Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases
Abstract
:1. Introduction
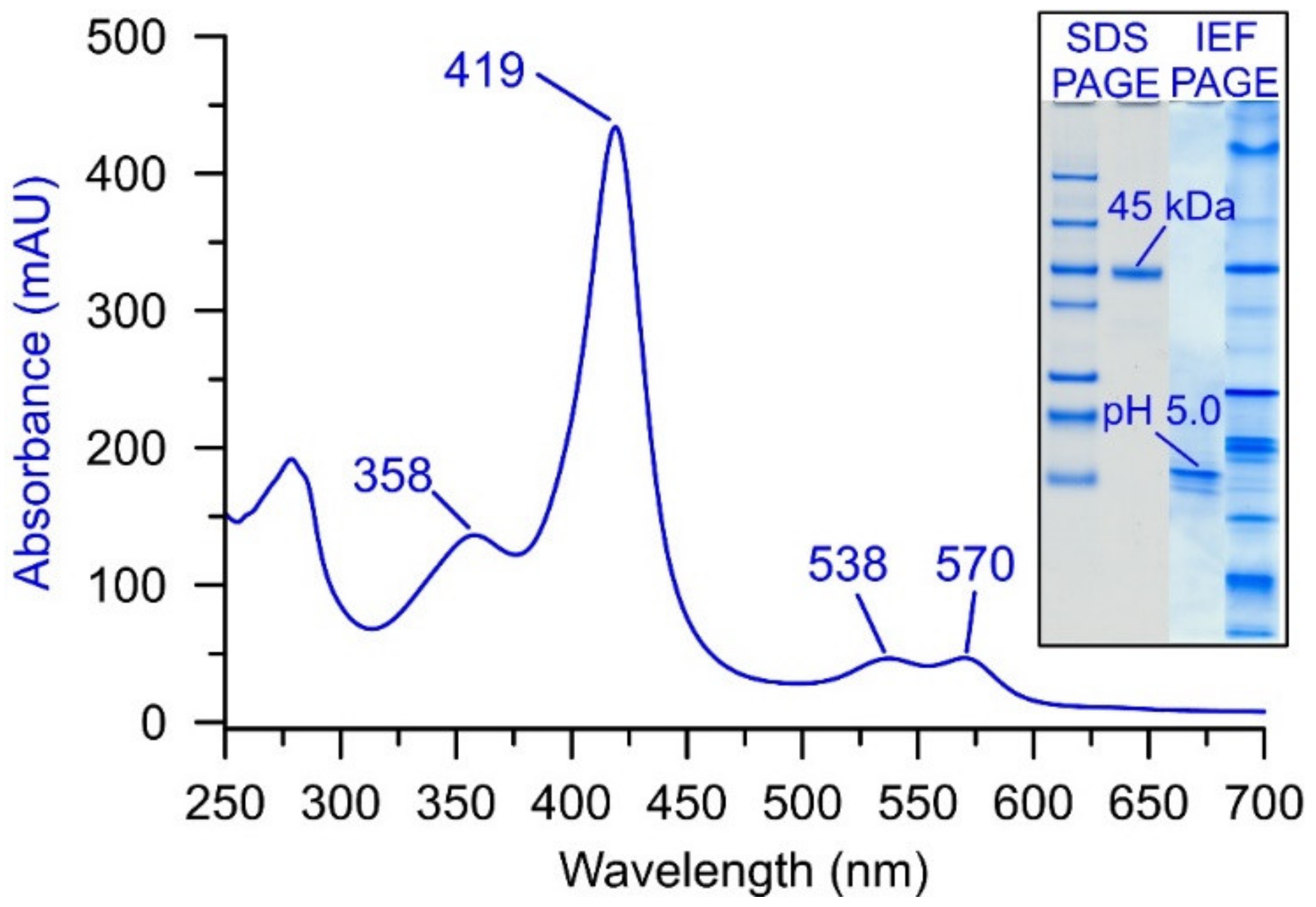
2. UPO Production and Purification
| Enzyme | Donating Organism | Expression System | Mutant | Molecular Weight (kDa) | Secretion Level (mg L−1) | Ref. |
|---|---|---|---|---|---|---|
| AaeUPO | Agrocybe aegerita (syn. Cyclocybe) | Wild-type | No | 45–46 (37 *) | 10 | [5,32,39] |
| rAaeUPO (PaDa-I) | A. aegerita | Saccharomyces cerevisiae, Pichia pastoris | Yes | 51 | 300 | [45,46,53] |
| rAaeUPO | A. aegerita | P. pastoris | No | - | 290 | [54] |
| rAniUPO | Aspergillus niger | P. pastoris | No | 60 | - | [55] |
| rCciUPO | Coprinopsis cinerea | Aspergillus oryzae | No | 44 | - | [12,18] |
| CglUPO | Chaetomium globosum | Wild-type | No | 36 | 44 | [38] |
| rCglUPO | C. globosum | A. oryzae | No | - | - | [18] |
| CPO (LfuUPO) | Leptoxyphium fumago (syn. Caldariomyces) | Wild-type | No | 40–42 | 600 | [56,57,58] |
| rLfuUPO | L. fumago | A. niger | No | 45-50 | 10 | [59] |
| CraUPO | Coprinellus radians | Wild-type | No | 43–45 (27 *) | - | [36] |
| CveUPO | Coprinopsis verticillata | Wild-type | No | 40 (23 *) | - | [41] |
| rCviUPO | Collariella virescens | A. oryzae, Escherichia coli | No | - | 7 | [18,60] |
| rDcaUPO | Daldinia caldariorum | A. oryzae, E. coli | No | - | 3 | [18,60] |
| rHinUPO | Humicola insolens | A. oryzae | No | - | - | [18,38] |
| HspUPO | Hypoxylon sp. EC38 | S. cerevisiae | No | 55 (28 *) | 200 | [61] |
| MroUPO | Marasmius rotula | Wild-type | No | 32 (27 *); 64 ** | 450 | [34,62] |
| MweUPO | M. wettsteinii | Wild-type | No | 32; 62 ** | - | [35] |
| rMfeUPO | Myceliophthora fergusii | A. oryzae | No | - | - | [18] |
| rMhiUPO | M. hinnulea | A. oryzae | No | - | - | [18] |
| PabUPO | Candolleomyces aberdarensis (syn. Psathyrella aberdarensis) | Wild-type | No | 40–41 | - | [29,63] |
| rPabUPO-I (Grogu) | C. aberdarensis | S. cerevisiae P. pastoris | Yes Yes | 47 - | 14 290 | [64] [64] |
| rPabUPO-II | C. aberdarensis | S. cerevisiae | No | 45 | 5.4 | [64] |
| rPviUPO | Pestalotiopsis virgatula | A. oryzae | No | - | - | [18] |
| rThyUPO | Thielavia hyrcaniae | A. oryzae | No | - | - | [18] |
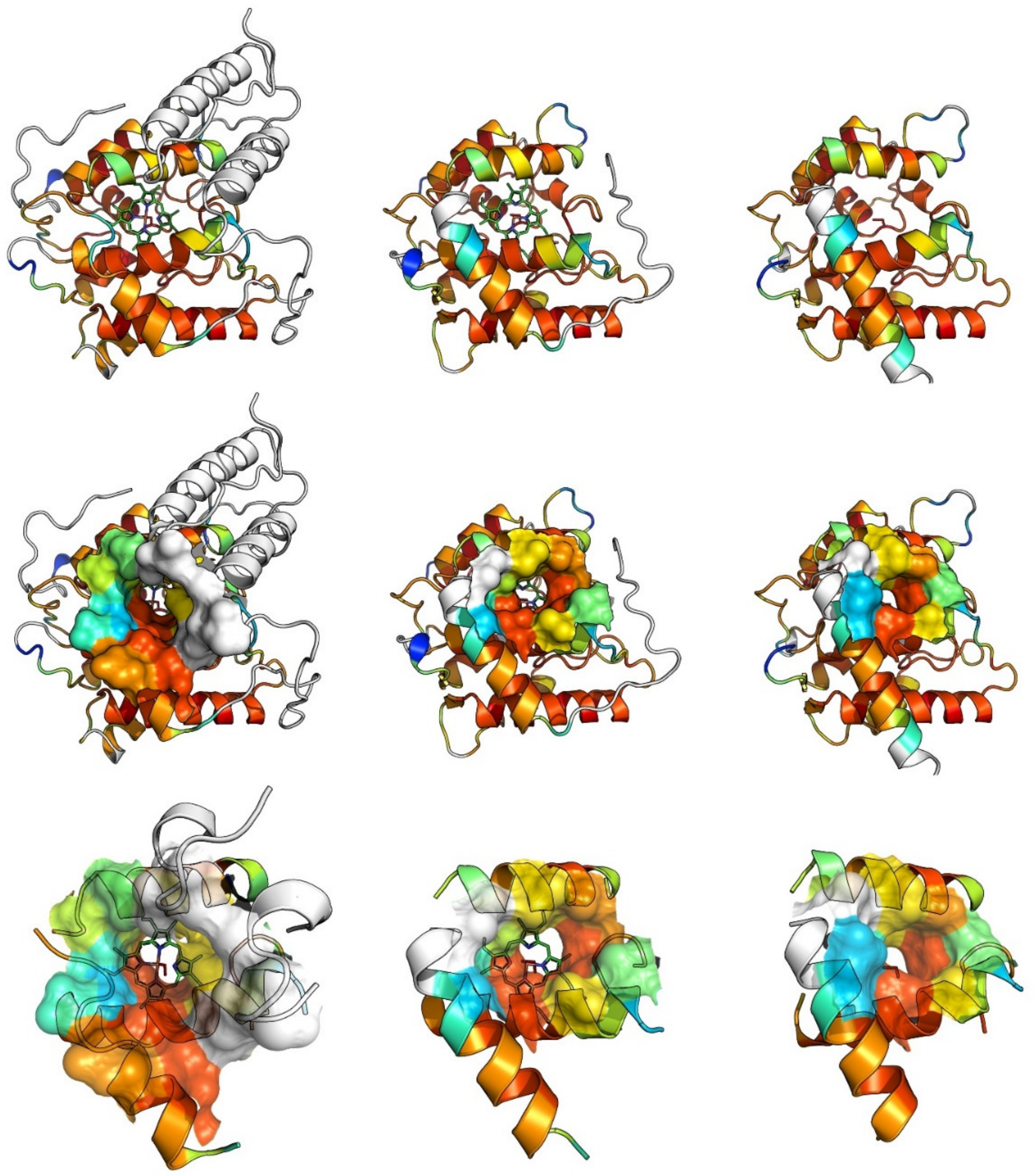
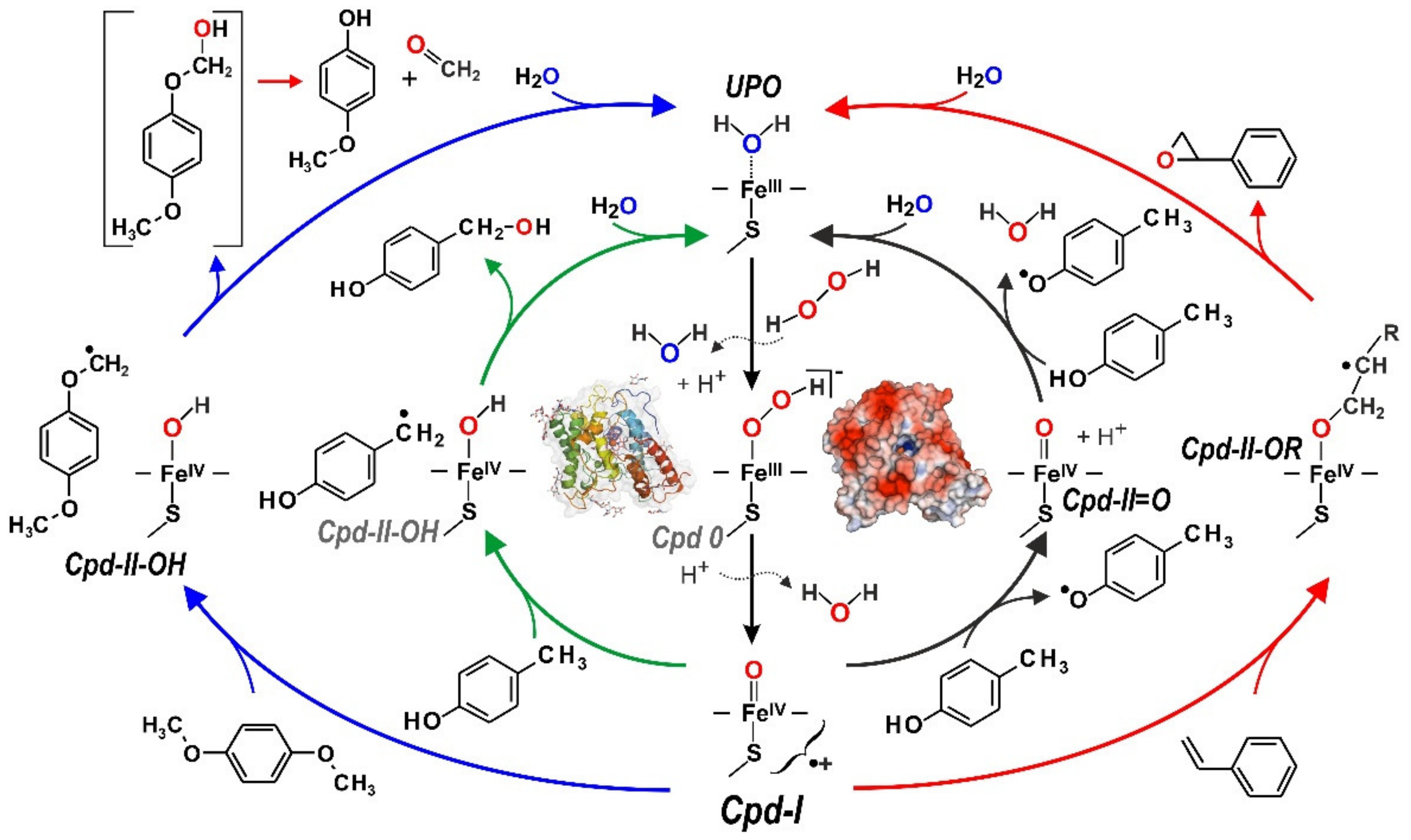
3. UPO Characteristics and Catalytic Cycles
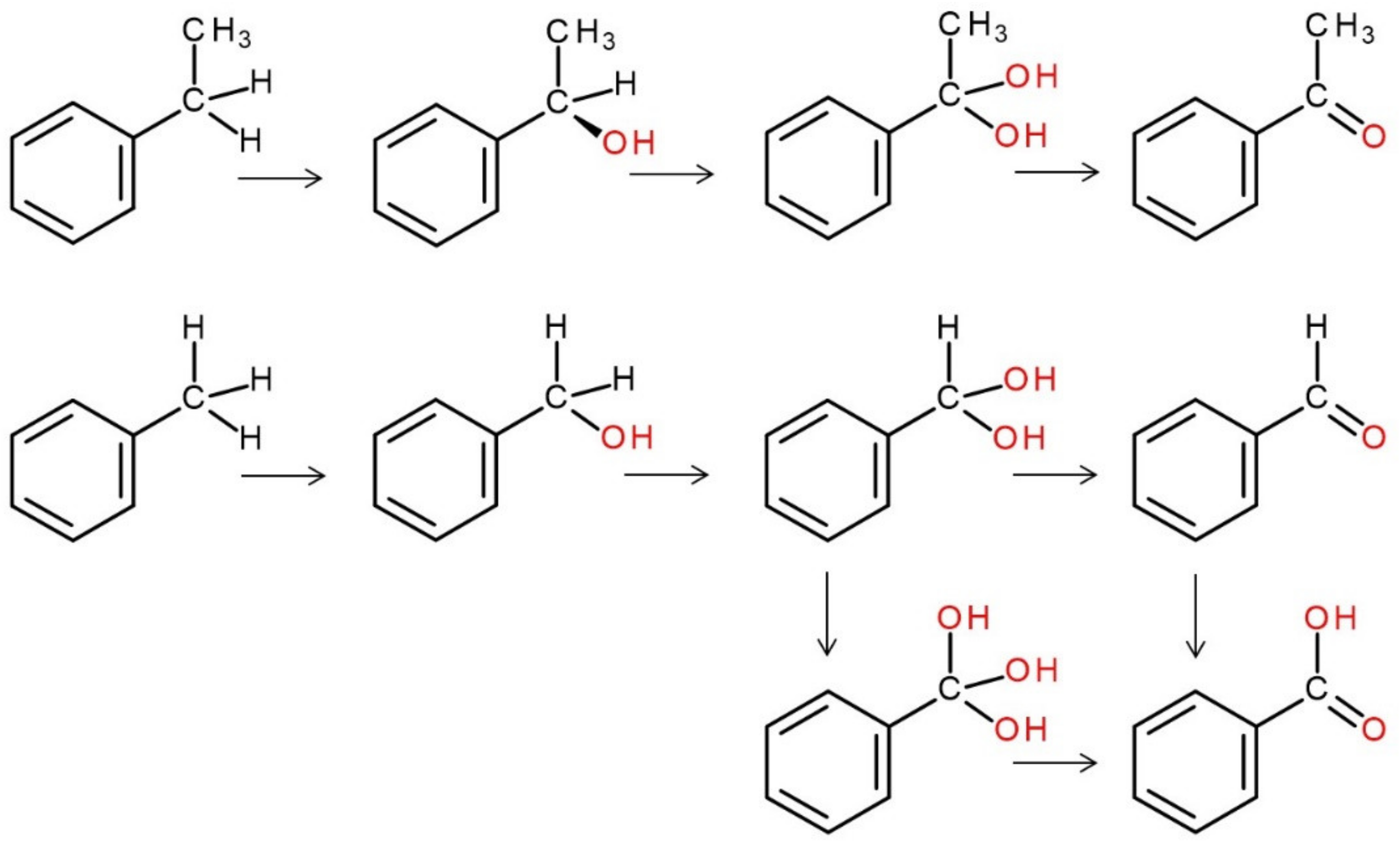
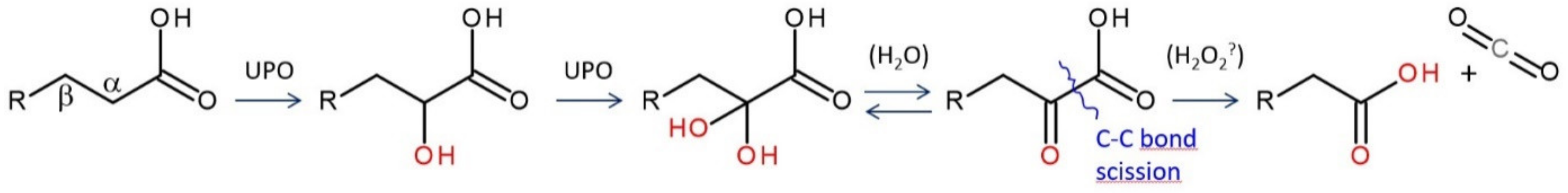
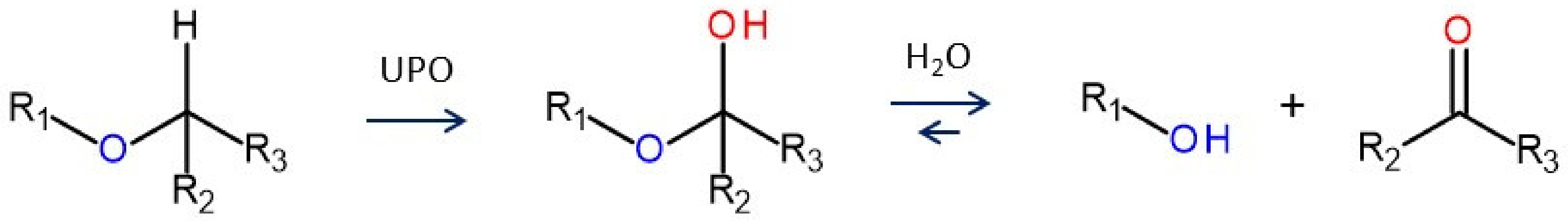
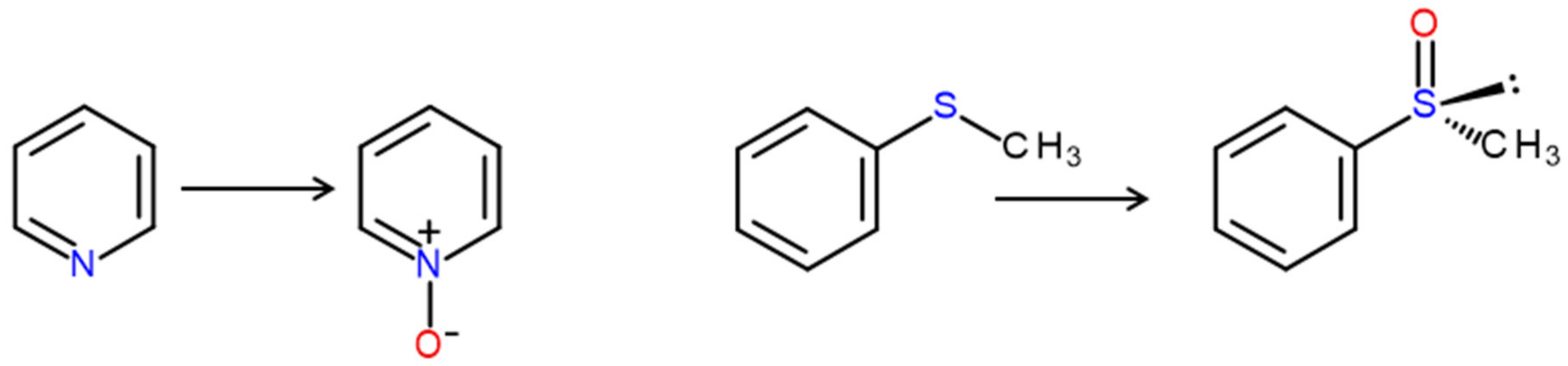
4. Selected Reactions Catalyzed by UPOs
5. Occurrence in the Fungal Kingdom and Phylogeny
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schomburg, D.; Schomburg, I. Class 1 Oxidoreductases EC 1, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 504–516. [Google Scholar]
- Hofrichter, M.; Kellner, H.; Pecyna, M.J.; Ullrich, R. Fungal unspecific peroxygenases: Heme-thiolate proteins that combine peroxidase and cytochrome P450 properties. In Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450; Hrycay, E.G., Bandiera, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 341–368. [Google Scholar]
- Ramirez-Escudero, M.; Molina-Espeja, P.; Gomez de Santos, P.; Hofrichter, M.; Sanz-Aparicio, J.; Alcalde, M. Structural insights into the substrate promiscuity of a laboratory-evolved peroxygenase. ACS Chem. Biol. 2018, 13, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Püllmann, P.; Zhang, W.; Weissenborn, M.J. Enzymatic hydroxylations of sp3-carbons. ACS Catal. 2021, 11, 9168–9203. [Google Scholar] [CrossRef]
- Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheny, P.B.; Curtis, J.M.; Hofstetter, V.; Aime, M.C.; Moncalvo, J.-M.; Ge, Z.-W.; Yang, Z.-L.; Slot, J.C.; Ammirati, J.F.; Baroni, T.J.; et al. Major clades of Agaricales: A multilocus phylogenetic overview. Mycologia 2006, 98, 982–995. [Google Scholar] [CrossRef]
- Kiebist, J.; Hofrichter, M.; Zuhse, R.; Scheibner, K. Oxyfunctionalization of pharmaceuticals by fungal peroxygenases. In Pharmaceutical Biocatalysis: Chemoenzymatic Synthesis of Active Pharmaceutical Ingredients; Grunwald, P., Grunwald, P., Eds.; Pan Stanford Series on Biocatalysis; Jenny Stanford Publishing: Singapore, 2019; Volume 5. [Google Scholar]
- Ullrich, R.; Karich, A.; Hofrichter, M. Fungal peroxygenases—A versatile tool for biocatalysis. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; pp. 260–280. [Google Scholar]
