Oxidative Modification Status of Human Serum Albumin Caused by Chronic Low-Dose Radiation Exposure in Mamuju, Sulawesi, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Blood Sampling from the Residents
2.3. External and Internal Radiation Exposure Dose Assessments to Estimate the Total Effective Dose
2.4. LC-MS/MS and High-Resolution Multiple Reaction Monitoring (MRM-HR)
2.5. Statistical Analyses
3. Results
3.1. The Re-Assessment of Each Annual Effective Dose
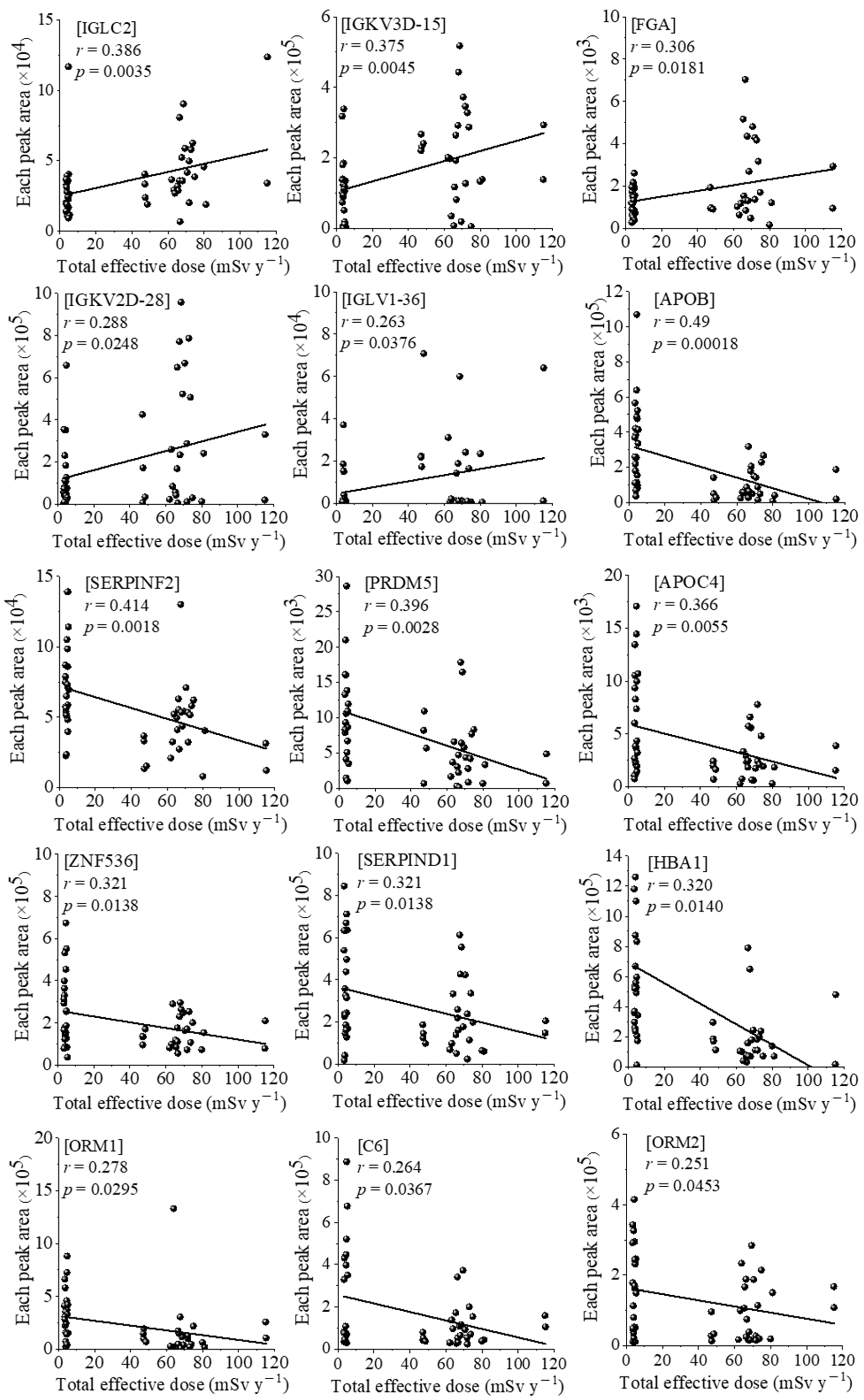
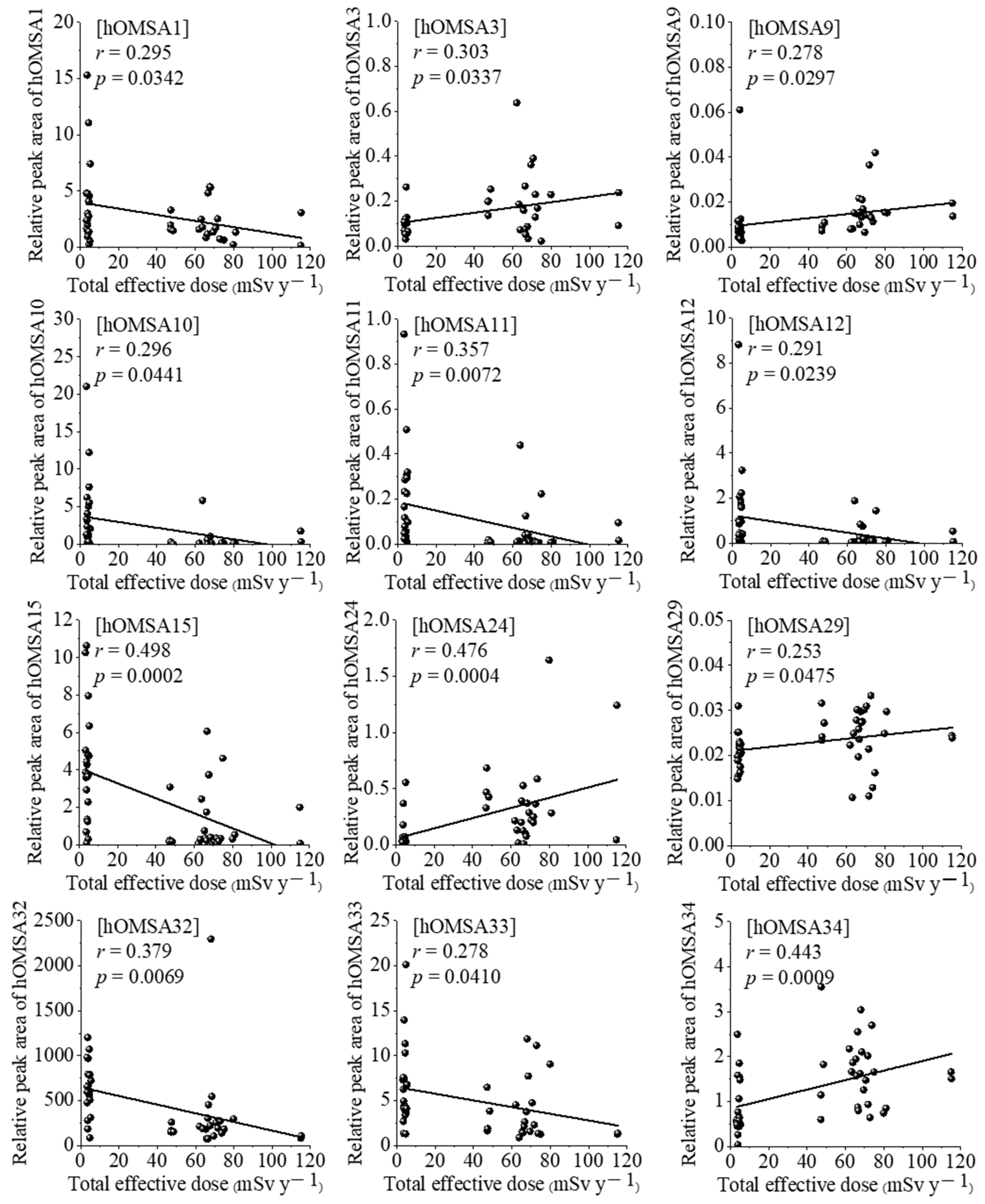
3.2. Correlations between Serum Proteomic and Oxidative Modification of HSA Profiling, and Individual Effective Doses of Study Subjects Living in Both Areas
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, D.; Jain, V.; Das, B. Evaluation of natural chronic low dose radiation exposure on telomere length and transcriptional response of shelterin complex in individuals residing in Kerala coast, India. Mutat. Res. 2022, 825, 111797. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.H.; Simon, S.L.; Wojcik, A.; Sohrabi, M.; Burkart, W.; Cardis, E.; Laurier, D.; Tirmarche, M.; Hayata, I. Human exposure to high natural background radiation: What can it teach us about radiation risks? J. Radiol Prot. 2009, 29, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Syaeful, H.; Sukadana, I.G.; Sumaryanto, A. Radiometric Mapping for Naturally Occurring Radioactive