Conditioned Medium from H2O2-Preconditioned Human Adipose-Derived Stem Cells Ameliorates UVB-Induced Damage to Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. H2O2-Preconditioning of hASCs
2.3. Ultraviolet B Irradiation
2.4. hFF Treatment with HC016-Conditioned Medium
2.5. Proliferation Assays
2.6. Measurement of ROS
2.7. Cytotoxicity Assays
2.8. Analysis of Nrf2 Expression by Western Blot
2.9. Cell Localization of Nrf2 by Immunofluorescence Assay
2.10. Fluorescent Labeling of Cytoskeletal Proteins
2.11. Apoptosis Analysis
2.12. Senescence Evaluation by SA-β-Gal Staining
2.13. Analysis of IL-6 and Collagen Type I Expression by qRT-PCR
2.14. Protein Quantification
2.15. Cell Migration Assays
2.16. Statistical Analysis
3. Results
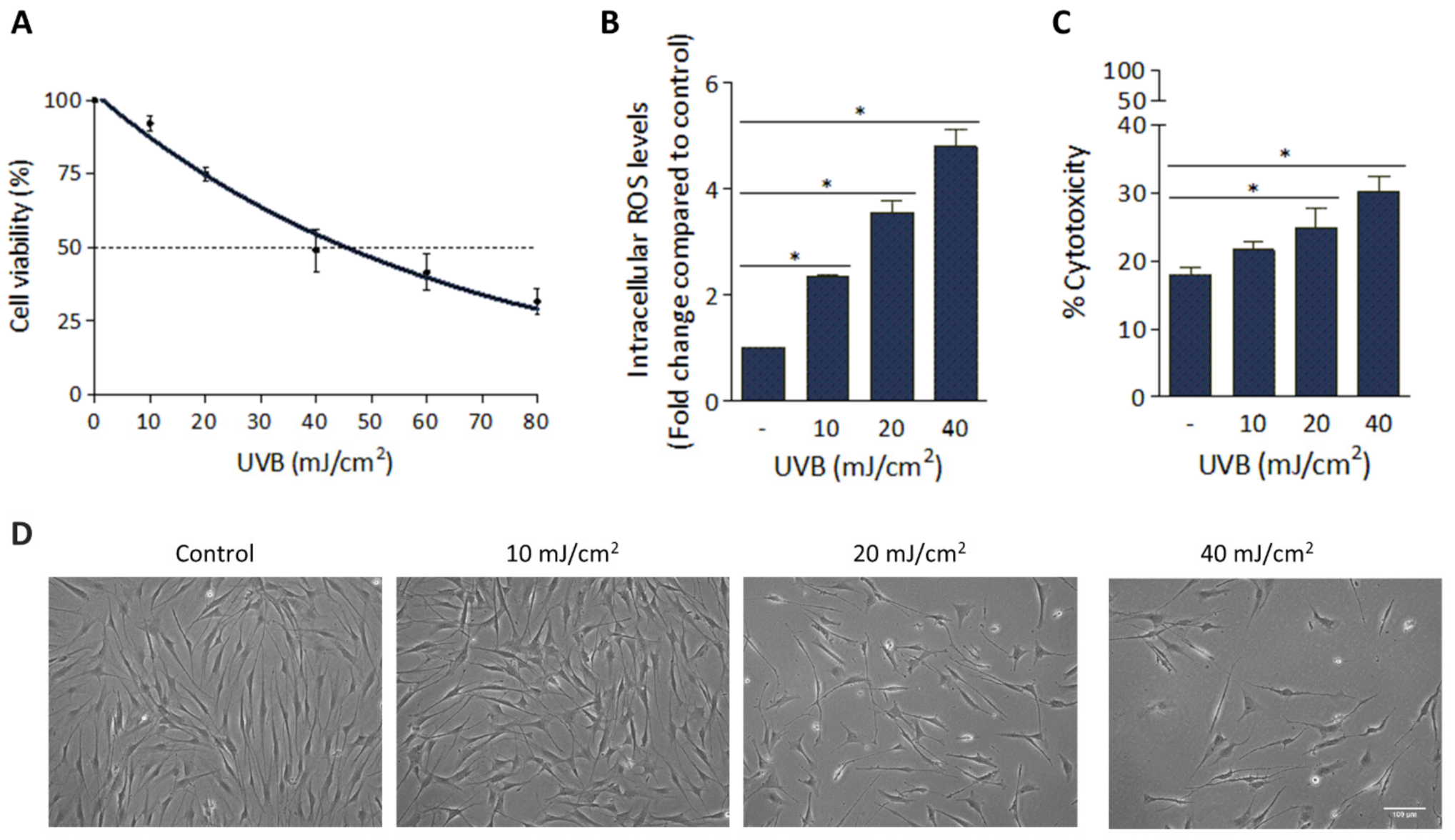
3.1. Deleterious Effect of UVB Irradiation on hFFs
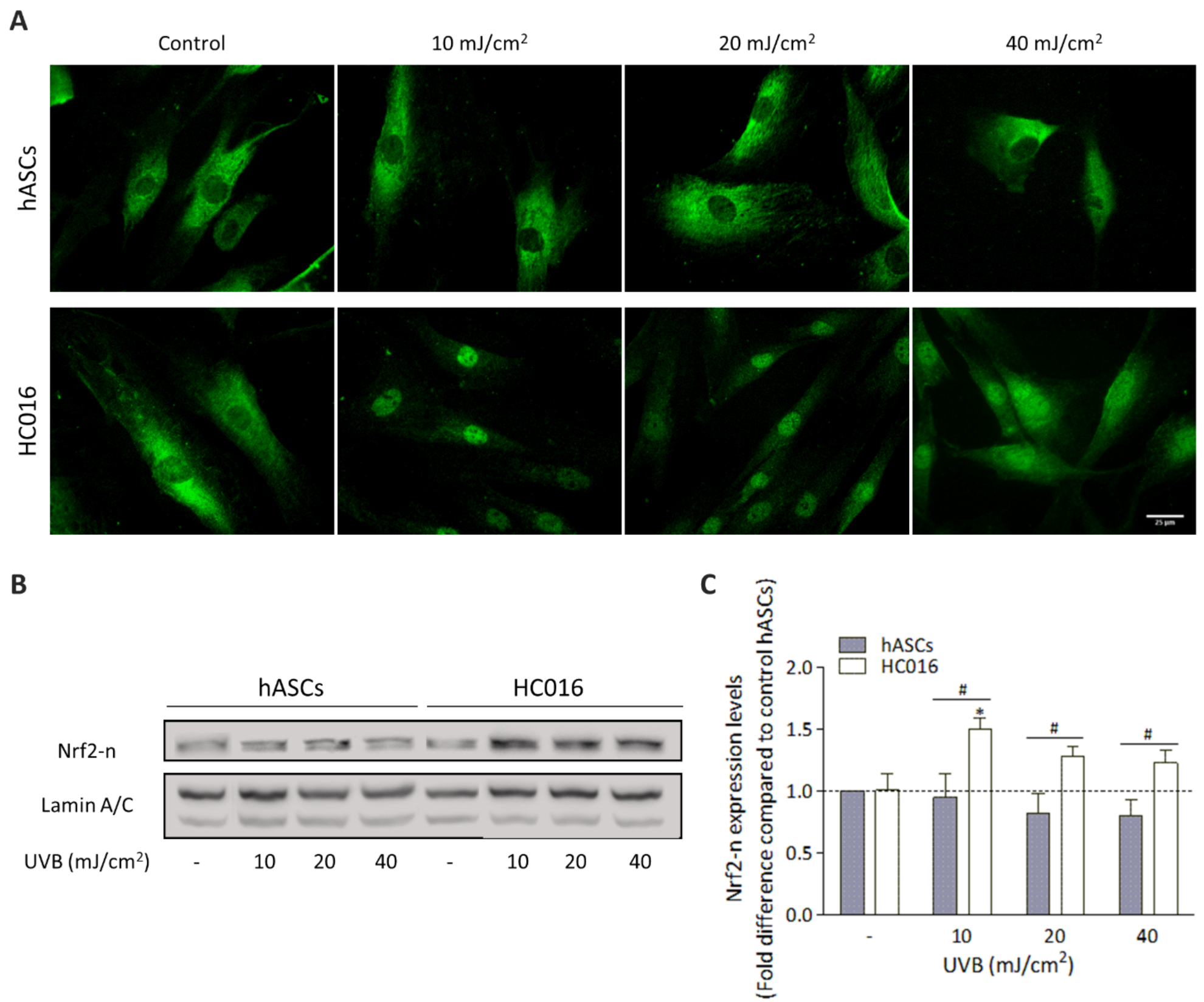
3.2. H2O2-Preconditioning Reduces UVB-Induced Damage in hASCs
3.3. H2O2-Preconditioning Induces Nrf2 Expression in Response to UVB Exposure
3.4. Effect of HC016-CM Treatment on hFFs
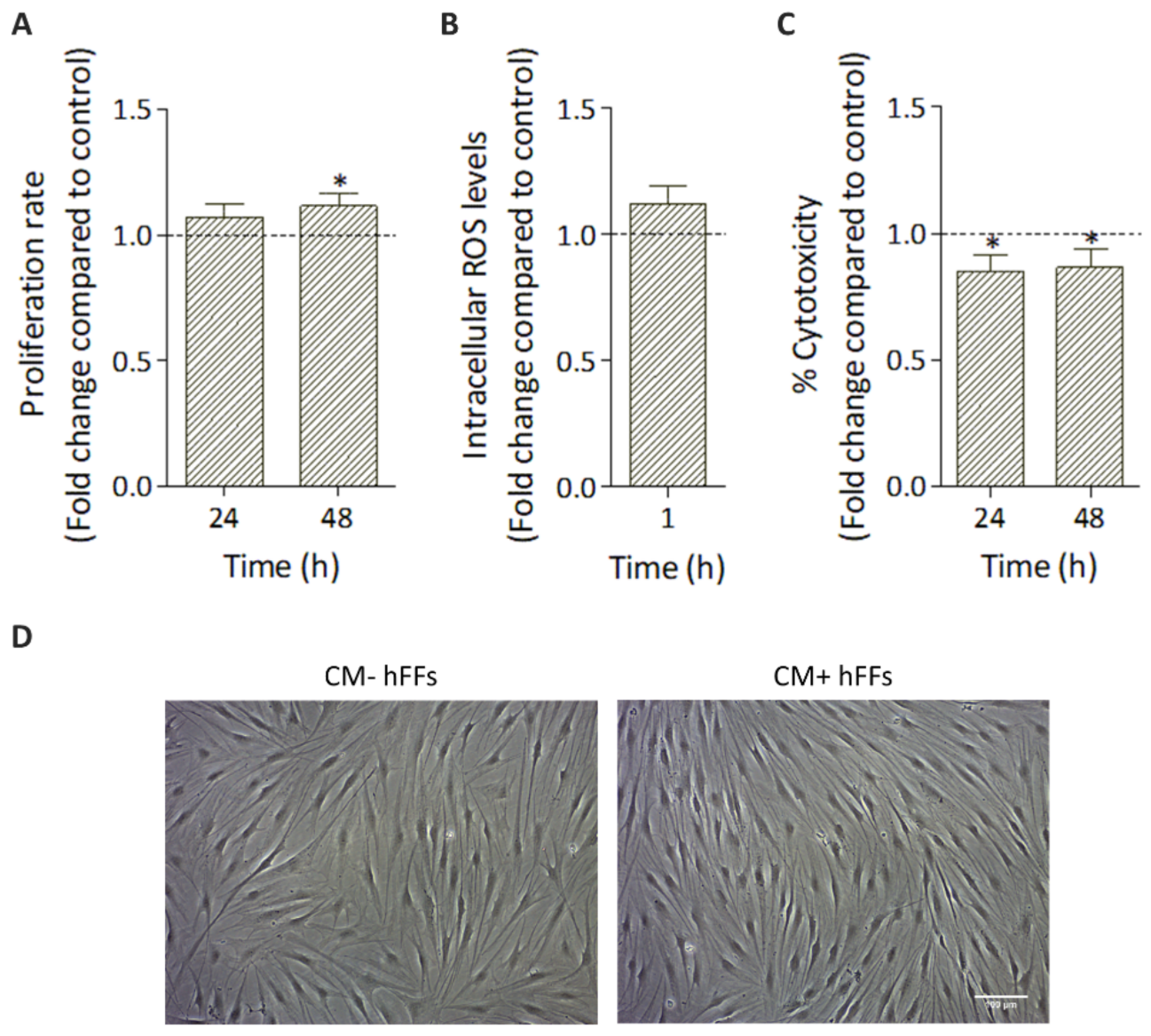
3.4.1. HC016-CM Does Not Alter hFF Basic Cell Culture Parameters
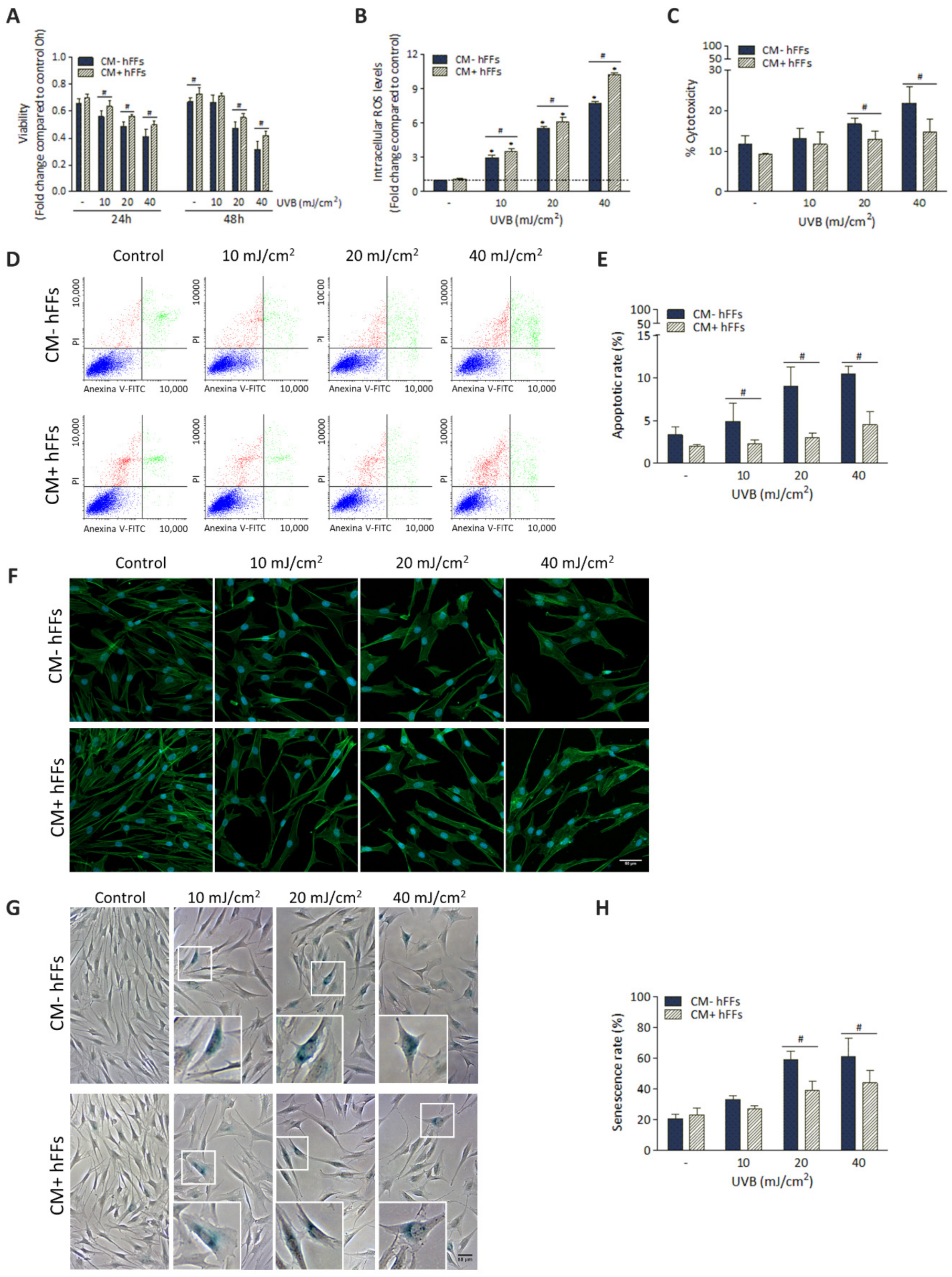
3.4.2. HC016-CM Protects hFFs against UVB-Induced Damage
3.5. HC016-CM Treatment Reduces UVB-Induced Alterations in mRNA Expression and Synthesis of IL-6 and Pro-Collagen Type I in hFFs
3.6. HC016-CM Treatment Attenuates the Deleterious Effect of UVB Radiation on hFF Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Natural Exogenous Antioxidant Defense against Changes in Human Skin Fibroblast Proteome Disturbed by UVA Radiation. Oxid. Med. Cell. Longev. 2020, 2020, 3216415. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic Effects of Ultraviolet Radiation on the Skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, H.; Peng, S.; Zhao, W.; Wang, B. Proteome Analysis of Ultraviolet-B-Induced Protein Expression in Vitro Human Dermal Fibroblasts. Photodermatol. Photoimmunol. Photomed. 