- Manoj, K.M.; Hager, L.P. Chloroperoxidase, a Janus enzyme. Biochemistry 2008, 47, 2997–3003. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R. Heme-thiolate haloperoxidases: Versatile biocatalysts with biotechnological and environmental significance. Appl. Microbiol. Biotechnol. 2006, 71, 276–288. [Google Scholar] [CrossRef]
- Wang, X.; Peter, S.; Kinne, M.; Hofrichter, M.; Groves, J.T. Detection and kinetic characterization of a highly reactive heme–thiolate peroxygenase compound I. J. Am. Chem. Soc. 2012, 134, 12897–12900. [Google Scholar] [CrossRef] [Green Version]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Oxyfunctionalization of aliphatic compounds by a recombinant peroxygenase from Coprinopsis cinerea. Biotechnol. Bioeng. 2013, 110, 2323–2332. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; de Santos, P.G.; Alcalde, M. Directed evolution of unspecific peroxygenase. In Directed Enzyme Evolution: Advances and Applications; Alcalde, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 127–143. [Google Scholar]
- Ullrich, R.; Poraj-Kobielska, M.; Herold-Majumdar, O.M.; Vind, J.; Hofrichter, M. Synthesis of indigo-dyes from indole derivatives by unspecific peroxygenases and their application for in-situ dyeing. Catalysts 2021, 11, 1495. [Google Scholar] [CrossRef]
- Hofrichter, M.; Scheibner, K.; Nüske, J.; Ullrich, R. Enzymatic Production of Acids with Intermediate Formation of Aldehydes in a Single Stage Reaction from Alcohols, Involves Addition of Fresh Arylalcohol-Arylaldehyde-Peroxidases and an Oxidant. German Patent DE10332065A1, 11 July 2003. [Google Scholar]
- Hofrichter, M.; Scheibner, K.; Ullrich, R.; Kinne, M.; Peter, S.; Lund, H.; Kalum, L. Regioselective Hydroxylation of Aliphatic Hydrocarbons Employing a Fungal Peroxygenase. EU Patent EP2553109B1, 28 May 2010. [Google Scholar]
- Lund, H.; Kalum, L.; Hofrichter, M.; Peter, S. Epoxidation Using Peroxygenase. Patents PCT/EP2013/056326, US9663806, U9908860B2, US10358429B2, 31 May 2012. [Google Scholar]
- Pecyna, M.; Schnorr, K.M.; Ullrich, R.; Scheibner, K.; Kluge, M.; Hofrichter, M. Fungal Peroxygenases and Methods of Application. Patent WO 2008/119780 A2, 30 May 2007. [Google Scholar]
- Landvik, S.; Oestergaard, L.H.; Kalum, L. Poplypeptides Having Peroxygenase Activity and Polynucleotides Encoding Same. U.S. Patent 14,361,390, 2 December 2011. [Google Scholar]
- Molina-Espeja, P.; Plougasca, F.J.; Alcalde, M.; Gomez de Santos, P. Nonspecific Peroxygenase Mutants with High Monooxygenase Activity and Their Uses. Patents WO 2017/081355 A1, PCT/ES2016/070809, 13 November 2015. [Google Scholar]
- Fernández-Fueyo, E.; Aranda, C.; Gutiérrez, A.; Martínez, A.T. Method of Heterologous Expression of Active Fungal Unspecific Peroxygenase in Bacterial Host Cells for Fatty-Acid Epoxidation and Other Oxygenation Reactions. Patents WO 2020/011847 A1, PCT/EP2019/068519, 10 July 2018. [Google Scholar]
- Schramm, M.; Schmidtke, K.-U.; Scheibner, K. Process for the Cell-Free Preparation of Non-Specific Peroxygenases and Their Use. German Patent DE 10 2021 209 758.8, 3 September 2021. [Google Scholar]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef]
- Hofrichter, M.; Ullrich, R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef]
- Püllmann, P.; Weissenborn, M.J. Pilzliche Peroxygenasen: Der Schlüssel zu C-H-Hydroxylierungen und mehr? BIOspektrum 2019, 25, 572–574. [Google Scholar] [CrossRef] [Green Version]
- Sigmund, M.-C.; Poelarends, G.J. Current state and future perspectives of engineered and artificial peroxygenases for the oxyfunctionalization of organic molecules. Nat. Catal. 2020, 3, 690–702. [Google Scholar] [CrossRef]
- Scheibner, K.; Ullrich, R.; Kiebist, J.; Kellner, H.; Hofrichter, M. Unspezifische Peroxygenasen—Oxyfunktionalisierung außerhalb der Pilzhyphe. BIOspektrum 2020, 26, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Liers, C.; Scheibner, K.; Kimani, V.W.; Ullrich, R. Fungal Peroxygenases: A Phylogenetically Old Superfamily of Heme Enzymes with Promiscuity for Oxygen Transfer Reactions. In Grand Challenges in Fungal Biotechnology; Grand Challenges in Biology and Biotechnology; Nevalainen, H., Rampelotto, P.H., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 1, pp. 369–403. [Google Scholar]
