Protein Oxidation in Muscle Foods: A Comprehensive Review
Abstract
:1. Introduction
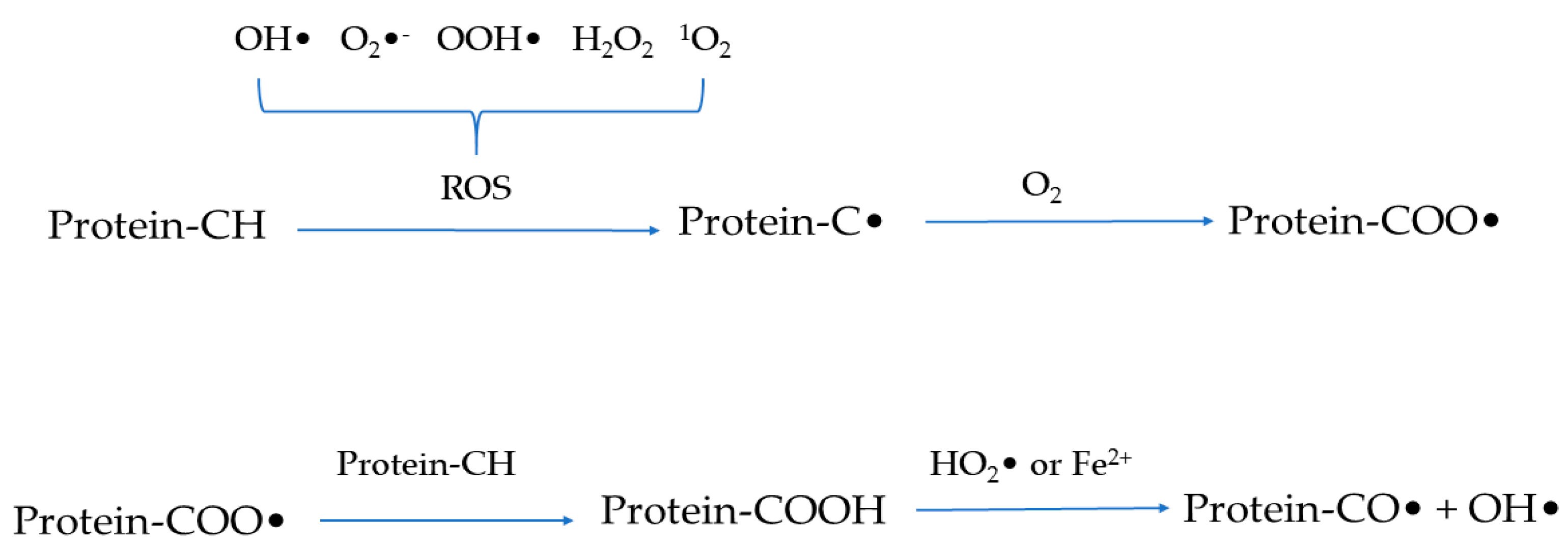
2. Mechanisms of Protein Oxidation
2.1. Protein Oxidation Process
2.2. Protein Photo-Oxidation
2.3. Metal-Catalyzed Protein Oxidation
2.4. Enzyme-Catalyzed Protein Oxidation
3. Factors Affecting Protein Oxidation
3.1. Intrinsic Factors: Composition of Muscle Foods
3.2. Extrinsic Factors
3.2.1. Salting and Curing
| Product | Treatment | Effect | Ref. |
|---|---|---|---|
| Fresh beef tenderloin | Salting (NaCl) + TPP or mixture | Carbonyl content increase and tryptophan fluorescence intensity loss. Promoted formation of CML and CEL. Lower cooking loss and higher moisture content. | [46] |
| Pork meat Chinese dry sausages | Salting (NaCl) (2–4%) | Higher doses facilitate the protein oxidation, lipid hydrolysis and oxidation (higher lipase activity, higher TBARS values, and higher LOX activity). | [47] |
| Spanish ham | Dry curing (9–24 months) | Promoting role on lipid oxidation (higher TBARS values), major peptidyl PTMs and release of FAAs. | [49] |
| Pork ham | Dry curing—NaCl replacement with KCl, CaCl2 and MgCl2 | No significant differences in acid lipase activity or lipid oxidation. | [50] |
| Mutton ham | Dry curing (0–180 days) | Increased proteins’ surface hydrophobicity, carbonyl content increase, and thiol content decrease. | [51] |
| BF & SM muscles ham | Salting + cold smoking + drying + ripening | Higher proteolytic, protein oxidation and total FAAs content in BF than in SM. | [52] |
| Beef jerky | Fermentation | Carbonyl content and TBARS increase in normal fermentation but at lower levels when starter cultures where used. | [53] |
| Harbin dry sausages | Fermentation | Carbonyl compounds formation and sulfhydryl loss decreased using mixed cultured starters. | [54,55] |
| Minced beef | Cold treatment (4 °C) | Carbonyl compounds increase. Free and total thiols decrease. | [56] |
| Obscure pufferfish (Takifugu obscurus) | Freezing-thawing cycles + LE + OC | Increased of sulfhydryl and tryptophan loss. Cross-linkage formation. | [57] |
| Dry-cured pork loins | Dry curing + freezing 18 °C, 5 months/thawing 12 h, 4 °C | Increased cross linkage through Schiff bases formation. | [58] |
| Pork loins | Aging (1ºC, 19 days) + fast-freezing (−80 °C) | Carbonyl content and TBARS increased through time. Increased lipid oxidation. | [59] |
| Pork sausages | Heat treatment | SH groups decrease. Carbonyls and SeS groups increase. | [60] |
| Bigeye tuna (Thunnus obesus) | Salting + Freezing | Synergistic effect on lipid oxidation: TBARS increase. Increased protein cross-linking formation. | [61] |
| Chicken leg and breast meats | Freezing (−7, −12, −18 °C) | Higher carbonyl compounds increase at higher temperatures. Decreased sulfhydryl loss at lower temperatures. | [62] |
| Yak meat | Air-drying | Carbonyl compounds increase. Sulfide bond content increase and total sulfhydryl group decrease. | [42] |
| Rabbit meat | Refrigerated vs. superchilled storage | Superchilled conditions showed TBARS decrease, lower metmyoglobin percentage, carbonyl content, and sulfhydryl loss. | [63] |
| Rhea americana meat | Air- (5 days) and vacuum- storage (28 days) | No evolution of protein and lipid oxidation when vacuum storage was used. | [64] |
| Pork patties | Guarana seeds extract incorporation | Carbonyl compounds and TBARS decrease. | [65] |
| Burger beef patties | Rosa canina L. extract incorporation | Tryptophan oxidation decrease. Increase formation of Schiff bases. | [66] |
| Duck breast muscle | Dietary curcumin supplementation | Carbonyl compounds and TBARS decrease. Free amino groups on myofibrillar protein increase. | [67] |