2010, 26, 318–326. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.X.; Xing, W.M.; Wen, X.L.; Jia, B.B.; Yang, Z.X.; Wang, Y.Z.; Jin, X.Q.; Wang, G.F.; Yan, J. Salidroside Protects against Premature Senescence Induced by Ultraviolet B Irradiation in Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 2015, 37, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.C.; Jin, Y.S.; Hai, R.C.; Mi, K.L.; Choon, S.Y.; Rhie, G.E.; Kwang, H.C.; Kyu, H.K.; Kyung, C.P.; Hee, C.E. Modulation of Skin Collagen Metabolism in Aged and Photoaged Human Skin in Vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [Green Version]
- Cavinato, M.; Jansen-Dürr, P. Molecular Mechanisms of UVB-Induced Senescence of Dermal Fibroblasts and Its Relevance for Photoaging of the Human Skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet Radiation-Induced Skin Aging: The Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [Green Version]
- Samivel, R.; Nagarajan, R.P.; Subramanian, U.; Khan, A.A.; Masmali, A.; Almubrad, T.; Akhtar, S. Inhibitory Effect of Ursolic Acid on Ultraviolet B Radiation-Induced Oxidative Stress and Proinflammatory Response-Mediated Senescence in Human Skin Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2020, 2020, 1246510. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Panich, U.; Slominski, A.T. Editorial: Redox Biology of Skin Aging and Carcinogenesis: The Role of Natural Antioxidants as Potential Protective Agents. Front. Pharmacol. 2020, 11, 249. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxidants Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Park, S.Y.; Hwang, E.; Park, B.; Seo, S.A.; Cho, J.G.; Zhang, M.; Yi, T.H. Dietary Foeniculum Vulgare Mill Extract Attenuated UVB Irradiation-Induced Skin Photoaging by Activating of Nrf2 and Inhibiting MAPK Pathways. Phytomedicine 2016, 23, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Halabian, R.; Gharehbaghian, A.; Amirizadeh, N.; Jahanian-Najafabadi, A.; Roushandeh, A.M.; Roudkenar, M.H. Nrf-2 Overexpression in Mesenchymal Stem Cells Reduces Oxidative Stress-Induced Apoptosis and Cytotoxicity. Cell Stress Chaperones 2012, 17, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Rajnochová Svobodová, A. Effect of UVA Radiation on the Nrf2 Signalling Pathway in Human Skin Cells. J. Photochem. Photobiol. B Biol. 2020, 209, 111948. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) against Ultraviolet (UV) Radiation Effects and the Skin Photoaging. Biomedicines 2021, 9, 532. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef]
- Amin, R.M.; Elfeky, S.A.; Verwanger, T.; Krammer, B. A New Biocompatible Nanocomposite as a Promising Constituent of Sunscreens. Mater. Sci. Eng. C 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Charles-De-Sá, L.; Gontijo-De-Amorim, N.F.; Rigotti, G.; Sbarbati, A.; Bernardi, P.; Benati, D.; Bizon Vieira Carias, R.; Maeda Takiya, C.; Borojevic, R. Photoaged Skin Therapy with Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2020, 145, 1037e–1049e. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Musiał-Wysocka, A.; Lasota, M.; Ulman, A.; Majka, M. Secretion, Migration and Adhesion as Key Processes in the Therapeutic Activity of Mesenchymal Stem Cells. Acta Biochim. Pol. 2019, 66, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Boulestreau, J.; Maumus, M.; Rozier, P.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell Derived Extracellular Vesicles in Aging. Front. Cell Dev. Biol. 