- Mahor, D.; Cong, Z.; Weissenborn, M.J.; Hollmann, F.; Zhang, W. Valorization of small alkanes by biocatalytic oxyfunctionalization. ChemSusChem 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Hobisch, M.; Holtmann, D.; Gomez de Santos, P.; Alcalde, M.; Hollmann, F.; Kara, S. Recent developments in the use of peroxygenases—Exploring their high potential in selective oxyfunctionalisations. Biotechnol. Adv. 2021, 51, 107615. [Google Scholar] [CrossRef]
- Ullrich, R.; Liers, C.; Schimpke, S.; Hofrichter, M. Purification of homogeneous forms of fungal peroxygenase. Biotechnol. J. 2009, 4, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.K.; Rühl, M.; Mishra, B.; Kleofas, V.; Hofrichter, M.; Herzog, R.; Pecyna, M.J.; Sharma, R.; Kellner, H.; Hennicke, F.; et al. The genome sequence of the commercially cultivated mushroom Agrocybe aegerita reveals a conserved repertoire of fruiting-related genes and a versatile suite of biopolymer-degrading enzymes. BMC Genom. 2018, 19, 48. [Google Scholar] [CrossRef]
- Gröbe, G.; Ullrich, R.; Pecyna, M.J.; Kapturska, D.; Friedrich, S.; Hofrichter, M.; Scheibner, K. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express 2011, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Poraj-Kobielska, M.; Scholze, S.; Halbout, C.; Sandvoss, M.; Pecyna, M.J.; Scheibner, K.; Hofrichter, M. Side chain removal from corticosteroids by unspecific peroxygenase. J. Inorg. Biochem. 2018, 183, 84–93. [Google Scholar] [CrossRef]
- Anh, D.H.; Ullrich, R.; Benndorf, D.; Svatoś, A.; Muck, A.; Hofrichter, M. The coprophilous mushroom Coprinus radians secretes a haloperoxidase that catalyzes aromatic peroxygenation. Appl. Environ. Microbiol. 2007, 73, 5477–5485. [Google Scholar] [CrossRef] [Green Version]
- Melzer, A.; Kimani, V.W.; Ullrich, R. Psathyrella aberdarensis, a new species of Psathyrella (Agaricales) from a Kenyan National Park. Austrian J. Mycol. 2018, 27, 23–30. [Google Scholar]
- Kiebist, J.; Schmidtke, K.-U.; Zimmermann, J.; Kellner, H.; Jehmlich, N.; Ullrich, R.; Zänder, D.; Hofrichter, M.; Scheibner, K. A peroxygenase from Chaetomium globosum catalyzes the selective oxygenation of testosterone. ChemBioChem 2017, 18, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecyna, M.; Ullrich, R.; Bittner, B.; Clemens, A.; Scheibner, K.; Schubert, R.; Hofrichter, M. Molecular characterization of aromatic peroxygenase from Agrocybe aegerita. Appl. Microbiol. Biotechnol. 2009, 84, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Piontek, K.; Strittmatter, E.; Ullrich, R.; Gröbe, G.; Pecyna, M.J.; Kluge, M.; Scheibner, K.; Hofrichter, M.; Plattner, D.A. Structural basis of substrate conversion in a new aromatic peroxygenase: Cytochrome P450 functionalities with benefits. J. Biol. Chem. 2013, 288, 34767–34776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, D.H. Novel Extracellular Haloperoxidase-Peroxygenases from the Coprophilous Fungi Coprinus radians and Coprinus verticillatus: Production, Purification and Biochemical Characterization. Ph.D. Thesis, International Graduate School of Zittau, Zittau, Germany, 2008. [Google Scholar]
- Pecyna, M. Molecular Biological Charaterization of Heme-Thiolate and DyP-Type Peroxidases of Selected Basidiomycetes. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2016. [Google Scholar]
- Wang, X.W.; Yang, F.Y.; Meijer, M.; Kraak, B.; Sun, B.D.; Jiang, Y.L.; Wu, Y.M.; Bai, F.Y.; Seifert, K.A.; Crous, P.W.; et al. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Stud. Mycol. 2019, 93, 65–153. [Google Scholar] [CrossRef]
- Aranda, C.; Municoy, M.; Guallar, V.; Kiebist, J.; Scheibner, K.; Ullrich, R.; del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of 4-hydroxyisophorone and 4-ketoisophorone by fungal peroxygenases. Catal. Sci. Technol. 2019, 9, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Garcia-Ruiz, E.; Gonzalez-Perez, D.; Ullrich, R.; Hofrichter, M.; Alcalde, M. Directed evolution of unspecific peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80, 3496–3507. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Ma, S.; Mate, D.M.; Ludwig, R.; Alcalde, M. Tandem-yeast expression system for engineering and producing unspecific peroxygenase. Enzyme Microb. Technol. 2015, 73–74, 29–33. [Google Scholar] [CrossRef]