| Frozen-thawed duck breast muscle | Dietary resveratrol supplementation | Carbonyl compounds decrease and decreased sulfhydryl loss. | [68] |
3.2.2. Fermentation
3.2.3. Thermal Treatments
3.2.4. Storage Conditions
4. Analytical Methods for the Protein Oxidation Quantification
4.1. Determination of Protein Carbonylation
4.2. Detection of Loss of Sulfhydryl Groups and Loss of Tryptophan
4.3. Assessment of Cross-Linking and Fragmentation of Proteins
5. Effects of Protein Oxidation on Food Quality
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund, M.N.; Baron, C.P. Protein oxidation in foods and food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 33–69. ISBN 9781845694951. [Google Scholar]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Luna, C. Dietary protein oxidation: A silent threat to human health? Crit. Rev. Food Sci. Nutr. 2017, 57, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chem.-Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2021, 10, 40. [Google Scholar] [CrossRef]
- Bohrer, B.M. Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, Z.; He, Z.; Wang, Z.; Qin, F.; Zeng, M.; Chen, J. Effect of Freeze-Thaw Cycles on the Oxidation of Protein and Fat and Its Relationship with the Formation of Heterocyclic Aromatic Amines and Advanced Glycation End Products in Raw Meat. Molecules 2021, 26, 1264. [Google Scholar] [CrossRef]
- Agim, Z.S.; Cannon, J.R. Alterations in the nigrostriatal dopamine system after acute systemic PhIP exposure. Toxicol. Lett. 2018, 287, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, A.; Agim, Z.S.; Montenegro, P.C.; McCabe, G.P.; Rochet, J.C.; Cannon, J.R. Selective dopaminergic neurotoxicity of three heterocyclic amine subclasses in primary rat midbrain neurons. Neurotoxicology 2018, 65, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Sharman, E.H. Reactive oxygen species and protein oxidation in neurodegenerative disease. In Inflammation, Aging, and Oxidative Stress; Bondy, S.C., Campbell, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 199–212. [Google Scholar]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein Oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Thompson, L.D.V.; Durand, D.; Fugere, N.A.; Ferrington, D.A. Myosin and actin expression and oxidation in aging muscle. J. Appl. Physiol. 2006, 101, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Schaich, K.M.; Pryor, W.A. Free radical initiation in proteins and amino acids by ionizing and ultraviolet radiations and lipid oxidation—Part III: Free radical transfer from oxidizing lipids. Crit. Rev. Food Sci. Nutr. 1980, 13, 189–244. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef]
- Douny, C.; Tihon, A.; Bayonnet, P.; Brose, F.; Degand, G.; Rozet, E.; Milet, J.; Ribonnet, L.; Lambin, L.; Larondelle, Y.; et al. Validation of the Analytical Procedure for the Determination of Malondialdehyde and Three Other Aldehydes in Vegetable Oil Using Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS) and Application to Linseed Oil. Food Anal. Methods 2014, 8, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.I.; Lynn, B.C.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1094–1099. [Google Scholar] [CrossRef]
- Mello, C.F.; Sultana, R.; Piroddi, M.; Cai, J.; Pierce, W.M.; Klein, J.B.; Butterfield, D.A. Acrolein induces selective protein carbonylation in synaptosomes. Neuroscience 2007, 147, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traverso, N.; Menini, S.; Cottalasso, D.; Odetti, P.; Marinari, U.M.; Pronzato, M.A. Mutual interaction between glycation and oxidation during non-enzymatic protein modification. Biochim. Biophys. Acta-Gen. Subj. 1997, 1336, 409–418. [Google Scholar] [CrossRef]
