidants, Omega-3 Fatty Acids, in Mice. Antioxidants. 2022; 11(1):119. https://doi.org/10.3390/antiox11010119
Chicago/Turabian StyleZhou, Guodong, Weiwu Jiang, Guobin Xia, Lihua Wang, Molly Richardson, Chun Chu, and Bhagavatula Moorthy. 2022. "Attenuation of Polycyclic Aromatic Hydrocarbon (PAH)-Mediated Pulmonary DNA Adducts and Cytochrome P450 (CYP)1B1 by Dietary Antioxidants, Omega-3 Fatty Acids, in Mice" Antioxidants 11, no. 1: 119. https://doi.org/10.3390/antiox11010119
APA StyleZhou, G., Jiang, W., Xia, G., Wang, L., Richardson, M., Chu, C., & Moorthy, B. (2022). Attenuation of Polycyclic Aromatic Hydrocarbon (PAH)-Mediated Pulmonary DNA Adducts and Cytochrome P450 (CYP)1B1 by Dietary Antioxidants, Omega-3 Fatty Acids, in Mice. Antioxidants, 11(1), 119. https://doi.org/10.3390/antiox11010119