- Molina-Espeja, P.; Cañellas, M.; Plou, F.J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. Synthesis of 1-naphthol by a natural peroxygenase engineered by directed evolution. ChemBioChem 2016, 17, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Mate, D.M.; Palomino, M.A.; Molina-Espeja, P.; Martin-Diaz, J.; Alcalde, M. Modification of the peroxygenative:peroxidative activity ratio in the unspecific peroxygenase from Agrocybe aegerita by structure-guided evolution. Prot. Eng. Des. Sel. 2017, 30, 191–198. [Google Scholar] [CrossRef]
- Kinne, M.; Poraj-Kobielska, M.; Aranda, E.; Ullrich, R.; Hammel, K.E.; Scheibner, K.; Hofrichter, M. Regioselective preparation of 5-hydroxypropranolol and 4′-hydroxydiclofenac with a fungal peroxygenase. Bioorg. Med. Chem. Lett. 2009, 19, 3085–3087. [Google Scholar] [CrossRef]
- Poraj-Kobielska, M. Conversion of Pharmaceuticals and Other Drugs by Fungal Peroxygenases. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2013. [Google Scholar]
- Molina-Espeja, P.; Beltran-Nogal, A.; Alfuzzi, M.A.; Guallar, V.; Alcalde, M. Mapping potential determinants of peroxidative activity in an evolved fungal peroxygenase from Agrocybe aegerita. Front. Bioeng. Biotechnol. 2021, 9, 1282. [Google Scholar] [CrossRef]
- Martin-Diaz, J.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Alcalde, M. Directed evolution of unspecific peroxygenase in organic solvents. Biotechnol. Bioeng. 2021, 118, 3002–3014. [Google Scholar] [CrossRef]
- Molina-Espeja, P.; Santos-Moriano, P.; García-Ruiz, E.; Ballesteros, A.; Plou, F.J.; Alcalde, M. Structure-guided immobilization of an evolved unspecific peroxygenase. Int. J. Mol. Sci. 2019, 20, 1627. [Google Scholar] [CrossRef] [Green Version]
- Tonin, F.; Tieves, F.; Willot, S.; van Troost, A.; van Oosten, R.; Breestraat, S.; van Pelt, S.; Alcalde, M.; Hollmann, F. Pilot-Scale poduction of peroxygenase from Agrocybe aegerita. Org. Process. Res. Dev. 2021, 25, 1414–1418. [Google Scholar] [CrossRef]
- Bormann, S.; Kellner, H.; Hermes, J.; Herzog, R.; Ullrich, R.; Liers, C.; Ulber, R.; Holtmann, D. Broadening the biocatalytic toolbox—Screening and expression of new unspecific peroxygenases. Antioxidants, 2022; under review. [Google Scholar]
- Pickard, M.A.; Kadima, T.A.; Carmichael, R.D. Chloroperoxidase, a peroxidase with potential. J. Ind. Microbiol. 1991, 7, 235–241. [Google Scholar] [CrossRef]
- Morris, D.R.; Hager, L.P. Chloroperoxidase: I. Isolation and properties of the chrystaline glycoprotein. J. Biol. Chem. 1966, 241, 1763–1768. [Google Scholar] [CrossRef]
- Sae, A.S.W.; Cunningham, B.A. Isolation and properties of chloroperoxidase isozymes. Phytochemistry 1979, 18, 1785–1787. [Google Scholar] [CrossRef]
- Conesa, A.; van de Velde, F.; van Rantwijk, F.; Sheldon, R.A.; van den Hondel, C.A.M.J.J.; Punt, P.J. Expression of the Caldariomyces fumago chloroperoxidase in Aspergillus niger and characterization of the recombinant enzyme. J. Biol. Chem. 2001, 276, 17635–17640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linde, D.; Olmedo, A.; González-Benjumea, A.; Estévez, M.; Renau-Mínguez, C.; Carro, J.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Two new unspecific peroxygenases from heterologous expression of fungal genes in Escherichia coli. Appl. Environ. Microbiol. 2020, 86, e02899-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotilio, L.; Swoboda, A.; Ebner, K.; Rinnofner, C.; Glieder, A.; Kroutil, W.; Mattevi, A. Structural and biochemical studies enlighten the unspecific peroxygenase from Hypoxylon sp. EC38 as an efficient oxidative biocatalyst. ACS Catal. 2021, 11, 11511–11525. [Google Scholar] [CrossRef]
- Olmedo, A.; Río, J.C.d.; Kiebist, J.; Ullrich, R.; Hofrichter, M.; Scheibner, K.; Martínez, A.T.; Gutiérrez, A. Fatty acid chain shortening by a fungal peroxygenase. Chem. Eur. J. 2017, 23, 16985–16989. [Google Scholar] [CrossRef] [Green Version]
- Kimani, V.W. New Secretory Peroxidases and Peroxygenases from Saprotrophic Fungi of Kenyan Forests. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2019. [Google Scholar]
- Gomez de Santos, P.; Hoang, M.D.; Kiebist, J.; Kellner, H.; Ullrich, R.; Scheibner, K.; Hofrichter, M.; Liers, C.; Alcalde, M. Functional expression of two unusual acidic peroxygenases from Candolleomyces aberdarensis in yeasts by adopting evolved secretion mutations. Appl. Environ. Microbiol. 2021, 87, e0087821. [Google Scholar] [CrossRef]