Materials (NORM) Assessment in Mamuju, West Sulawesi. Atom. Indones 2014, 40, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Syaifudin, M.; Purnami, S.; Rahardjo, T.; Kurnia, I.; Rahajeng, N.; Darlina; Nurhayati, S.; Mailana, W.; Ramadhani, D.; Suvifan, V.A.; et al. Cytogenetic and Molecular Damages in Blood Lymphocyte of Inhabitants Living in High Level Natural Radiation Area (HLNRA) of Botteng Village, Mamuju, West Sulawesi, Indonesia. Radiat Environ. Med. 2018, 7, 65–76. [Google Scholar]
- Basri, I.K.H.; Yusuf, D.; Rahardjo, T.; Nurhayati, S.; Tetriana, D.; Ramadhani, D.; Alatas, Z.; Purnami, S.; Kisnanto, T.; Lusiyanti, Y.; et al. Study of γ-H2AX as DNA double strand break biomarker in resident living in high natural radiation area of Mamuju, West Sulawesi. J. Environ. Radioact. 2017, 171, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, D.; Nurhayati, S.; Rahardjo, T.; Pudjadi, E.; Syaifudin, M. Lymphocyte Proliferation Kinetics in Inhabitant of Takandeang Village, Mamuju: A High Background Radiation Areas in Indonesia. Indones Biomed. J. 2018, 10, 66–73. [Google Scholar] [CrossRef]
- Hosoda, M.; Nugraha, E.D.; Akata, N.; Yamada, R.; Tamakuma, Y.; Sasaki, M.; Kelleher, K.; Yoshinaga, S.; Suzuki, T.; Pornnumpa, C.; et al. A unique high natural background radiation area—Dose assessment and perspectives. Sci. Total Environ. 2021, 750, 142346. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources, Effects and Risks of Ionizing Radiation, UNSCEAR 2017 Report; United Nations Publication: New York, NY, USA, 2018. [Google Scholar]