- Davies, M.J. Oxidative damage to proteins. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; American Cancer Society: New York, NY, USA, 2012; pp. 1425–1457. [Google Scholar]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Trnková, L.; Dršata, J.; Boušová, I. Oxidation as an important factor of protein damage: Implications for Maillard reaction. J. Biosci. 2015, 40, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Oliver, C.N. Metal-catalyzed oxidation of proteins. Physiological consequences. J. Biol. Chem. 1991, 266, 2005–2008. [Google Scholar] [CrossRef]
- Bamm, V.V.; Tsemakhovich, V.A.; Shaklai, N. Oxidation of low-density lipoprotein by hemoglobin–hemichrome. Int. J. Biochem. Cell Biol. 2003, 35, 349–358. [Google Scholar] [CrossRef]
- Grinshtein, N.; Bamm, V.V.; Tsemakhovich, V.A.; Shaklai, N. Mechanism of Low-Density Lipoprotein Oxidation by Hemoglobin-Derived Iron. Biochemistry 2003, 42, 6977–6985. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, Y.L.; Sato, H.; Kumazawa, Y. Controlled Cross-Linking with Glucose Oxidase for the Enhancement of Gelling Potential of Pork Myofibrillar Protein. J. Agric. Food Chem. 2016, 64, 9523–9531. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of Phenols, Anilines, and Benzenethiols by Fungal Laccases: Correlation between Activity and Redox Potentials as Well as Halide Inhibition†. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From Isolation to Application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kussendrager, K.D.; Van Hooijdonk, A.C.M. Lactoperoxidase: Physico-chemical properties, occurrence, mechanism of action and applications. Br. J. Nutr. 2000, 84, 19–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Traore, S.; Aubry, L.; Gatellier, P.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Santé-Lhoutellier, V. Higher drip loss is associated with protein oxidation. Meat Sci. 2012, 90, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT 2021, 142, 110984. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Guo, X.; Zhu, X.; Mao, X.; Guo, X.; Deng, X.; Zhang, J. μ-Calpain oxidation and proteolytic changes on myofibrillar proteins from Coregonus peled in vitro. Food Chem. 2021, 361, 130100. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of early postmortem protein oxidation on beef quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar] [CrossRef]
- Cropotova, J.; Tappi, S.; Genovese, J.; Rocculi, P.; Dalla Rosa, M.; Rustad, T. The combined effect of pulsed electric field treatment and brine salting on changes in the oxidative stability of lipids and proteins and color characteristics of sea bass (Dicentrarchus labrax). Heliyon 2021, 7, e05947. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, C.; Quan, W.; Qin, F.; Wang, Z.; He, Z.; Zeng, M.; Chen, J. Assessment the influence of salt and polyphosphate on protein oxidation and Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine formation in roasted beef patties. Meat Sci. 2021, 177, 108489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Cittadini, A.; Domínguez, R.; Lorenzo, J.M. Metallic-based salt substitutes to reduce sodium content in meat products. Curr. Opin. Food Sci. 2021, 38, 21–31. [Google Scholar] [CrossRef]
- Li, C.; Mora, L.; Gallego, M.; Aristoy, M.C.; Toldrá, F. Evaluation of main post-translational modifications occurring in naturally generated peptides during the ripening of Spanish dry-cured ham. Food Chem. 2020, 332, 127388. [Google Scholar] [CrossRef] [PubMed]
- Ripollés, S.; Campagnol, P.C.B.; Armenteros, M.; Aristoy, M.C.; Toldrá, F. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on lipolysis and lipid oxidation in dry-cured ham. Meat Sci. 2011, 89, 58–64. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in proteolysis, protein oxidation, flavor, color and texture of dry-cured mutton ham during storage. LWT 2021, 149, 111860. [Google Scholar] [CrossRef]