- Püllmann, P.; Knorrscheidt, A.; Münch, J.; Palme, P.R.; Hoehenwarter, W.; Marillonnet, S.; Alcalde, M.; Westermann, B.; Weissenborn, M.J. A modular two yeast species secretion system for the production and preparative application of unspecific peroxygenases. Commun. Biol. 2021, 4, 562. [Google Scholar] [CrossRef]
- Knorrscheidt, A.; Soler, J.; Hünecke, N.; Püllmann, P.; Garcia-Borràs, M.; Weissenborn, M.J. Accessing chemo- and regioselective benzylic and aromatic oxidations by protein engineering of an unspecific peroxygenase. ACS Catal. 2021, 11, 7327–7338. [Google Scholar] [CrossRef]
- Püllmann, P.; Weissenborn, M.J. Improving the heterologous production of fungal peroxygenases through an episomal Pichia pastoris promoter and signal peptide shuffling system. ACS Synth. Biol. 2021, 10, 1360–1372. [Google Scholar] [CrossRef]
- Berka, R.M.; Grigoriev, I.V.; Otillar, R.; Salamov, A.; Grimwood, J.; Reid, I.; Ishmael, N.; John, T.; Darmond, C.; Moisan, M.-C.; et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 2011, 29, 922–927. [Google Scholar] [CrossRef]
- Singh, B. Myceliophthora thermophila syn. Sporotrichum thermophile: A thermophilic mould of biotechnological potential. Crit. Rev. Biotechnol. 2016, 36, 59–69. [Google Scholar] [CrossRef]
- Cregg, J.M.; Tolstorukov, I.; Kusari, A.; Sunga, J.; Madden, K.; Chappell, T. Expression in the yeast Pichia pastoris. In Methods in Enzymology, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 169–189. [Google Scholar]
- Karich, A.; Kleeberg, S.; Ullrich, R.; Hofrichter, M. Enzymatic preparation of 2,5-furandicarboxylic acid (FDCA)—A substitute of terephthalic acid—By the joined action of three fungal enzymes. Microorganisms 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Kinner, A.; Rosenthal, K.; Lütz, S. Identification and expression of new unspecific peroxygenases—Recent advances, challenges and opportunities. Front. Bioeng. Biotechnol. 2021, 9, 5630. [Google Scholar] [CrossRef]
- Chong, S. Overview of cell-free protein synthesis: Historic landmarks, commercial Systems, and expanding applications. Curr. Protoc. Mol. Biol. 2014, 108, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Dondapati, S.K.; Stech, M.; Zemella, A.; Kubick, S. Cell-free protein synthesis: A promising option for future drug development. BioDrugs 2020, 34, 327–348. [Google Scholar] [CrossRef] [Green Version]
- Karich, A.; Scheibner, K.; Ullrich, R.; Hofrichter, M. Exploring the catalase activity of unspecific peroxygenases and the mechanism of peroxide-dependent heme destruction. J. Mol. Catal. B Enzym. 2016, 134, 238–246. [Google Scholar] [CrossRef]
- Munro, A.W.; Girvan, H.M.; McLean, K.J. Variations on a (t)heme—novel mechanisms, redox partners and catalytic functions in the cytochrome P450 superfamily. Nat. Prod. Rep. 2007, 24, 585–609. [Google Scholar] [CrossRef]
- Piontek, K.; Ullrich, R.; Liers, C.; Diedrichs, K.; Plattner, A.D.; Hofrichter, M. Crystallization of a 45 kDa peroxygenase/peroxidase from the mushroom Agrocybe aegerita and structure determination by SAD utilizing only the haem iron. Acta Crystallogr. F Struct. Biol. Commun. 2010, 66, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold—Making protein folding accessible to all. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Janson, G.; Paiardini, A. PyMod 3: A complete suite for structural bioinformatics in PyMOL. Bioinformatics 2020, 37, 1471–1472. [Google Scholar] [CrossRef] [PubMed]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Paiardini, A.; Bossa, F.; Pascarella, S. CAMPO, SCR_FIND and CHC_FIND: A suite of web tools for computational structural biology. Nucleic Acids Res. 2005, 33, W50–W55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, X.; Krone, M.; Alharbi, N.; Rose, A.S.; Laramee, R.S.; O’Donoghue, S.; Baaden, M.; Chavent, M. Molecular graphics: Bridging structural biologists and computer scientists. Structure 2019, 27, 1617–1623. [Google Scholar] [CrossRef]
- Hameduh, T.; Haddad, Y.; Adam, V.; Heger, Z. Homology modeling in the time of collective and artificial intelligence. Comput. Struct. Biotechnol. J. 2020, 18, 3494–3506. [Google Scholar] [CrossRef]