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.P.; Avila, F.R.; Torres, R.A.; Maita, K.C.; Eldaly, A.S.; Rinker, B.D.; Zubair, A.C.; Forte, A.J.; Sarabia-Estrada, R. Hypoxia-Preconditioning of Human Adipose-Derived Stem Cells Enhances Cellular Proliferation and Angiogenesis: A Systematic Review. J. Clin. Transl. Res. 2022, 8, 61–70. [Google Scholar]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from Hypoxia-Treated Human Adipose-Derived Mesenchymal Stem Cells Enhance Angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Bai, Y.; Han, Y.; Yan, X.; Ren, J.; Zeng, Q.; Li, X.; Pei, X.; Han, Y. Adipose Mesenchymal Stem Cell-Derived Exosomes Stimulated by Hydrogen Peroxide Enhanced Skin Flap Recovery in Ischemia-Reperfusion Injury. Biochem. Biophys. Res. Commun. 2018, 500, 310–317. [Google Scholar] [CrossRef]
- Nouri, F.; Nematollahi-Mahani, S.N.; Sharifi, A.M. Preconditioning of Mesenchymal Stem Cells with Non-Toxic Concentration of Hydrogen Peroxide Against Oxidative Stress Induced Cell Death: The Role of Hypoxia-Inducible Factor-1. Adv. Pharm. Bull. 2019, 9, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Martinez-Redondo, D.; Gartzia, I.; Alonso-Varona, A.; Garrido, P.; Palomares, T. Cryopreserved H 2 O 2 -Preconditioned Human Adipose-Derived Stem Cells Exhibit Fast Post-Thaw Recovery and Enhanced Bioactivity against Oxidative Stress. J. Tissue Eng. Regen. Med. 2019, 13, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. H2O2-Preconditioned Human Adipose-Derived Stem Cells (HC016) Increase Their Resistance to Oxidative Stress by Overexpressing Nrf2 and Bioenergetic Adaptation. Stem Cell Res. Ther. 2020, 11, 335. [Google Scholar] [CrossRef]
- Garrido-Pascual, P.; Alonso-Varona, A.; Castro, B.; Burón, M.; Palomares, T. Hydrogen Peroxide-Preconditioned Human Adipose-Derived Stem Cells Enhance the Recovery of Oligodendrocyte-like Cells after Oxidative Stress-Induced Damage. Int. J. Mol. Sci. 2020, 21, 9513. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Skrzydlewska, E.; Figaszewski, Z.A. Effects of UVB Radiation on the Physicochemical Properties of Fibroblasts and Keratinocytes. J. Membr. Biol. 2016, 249, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, R.H.; Rusdiana, T.; Wathoni, N. Mesenchymal Stem Cell Secretome for Dermatology Application: A Review. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1401–1412. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T. Metabolic Regulation of Mesenchymal Stem Cell in Expansion and Therapeutic Application. Biotechnol. Prog. 2015, 31, 468–481. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The Role of Reactive Oxygen Species in Mesenchymal Stem Cell Adipogenic and Osteogenic Differentiation: A Review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Peng, W.; Zhang, J.; Dong, W.; Yuan, D.; Zheng, Y.; Wang, Z. New Strategy of Bone Marrow Mesenchymal Stem Cells against Oxidative Stress Injury via Nrf2 Pathway: Oxidative Stress Preconditioning. J. Cell. Biochem. 2019, 120, 19902–19914. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Deng, Y.B.; Feng, J.Q.; Ye, W.B. Oxidative Preconditioning Promotes Bone Marrow Mesenchymal Stem Cells Migration and Prevents Apoptosis. Cell Biol. Int. 2009, 33, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Du, J.; Yuan, D.F.; Zhang, Y.; Zhang, S.; Zhang, H.C.; Mi, J.W.; Ning, Y.L.; Chen, M.J.; Wen, D.L.; et al. Optimal H2O2preconditioning to Improve Bone Marrow Mesenchymal Stem Cells’ Engraftment in Wound Healing. Stem Cell Res. Ther. 2020, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Kim, W.-S.; Park, B.-S.; Park, J.-S.; Sung, J.-H.; Kwak, S.-J.; Yang, J.-M. Wound Healing Effect of Adipose-Derived Stem Cells: A Critical Role of Secretory Factors on Human Dermal Fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar]