- Poljanec, I.; Marušić Radovčić, N.; Petričević, S.; Karolyi, D.; Listeš, E.; Medić, H. Proteolysis and protein oxidation throughout the smoked dry-cured ham process. Food Chem. 2021, 362, 130207. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Sun, F.; Wang, Y.; Chen, Q.; Kong, B. Evaluation the potential of lactic acid bacteria isolates from traditional beef jerky as starter cultures and their effects on flavor formation during fermentation. LWT 2021, 142, 110982. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Liu, Q.; Xu, L. The role of bacterial fermentation in the hydrolysis and oxidation of sarcoplasmic and myofibrillar proteins in Harbin dry sausages. Meat Sci. 2016, 121, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kong, B.; Sun, Q.; Dong, F.; Liu, Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: In vitro and in a sausage model. Meat Sci. 2015, 110, 180–188. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, R.; Zhang, W.; Li, Y.; Wang, J.; Zhou, G. Effects of Different Packaging Systems on Beef Tenderness Through Protein Modifications. Food Bioprocess Technol. 2015, 8, 580–588. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, F.; Zhang, L.; Wang, H.; Wang, X. chang Effect of different extent of protein oxidation on the frozen storage stability of muscle protein in obscure pufferfish (Takifugu obscurus). LWT 2021, 137, 110416. [Google Scholar] [CrossRef]
- Lorido, L.; Ventanas, S.; Akcan, T.; Estévez, M. Effect of protein oxidation on the impaired quality of dry-cured loins produced from frozen pork meat. Food Chem. 2016, 196, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.H.; Seo, J.K.; Setyabrata, D.; Kim, Y.H.B. Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Sci. 2018, 139, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cui, C.; Zhao, M.; Zhao, Q.; Yang, B. Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chem. 2011, 124, 336–341. [Google Scholar] [CrossRef]
- Jiang, Q.; Nakazawa, N.; Hu, Y.; Wang, X.; Osako, K.; Okazaki, E. Evolution of tissue microstructure, protein properties, and oxidative stability of salted bigeye tuna (Thunnus obesus) meat during frozen storage. LWT 2021, 149, 111848. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmiş, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Filgueras, R.S.; Gatellier, P.; Aubry, L.; Thomas, A.; Bauchart, D.; Durand, D.; Zambiazi, R.C.; Santé-Lhoutellier, V. Colour, lipid and protein stability of Rhea americana meat during air- and vacuum-packaged storage: Influence of muscle on oxidative processes. Meat Sci. 2010, 86, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Morcuende, D.; Ganhão, R.; Estévez, M. Role of Phenolics Extracting from Rosa canina L. on Meat Protein Oxidation During Frozen Storage and Beef Patties Processing. Food Bioprocess Technol. 2015, 8, 854–864. [Google Scholar] [CrossRef]
- Jin, S.; Pang, Q.; Liu, R.; Yang, H.; Liu, F.; Wang, M.; Wang, Y.; Feng, X.; Shan, A. Dietary curcumin decreased lipid oxidation and enhanced the myofibrillar protein structure of the duck (Anas Platyrhynchos) breast muscle when subjected to storage. LWT 2020, 133, 109986. [Google Scholar] [CrossRef]
- Jin, S.; Wang, M.; Yang, H.; Shan, A.; Feng, X. Dietary supplementation of resveratrol improved the oxidative stability and spatial conformation of myofibrillar protein in frozen-thawed duck breast meat. Food Biosci. 2021, 43, 101261. [Google Scholar] [CrossRef]