- Poraj-Kobielska, M.; Kinne, M.; Ullrich, R.; Scheibner, K.; Kayser, G.; Hammel, K.E.; Hofrichter, M. Preparation of human drug metabolites using fungal peroxygenases. Biochem. Pharmacol. 2011, 82, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Karich, A.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants. Front. Microbiol. 2017, 8, 1463. [Google Scholar] [CrossRef]
- Wang, X.; Ullrich, R.; Hofrichter, M.; Groves, J.T. Heme-thiolate ferryl of aromatic peroxygenase is basic and reactive. Proc. Natl. Acad. Sci. USA 2015, 112, 3686–3691. [Google Scholar] [CrossRef] [Green Version]
- Kinne, M.; Zeisig, C.; Ullrich, R.; Kayser, G.; Hammel, K.E.; Hofrichter, M. Stepwise oxygenations of toluene and 4-nitrotoluene by a fungal peroxygenase. Biochem. Biophys. Res. Commun. 2010, 397, 18–21. [Google Scholar] [CrossRef]
- Kinne, M.; Poraj-Kobielska, M.; Ralph, S.A.; Ullrich, R.; Hofrichter, M.; Hammel, K.E. Oxidative cleavage of diverse ethers by an extracellular fungal peroxygenase. J. Biol. Chem. 2009, 284, 29343–29349. [Google Scholar] [CrossRef] [Green Version]
- Kluge, M.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase of Agrocybe aegerita. Green Chem. 2012, 14, 440–446. [Google Scholar] [CrossRef]
- Huang, X.; Groves, J.T. Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C–H activation. J. Biol. Inorg. Chem. 2017, 22, 185–207. [Google Scholar] [CrossRef] [Green Version]
- Shalan, H.; Kato, M.; Cheruzel, L. Keeping the spotlight on cytochrome P450. Biochim. Biophys. Acta Proteins Proteom. BBA 2018, 1866, 80–87. [Google Scholar] [CrossRef]
- Bormann, S.; Gomez Baraibar, A.; Ni, Y.; Holtmann, D.; Hollmann, F. Specific oxyfunctionalisations catalysed by peroxygenases: Opportunities, challenges and solutions. Catal. Sci. Technol. 2015, 5, 2038–2052. [Google Scholar] [CrossRef] [Green Version]
- Hofrichter, M.; Ullrich, R. New Trends in Fungal Biooxidation. In Industrial Applications; Hofrichter, M., Ed.; Springer: Heidelberg, Germany, 2011; pp. 425–449. [Google Scholar]
- Ullrich, R.; Hofrichter, M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, D.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Aranda, C.; Carro, J.; González-Benjumea, A.; Babot, E.D.; Olmedo, A.; Linde, D.; Martínez, A.T.; Gutiérrez, A. Advances in enzymatic oxyfunctionalization of aliphatic compounds. Biotechnol. Adv. 2021, 51, 107703. [Google Scholar] [CrossRef]
- Peng, L.; Wollenberger, U.; Kinne, M.; Hofrichter, M.; Ullrich, R.; Scheibner, K.; Fischer, A.; Scheller, F.W. Peroxygenase based sensor for aromatic compounds. Biosens. Bioelectron. 2010, 26, 1432–1436. [Google Scholar] [CrossRef]
- Yarman, A.; Gröbe, G.; Neumann, B.; Kinne, M.; Gajovic-Eichelmann, N.; Wollenberger, U.; Hofrichter, M.; Ullrich, R.; Scheibner, K.; Scheller, F.W. The aromatic peroxygenase from Marasmius rotula—A new enzyme for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 405–412. [Google Scholar] [CrossRef]
- Grogan, G. Hemoprotein catalyzed oxygenations: P450s, UPOs, and progress toward scalable reactions. JACS Au 2021, 1, 1312–1329. [Google Scholar] [CrossRef]
- Wapshott-Stehli, H.L.; Grunden, A.M. In situ H2O2 generation methods in the context of enzyme biocatalysis. Enzym. Microb. Technol. 2021, 145, 109744. [Google Scholar] [CrossRef]
- Friedrich, S.; Gröbe, G.; Kluge, M.; Brinkmann, T.; Hofrichter, M.; Scheibner, K. Optimization of a biocatalytic process to gain (R)-1-phenylethanol by applying the software tool Sabento for ecological assessment during the early stages of development. J. Mol. Catal. B Enzym. 2014, 103, 36–40. [Google Scholar] [CrossRef]
- Peter, S.; Kinne, M.; Wang, X.; Ullrich, R.; Kayser, G.; Groves, J.T.; Hofrichter, M. Selective hydroxylation of alkanes by an extracellular fungal peroxygenase. FEBS J. 2011, 278, 3667–3675. [Google Scholar] [CrossRef]
- Peter, S.; Karich, A.; Ullrich, R.; Gröbe, G.; Scheibner, K.; Hofrichter, M. Enzymatic one-pot conversion of cyclohexane into cyclohexanone: Comparison of four fungal peroxygenases. J. Mol. Catal. B Enzym. 2014, 103, 47–51. [Google Scholar] [CrossRef]
- Kiebist, J.; Holla, W.; Heidrich, J.; Poraj-Kobielska, M.; Sandvoss, M.; Simonis, R.; Gröbe, G.; Atzrodt, J.; Hofrichter, M.; Scheibner, K. One-pot synthesis of human metabolites of SAR548304 by fungal peroxygenases. Bioorg. Med. Chem. 2015, 23, 4324–4332. [Google Scholar] [CrossRef]