- Kim, W.S.; Park, B.S.; Sung, J.H. Protective Role of Adipose-Derived Stem Cells and Their Soluble Factors in Photoaging. Arch. Dermatol. Res. 2009, 301, 329–336. [Google Scholar]
- Palomares, T.; Cordero, M.; Bruzos-Cidon, C.; Torrecilla, M.; Ugedo, L.; Alonso-Varona, A. The Neuroprotective Effect of Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells Is Impaired by N-Acetyl Cysteine Supplementation. Mol. Neurobiol. 2018, 55, 13–25. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Liu, X.; Xv, N.; Zhang, S. Protective Effects of Adipose-Derived Stem Cells Secretome on Human Dermal Fibroblasts from Ageing Damages. Int. J. Clin. Exp. Pathol. 2015, 8, 15739–15748. [Google Scholar]
- Kim, W.S.; Park, B.S.; Park, S.H.; Kim, H.K.; Sung, J.H. Antiwrinkle Effect of Adipose-Derived Stem Cell: Activation of Dermal Fibroblast by Secretory Factors. J. Dermatol. Sci. 2009, 53, 96–102. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 185. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Long, X.; Liu, W.; Yao, G.; Zhao, Y.; Hayashi, T.; Hattori, S.; Fujisaki, H.; Ogura, T.; Tashiro, S.; et al. Differential Levels of Reactive Oxygen Species in Murine Preadipocyte 3T3-L1 Cells Cultured on Type I Collagen Molecule-Coated and Gel-Covered Dishes Exert Opposite Effects on NF-ΚB-Mediated Proliferation and Migration. Free Radic. Res. 2018, 52, 913–928. [Google Scholar] [CrossRef]
- Huo, Y.; Qiu, W.Y.; Pan, Q.; Yao, Y.F.; Xing, K.; Lou, M.F. Reactive Oxygen Species (ROS) Are Essential Mediators in Epidermal Growth Factor (EGF)-Stimulated Corneal Epithelial Cell Proliferation, Adhesion, Migration, and Wound Healing. Exp. Eye Res. 2009, 89, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Shimi, T.; Goldman, R.D. Nuclear Lamins and Oxidative Stress in Cell Proliferation and Longevity. Adv. Exp. Med. Biol. 2014, 773, 415–430. [Google Scholar] [CrossRef] [Green