- Pedro, D.; Saldaña, E.; Lorenzo, J.M.; Pateiro, M.; Dominguez, R.; Dos Santos, B.A.; Cichoski, A.J.; Campagnol, P.C.B. Low-sodium dry-cured rabbit leg: A novel meat product with healthier properties. Meat Sci. 2021, 173, 108372. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Zhang, Y.; Pan, J.; Huang, S.; He, L.; Jin, G. Impact of salt content and hydrogen peroxide-induced oxidative stress on protein oxidation, conformational/morphological changes, and micro-rheological properties of porcine myofibrillar proteins. Food Chem. 2021, 370, 131074. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Pérez-Santaescolástica, C.; Munekata, P.E.S.; Lorenzo, J.M. Salt reduction strategies in meat products made from whole pieces. In Strategies for Obtaining Healthier Foods; Lorenzo, J.M., Carballo, F.J., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 267–289. ISBN 978-1-53612-159-9. [Google Scholar]
- Cittadini, A.; Domínguez, R.; Gómez, B.; Pateiro, M.; Pérez-Santaescolástica, C.; López-Fernández, O.; Sarriés, M.V.; Lorenzo, J.M. Effect of NaCl replacement by other chloride salts on physicochemical parameters, proteolysis and lipolysis of dry-cured foal “cecina”. J. Food Sci. Technol. 2020, 57, 1628–1635. [Google Scholar] [CrossRef]
- Nachtigall, F.M.; Vidal, V.A.S.; Pyarasani, R.D.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R.; Santos, L.S. Substitution effects of NaCl by KCl and CaCl2 on lipolysis of salted meat. Foods 2019, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Bermúdez, R.; Domínguez, R.; Guiotto, A.; Franco, D.; Purriños, L. Physicochemical and microbial changes during the manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Food Control 2015, 50, 763–769. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Lorenzo, J.M.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Campagnol, P.C.B.; Franco, D. Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina. Foods 2021, 10, 669. [Google Scholar] [CrossRef]
- Marti-quijal, F.J.; Remize, F.; Meca, G.; Barba, F.J.; Ferrer, E. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opin. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Dalmiş, Ü.; Soyer, A. Effect of processing methods and starter culture (Staphylococcus xylosus and Pediococcus pentosaceus) on proteolytic changes in Turkish sausages (sucuk) during ripening and storage. Meat Sci. 2008, 80, 345–354. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Setyabrata, D.; Kim, Y.H.B. Impacts of aging/freezing sequence on microstructure, protein degradation and physico-chemical properties of beef muscles. Meat Sci. 2019, 151, 64–74. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, B.; Guo, J.; Nie, W.; Zhou, H.; Li, P.; Zhou, K.; Xu, B. Effects of low voltage electrostatic field on the microstructural damage and protein structural changes in prepared beef steak during the freezing process. Meat Sci. 2021, 179, 108527. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Parra, V.; Estévez, M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014, 96, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Armenteros, M.; Ventanas, S.; Solano, F.; Estévez, M. Pre-freezing raw hams affects quality traits in cooked hams: Potential influence of protein oxidation. Meat Sci. 2012, 92, 596–603. [Google Scholar] [CrossRef]
- Dominguez-Hernandez, E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xu, Y.; Li, J.; Niu, S.; Wang, C.; Zhang, N.; Yang, M.; Zhou, K.; Chen, S.; He, L.; et al. Effect of oxidized lipids stored under different temperatures on muscle protein oxidation in Sichuan-style sausages during ripening. Meat Sci. 2019, 147, 144–154. [Google Scholar] [CrossRef]