- Karich, A.; Kluge, M.; Ullrich, R.; Hofrichter, M. Benzene oxygenation and oxidation by the peroxygenase of Agrocybe aegerita. AMB Express 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Aranda, C.; Ullrich, R.; Kiebist, J.; Scheibner, K.; del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of the resveratrol analogue 4,4′-dihydroxy-trans-stilbene and stilbenoids modification by fungal peroxygenases. Catal. Sci. Technol. 2018, 8, 2394–2401. [Google Scholar] [CrossRef] [Green Version]
- Kluge, M.; Ullrich, R.; Dolge, C.; Scheibner, K.; Hofrichter, M. Hydroxylation of naphthalene by aromatic peroxygenase from Agrocybe aegerita proceeds via oxygen transfer from H2O2 and intermediary epoxidation. Appl. Microbiol. Biotechnol. 2009, 81, 1071–1076. [Google Scholar] [CrossRef]
- Aranda, E.; Ullrich, R.; Hofrichter, M. Conversion of polycyclic aromatic hydrocarbons, methyl naphthalenes and dibenzofuran by two fungal peroxygenases. Biodegradation 2010, 21, 267–281. [Google Scholar] [CrossRef]
- Peter, S.; Kinne, M.; Ullrich, R.; Kayser, G.; Hofrichter, M. Epoxidation of linear, branched and cyclic alkenes catalyzed by unspecific peroxygenase. Enzym. Microb. Technol. 2013, 52, 370–376. [Google Scholar] [CrossRef]
- Carro, J.; González-Benjumea, A.; Fernández-Fueyo, E.; Aranda, C.; Guallar, V.; Gutiérrez, A.; Martínez, A.T. Modulating fatty acid epoxidation vs hydroxylation in a fungal peroxygenase. ACS Catal. 2019, 9, 6234–6242. [Google Scholar] [CrossRef] [Green Version]
- Peter, S. Oxyfunctionalization of Alkanes, Alkenes and Alkynes by Unspecific Peroxygenase (EC 1.11.2.1). Ph.D. Thesis, TU Dresden, Dresden, Germany, 2013. [Google Scholar]
- Municoy, M.; González-Benjumea, A.; Carro, J.; Aranda, C.; Linde, D.; Renau-Mínguez, C.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Gutiérrez, A.; et al. Fatty-acid oxygenation by fungal peroxygenases: From computational simulations to preparative regio- and stereoselective epoxidation. ACS Catal. 2020, 10, 13584–13595. [Google Scholar] [CrossRef]
- González-Benjumea, A.; Marques, G.; Herold-Majumdar, O.M.; Kiebist, J.; Scheibner, K.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. High epoxidation yields of vegetable oil hydrolyzates and methyl esters by selected fungal peroxygenases. Front. Bioeng. Biotechnol. 2021, 8, 1470. [Google Scholar] [CrossRef] [PubMed]
- González-Benjumea, A.; Carro, J.; Renau-Mínguez, C.; Linde, D.; Fernández-Fueyo, E.; Gutiérrez, A.; Martínez, A.T. Fatty acid epoxidation by Collariella virescens peroxygenase and heme-channel variants. Catal. Sci. Technol. 2020, 10, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Dolge, C.; Kluge, M.; Hofrichter, M. Pyridine as novel substrate for regioselective oxygenation with aromatic peroxygenase from Agrocybe aegerita. FEBS Lett. 2008, 582, 4100–4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinne, M. The Extracellular Peroxygenase of the Agaric Fungus Agrocybe aegerita: Catalytic Properties and Physiological Background with Particular Emphasis on Ether Cleavage. Ph.D. Thesis, International Graduate School of Zittau, Zittau, Germany, 2010. [Google Scholar]
- Wu, Y.; Wollenberger, U.; Hofrichter, M.; Ullrich, R.; Scheibner, K.; Scheller, F.W. Direct electron transfer of Agrocybe aegerita peroxygenase at electrodes modified with chitosan-capped Au nanoparticles and its bioelectrocatalysis to aniline. Sens. Actuators B Chem. 2011, 160, 1419–1426. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Vanoni, M.; Ottolina, G.; Riva, S.; Crotti, M.; Brenna, E.; Monti, D. Peroxygenase-catalyzed enantioselective sulfoxidations. Eur. J. Org. Chem. 2017, 2017, 7186–7189. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Li, P.; Zhang, X.; Ribitsch, D.; Alcalde, M.; Hollmann, F.; Wang, Y. Enantioselective sulfoxidation of thioanisole by cascading a choline oxidase and a peroxygenase in the presence of natural deep eutectic solvents. ChemPlusChem 2020, 85, 254–257. [Google Scholar] [CrossRef]
- Aranda, E.; Kinne, M.; Kluge, M.; Ullrich, R.; Hofrichter, M. Conversion of dibenzothiophene by the mushrooms Agrocybe aegerita and Coprinellus radians and their extracellular peroxygenases. Appl. Microbiol. Biotechnol. 2009, 82, 1057–1066. [Google Scholar] [CrossRef]
- Ullrich, R.; Hofrichter, M. The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene. FEBS Lett. 2005, 579, 6247–6250. [Google Scholar] [CrossRef] [Green Version]