Version]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The Impact of Cellular Senescence in Skin Ageing: A Notion of Mosaic and Therapeutic Strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T.H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents Uvb-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 49. [Google Scholar] [CrossRef]
- López-Diazguerrero, N.-E.; Martínez-Garduño, C.M.; Konigsberg-Fainstein, M. La Senescencia Replicatica Como Una Respuesta Celular Al Estrés. Mech. Ageing Dev. 2006, 24, 47–53. [Google Scholar]



| Gene | Accession Number | Primer Sequence (5′-3′) |
|---|---|---|
| IL-6 | NM_000600 | Fw: GCAGATGAGTACAAAAGTCCTGA Rv: TTCTGTGCCTGCAGCTTC |
| COL1A2 | NM_000089 | Fw: GTGAGAGAGGAGTTGTTGGAC Rv: CCTTCAATCCATCCAGACCAT |
| GAPDH | NM_002046 | Fw: ACATCGCTCAGACACCATG Rv: TGTAGTTGAGGTCAATGAAGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burón, M.; Palomares, T.; Garrido-Pascual, P.; Herrero de la Parte, B.; García-Alonso, I.; Alonso-Varona, A. Conditioned Medium from H2O2-Preconditioned Human Adipose-Derived Stem Cells Ameliorates UVB-Induced Damage to Human Dermal Fibroblasts. Antioxidants 2022, 11, 2011. https://doi.org/10.3390/antiox11102011
Burón M, Palomares T, Garrido-Pascual P, Herrero de la Parte B, García-Alonso I, Alonso-Varona A. Conditioned Medium from H2O2-Preconditioned Human Adipose-Derived Stem Cells Ameliorates UVB-Induced Damage to Human Dermal Fibroblasts. Antioxidants. 2022; 11(10):2011. https://doi.org/10.3390/antiox11102011
Chicago/Turabian StyleBurón, María, Teodoro Palomares, Patricia Garrido-Pascual, Borja Herrero de la Parte, Ignacio García-Alonso, and Ana Alonso-Varona. 2022. "Conditioned Medium from H2O2-Preconditioned Human Adipose-Derived Stem Cells Ameliorates UVB-Induced Damage to Human Dermal Fibroblasts" Antioxidants 11, no. 10: 2011. https://doi.org/10.3390/antiox11102011