- Goethals, S.; Van Hecke, T.; Vossen, E.; Vanhaecke, L.; Van Camp, J.; De Smet, S. Commercial luncheon meat products and their in vitro gastrointestinal digests contain more protein carbonyl compounds but less lipid oxidation products compared to fresh pork. Food Res. Int. 2020, 136, 109585. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ben Slima, S.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Ben Salah, R. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef]
- Carvalho, R.H.; Ida, E.I.; Madruga, M.S.; Martínez, S.L.; Shimokomaki, M.; Estévez, M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017, 215, 129–137. [Google Scholar] [CrossRef]
- Ferreira, V.C.S.; Morcuende, D.; Madruga, M.S.; Silva, F.A.P.; Estévez, M. Role of protein oxidation in the nutritional loss and texture changes in ready-to-eat chicken patties. Int. J. Food Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef]
- Fuentes, V.; Estévez, M.; Ventanas, J.; Ventanas, S. Impact of lipid content and composition on lipid oxidation and protein carbonylation in experimental fermented sausages. Food Chem. 2014, 147, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Heinonen, M.; Ollilainen, V.; Toldrá, F.; Estévez, M. Analysis of protein carbonyls in meat products by using the DNPH-method, fluorescence spectroscopy and liquid chromatography-electrospray ionisation-mass spectrometry (LC-ESI-MS). Meat Sci. 2009, 83, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Rysman, T.; Van Hecke, T.; De Smet, S.; Van Royen, G. Ascorbate and Apple Phenolics Affect Protein Oxidation in Emulsion-Type Sausages during Storage and in Vitro Digestion. J. Agric. Food Chem. 2016, 64, 4131–4138. [Google Scholar] [CrossRef]
- Baron, C.P.; Kjærsgård, I.V.H.; Jessen, F.; Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2007, 55, 8118–8125. [Google Scholar] [CrossRef]
- Eymard, S.; Baron, C.P.; Jacobsen, C. Oxidation of lipid and protein in horse mackerel (Trachurus trachurus) mince and washed minces during processing and storage. Food Chem. 2009, 114, 57–65. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.; Hao, G.; Zhang, Y. Effect of kappa-carrageenan oligosaccharides on myofibrillar protein oxidation in peeled shrimp (Litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef]
- Utrera, M.; Rodríguez-Carpena, J.G.; Morcuende, D.; Estévez, M. Formation of lysine-derived oxidation products and loss of tryptophan during processing of porcine patties with added avocado byproducts. J. Agric. Food Chem. 2012, 60, 3917–3926. [Google Scholar] [CrossRef]
- Nakyinsige, K.; Sazili, A.Q.; Aghwan, Z.A.; Zulkifli, I.; Goh, Y.M.; Abu Bakar, F.; Sarah, S.A. Development of microbial spoilage and lipid and protein oxidation in rabbit meat. Meat Sci. 2015, 108, 125–131. [Google Scholar] [CrossRef]
- Cai, W.Q.; Chen, Y.W.; Dong, X.P.; Shi, Y.G.; Wei, J.L.; Liu, F.J. Protein oxidation analysis based on comparative proteomic of Russian sturgeon (Acipenser gueldenstaedti) after sous-vide cooking. Food Control 2021, 121, 107594. [Google Scholar] [CrossRef]
- Hu, L.; Ren, S.; Shen, Q.; Chen, J.; Ye, X.; Ling, J. Proteomic study of the effect of different cooking methods on protein oxidation in fish fillets. RSC Adv. 2017, 7, 27496–27505. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Cui, J.; Yin, X.; Luo, Y.; Zhou, Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Control 2014, 40, 134–139. [Google Scholar] [CrossRef]
- Silva, F.A.P.; Ferreira, V.C.S.; Madruga, M.S.; Estévez, M. Effect of the cooking method (grilling, roasting, frying and sous-vide) on the oxidation of thiols, tryptophan, alkaline amino acids and protein cross-linking in jerky chicken. J. Food Sci. Technol. 2016, 53, 3137–3146. [Google Scholar] [CrossRef] [Green Version]
- Hellwig, M. Analysis of Protein Oxidation in Food and Feed Products. J. Agric. Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef]