- Dembitsky, V.M. Oxidation, epoxidation and sulfoxidation reactions catalysed by haloperoxidases. Tetrahedron 2003, 59, 4701–4720. [Google Scholar] [CrossRef]
- Kinne, M.; Poraj-Kobielska, M.; Ullrich, R.; Nousiainen, P.; Sipilä, J.; Scheibner, K.; Hammel, K.E.; Hofrichter, M. Oxidative cleavage of non-phenolic β-O-4 lignin model dimers by an extracellular aromatic peroxygenase. Holzforschung 2011, 65, 673–679. [Google Scholar] [CrossRef]
- Forsberg, Z.; Sørlie, M.; Petrović, D.; Courtade, G.; Aachmann, F.L.; Vaaje-Kolstad, G.; Bissaro, B.; Røhr, Å.K.; Eijsink, V.G.H. Polysaccharide degradation by lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2019, 59, 54–64. [Google Scholar] [CrossRef]
- Lara, E.; Moreira, D.; López-García, P. The environmental clade LKM11 and Rozella form the deepest branching clade of fungi. Protist 2010, 161, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagy, L.G.; Ohm, R.A.; Patyshakuliyeva, A.; Brun, A.; Aerts, A.L.; et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, E.; McGowan, J.; McCarthy, C.G.P.; Amini, A.; Grogan, H.; Fitzpatrick, D.A. Whole genome sequence of the commercially relevant mushroom strain Agaricus bisporus var. bisporus ARP23. G3 2019, 9, 3057–3066. [Google Scholar] [CrossRef] [Green Version]
- Savory, F.; Leonard, G.; Richards, T.A. The role of horizontal gene transfer in the evolution of the oomycetes. PLoS Pathog. 2015, 11, e1004805. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Andersen, C.B.; Vetukuri, R.R.; Dou, D.; Grenville-Briggs, L.J. Horizontal gene transfer and tandem duplication shape the unique CAZyme complement of the mycoparasitic oomycetes Pythium oligandrum and Pythium periplocum. Front. Microbiol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Kendrick, B. The Fifth Kingdom, 3rd ed.; Focus Publishing: New York, NY, USA, 2000. [Google Scholar]
- Danielson, P.B. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef]
- Hlavica, P. Evaluation of structural features in fungal cytochromes P450 predicted to rule catalytic diversification. Biochim. Biophys. Acta Proteins Proteom. BBA 2013, 1834, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.F.V.; Sheridan, G. Cytochromes P450, oxygen, and evolution. Sci. World J. 2001, 1, 698519. [Google Scholar] [CrossRef] [PubMed]
- Kalum, L.; Lund, H.; Hofrichter, M.; Ullrich, R. Enzymatic Preparation of Indigo Dyes and Intermediates. EU Patent PCT/EP2014/052103, 5 February 2013. [Google Scholar]
- Kont, R.; Bissaro, B.; Eijsink, V.G.H.; Väljamäe, P. Kinetic insights into the peroxygenase activity of cellulose-active lytic polysaccharide monooxygenases (LPMOs). Nat. Commun. 2020, 11, 5786. [Google Scholar] [CrossRef] [PubMed]
- Rieder, L.; Stepnov, A.A.; Sørlie, M.; Eijsink, V.G.H. Fast and specific peroxygenase reactions catalyzed by fungal mono-copper enzymes. Biochemistry 2021, 60, 3633–3643. [Google Scholar] [CrossRef] [PubMed]
- Kellner, H.; Luis, P.; Pecyna, M.J.; Barbi, F.; Kapturska, D.; Krüger, D.; Zak, D.R.; Marmeisse, R.; Vandenbol, M.; Hofrichter, M. Widespread occurrence of expressed fungal secretory peroxidases in forest soils. PLoS ONE 2014, 9, e95557. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, S.; Hoppe, B.; Stengel, E.; Noll, L.; Moll, J.; Bässler, C.; Dahl, A.; Buscot, F.; Hofrichter, M.; Kellner, H. Molecular fungal community and its decomposition activity in sapwood and heartwood of 13 temperate European tree species. PLoS ONE 2019, 14, e0212120. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Kiebist, J.; Scheibner, K.; Ullrich, R. Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases. Antioxidants 2022, 11, 163. https://doi.org/10.3390/antiox11010163
Hofrichter M, Kellner H, Herzog R, Karich A, Kiebist J, Scheibner K, Ullrich R. Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases. Antioxidants. 2022; 11(1):163. https://doi.org/10.3390/antiox11010163
Chicago/Turabian StyleHofrichter, Martin, Harald Kellner, Robert Herzog, Alexander Karich, Jan Kiebist, Katrin Scheibner, and René Ullrich. 2022. "Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases" Antioxidants 11, no. 1: 163. https://doi.org/10.3390/antiox11010163
APA StyleHofrichter, M., Kellner, H., Herzog, R., Karich, A., Kiebist, J., Scheibner, K., & Ullrich, R. (2022). Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases. Antioxidants, 11(1), 163. https://doi.org/10.3390/antiox11010163