- Schaich, K.M. Analysis of lipid and protein oxidation in fats, oils, and foods. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 133–156. ISBN 9781630670566. [Google Scholar]
- Cropotova, J.; Rustad, T. A novel fluorimetric assay for visualization and quantification of protein carbonyls in muscle foods. Food Chem. 2019, 297, 125006. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Ollilainen, V.; Heinonen, M. Analysis of protein oxidation markers α-Aminoadipic and γ-Glutamic semialdehydes in food proteins using liquid chromatography (LC)-Electrospray ionization (ESI)-Multistage tandem mass spectrometry (MS). J. Agric. Food Chem. 2009, 57, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef]
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Oxidation of proteins. In Chemical Changes during Processing and Storage of Foods; Rodríguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press, Elsevier: Oxford, UK, 2021; pp. 85–123. ISBN 9780128173800. [Google Scholar]
- Zhang, D.; Li, H.; Emara, A.M.; Hu, Y.; Wang, Z.; Wang, M.; He, Z. Effect of in vitro oxidation on the water retention mechanism of myofibrillar proteins gel from pork muscles. Food Chem. 2020, 315, 126226. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Heterocyclic aromatic amines in cooked food: Toxicology and analysis. In Food Toxicology and Forensics; Galanakis, C.M., Ed.; Academic Press, Elsevier: Oxford, UK, 2021; pp. 421–460. ISBN 978-0-12-822360-4. [Google Scholar]
- Kosmopoulos, M.; Drekolias, D.; Zavras, P.D.; Piperi, C.; Papavassiliou, A.G. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P.; Estévez, M.; Yuan, L.; Gao, R. Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5548–5569. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef]
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Sci. 2018, 135, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Estévez, M.; Estevez, M.; Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Chaijan, M. Review: Lipid and myoglobin oxidations in muscle foods. Songklanakarin J. Sci. Technol. 2008, 30, 47–53. [Google Scholar]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wen, P.; Yuan, Y.; Yu, X.; Chen, Y.; Xu, H.; Cui, G.; Wang, J. Effect of roasting temperature on lipid and protein oxidation and amino acid residue side chain modification of beef patties. RSC Adv. 2021, 11, 21629–21641. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Pan, N.; Kong, B.; Xia, X. Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 2021, 172, 108335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Huff Lonergan, E.; Zhang, W.; Lonergan, S.M. Biochemistry of postmortem muscle—Lessons on mechanisms of meat tenderization. Meat Sci. 2010, 86, 184–195. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Utrera, M.; Estévez, M. Oxidation of myofibrillar proteins and impaired functionality: Underlying mechanisms of the carbonylation pathway. J. Agric. Food Chem. 2012, 60, 8002–8011. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, C.; Ertbjerg, P. Relationship between proteolysis and water-holding of myofibrils. Meat Sci. 2017, 131, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Farouk, M.M.; Mustafa, N.M.; Wu, G.; Krsinic, G. The “sponge effect” hypothesis: An alternative explanation of the improvement in the waterholding capacity of meat with ageing. Meat Sci. 2012, 90, 670–677. [Google Scholar] [CrossRef]
- Sante-Lhoutellier, V.; Aubry, L.; Gatellier, P. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Zou, X.; He, J.; Xu, X.; Zhou, G.; Li, C. In vitro protein digestibility of pork products is affected by the method of processing. Food Res. Int. 2017, 92, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Santé-Lhoutellier, V.; Astruc, T.; Marinova, P.; Greve, E.; Gatellier, P. Effect of meat cooking on physicochemical state and in vitro digestibility of myofibrillar proteins. J. Agric. Food Chem. 2008, 56, 1488–1494. [Google Scholar] [CrossRef] [PubMed]


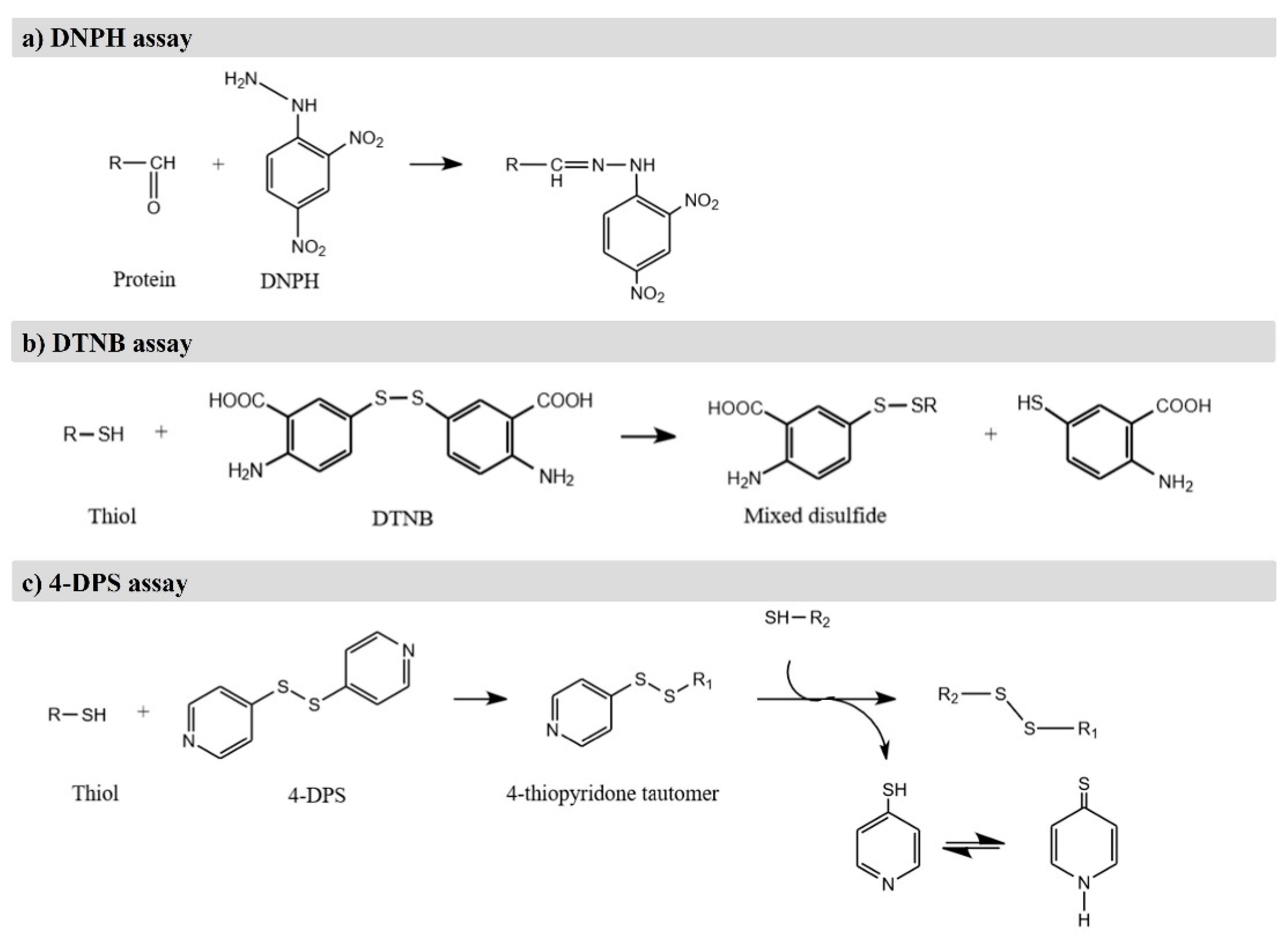
| Indicator | Food Product | Method | Results | Ref. |
|---|---|---|---|---|
| Carbonyls content A | Chicken breast | DNPH | 0.2−2.9 | [87] |
| Ready-to-eat chicken patties | 5−19 | [88] | ||
| Fermented sausages | 1.5−4.5 | [89] | ||
| Raw meat | 2.5 | [90] | ||
| Dry-cured ham | 8 | |||
| Dry-cured loin | 8 | |||
| Dry-cured sausage | 9 | |||
| Cooked sausage | 13 | |||
| Pork sausages | 2.36−3.35 | [91] | ||
| Sturgeon fillets | 2.1−10.6 | [88] | ||
| Rainbow trout | 2.8−2.9 | [92] | ||
| Horse mackerel fillets | 1.7−7.2 | [93] | ||
| Pacific white shrimp | 3−9.5 | [94] | ||
| Quantification of specific carbonyls A | Fermented sausages | HPLC-FLD | AAS: 0.17−0.35; GGS: 0.09−0.11 | [89] |
| Porcine patties | AAS: 0.27−1.09; AAA: 0.56−0.84 | [95] | ||
| Dry-cured loins | 0.13−1.10 | [58] | ||
| Beef patties | AAS: 131.07−857.61; AAA: 5.35−21.3 | [66] | ||
| Pork sausages | AAS: 1.47−1.57; GGS: 0.23−0.28 | [91] | ||
| Raw meat | LC–ESI–MS | AAS: 27; GGS: 30 | [90] | |
| Dry-cured ham | AAS: 23; GGS: 150 | |||
| Dry-cured loin | AAS: 23; GGS: 115 | |||
| Dry-cured sausage | AAS: 22; GGS: 120 | |||
| Cooked sausage | AAS: 28; GGS: 60 | |||
| Free thiol content B | Rabbit meat | DTNB | 24.3−34.7 | [96] |
| Sturgeon fillets | 17−35 | [97] | ||
| Sturgeon fillets | 0.3−0.8 | [98] | ||
| Horse mackerel fillets | 98.6−124.4 | [93] | ||
| Silver carp | 5.3−7.2 | [99] | ||
| Pacific white shrimp | 28−42 | [94] | ||
| Chicken breast | 4-DPS | 15−16 | [87] | |
| Jerky chicken | 25−55 | [100] | ||
| Ready-to-eat chicken patties | 21−52 | [88] | ||
| Pork sausages | 14.80−21.80 | [91] | ||
| Ground beef | 26.5−37.6 | [99] | ||
| Tryptophan content C | Chicken breast | FS | 11−13 | [87] |
| Jerky chicken | 8−10 * | [100] | ||
| Porcine patties | 0.14−0.77 | [95] | ||
| Beef patties | 0.16−2.54 | [66] | ||
| Ready-to-eat chicken patties | 100−170 | [88] | ||
| Cross linking proteins | Rabbit meat | SDS-PAGE | Reduced myofibrillar protein content | [96] |
| Ground beef | Oxidized proteins | [99] | ||
| Sturgeon fillets | Reduced myofibrillar protein content | [97] | ||
| Sturgeon fillets | Reduced myofibrillar protein content | [98] | ||
| Pacific white shrimp | Reduced myofibrillar protein content | [94] | ||
| Rainbow trout | SDS-PAGE and Immunoblotting | Oxidized proteins | [92] | |
| Horse mackerel fillets | Oxidized proteins | [93] | ||
| Cross linking proteins- Disulphide bonds D | Jerky chicken | Total-Free thiol difference | 12−27 | [100] |
| Ready-to-eat chicken patties | 5−17 | [88] | ||
| Ground beef | 7.0−11.5 | [99] | ||
| Cross linking proteins- Schiff bases E | Chicken breast | FS | 6−7 | [87] |
| Dry-cured loins | 420 | [58] | ||
| Jerky chicken | 600−780 | [100] | ||
| Porcine patties | 23.7−169.0 | [95] | ||
| Beef patties | 3383−992 | [66] | ||
| Ready-to-eat chicken patties | 410−900 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. https://doi.org/10.3390/antiox11010060
Domínguez R, Pateiro M, Munekata PES, Zhang W, Garcia-Oliveira P, Carpena M, Prieto MA, Bohrer B, Lorenzo JM. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants. 2022; 11(1):60. https://doi.org/10.3390/antiox11010060
Chicago/Turabian StyleDomínguez, Rubén, Mirian Pateiro, Paulo E. S. Munekata, Wangang Zhang, Paula Garcia-Oliveira, Maria Carpena, Miguel A. Prieto, Benjamin Bohrer, and José M. Lorenzo. 2022. "Protein Oxidation in Muscle Foods: A Comprehensive Review" Antioxidants 11, no. 1: 60. https://doi.org/10.3390/antiox11010060