- Tubiana, M.; Feinendegen, L.E.; Yang, C.; Kaminski, J.M. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology 2009, 251, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Tatara, Y.; Nugraha, E.D.; Ramadhani, D.; Tamakuma, Y.; Sato, Y.; Miura, T.; Hosoda, M.; Yoshinaga, S.; Syaifudin, M.; et al. Detection of biological responses to low-dose radiation in humans. Free Radic. Biol. Med. 2022, 184, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Tatara, Y.; Nugraha, E.D.; Sato, Y.; Miura, T.; Hosoda, M.; Syaifudin, M.; Tokonami, S.; Kashiwakura, I. Serum Proteomic and Oxidative Modification Profiling in Mice Exposed to Total Body X-Irradiation. Antioxidants 2022, 11, 1710. [Google Scholar] [CrossRef]
- Nugraha, E.D.; Hosoda, M.; Kusdiana; Untara; Mellawati, J.; Nurokhim; Tamakuma, Y.; Ikram, A.; Syaifudin, M.; Yamada, R.; et al. Comprehensive Exposure Assessments from the Viewpoint of Health and Human Activities in a Unique High Natural Background Radiation Area, Mamuju, Indonesia. Sci. Rep. 2021, 11, 14578. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, E.D.; Hosoda, M.; Tamakuma, Y.; Kranrod, C.; Mellawati, J.; Akata, N.; Tokonami, S. A unique high natural background radiation area in Indonesia: A brief review from the viewpoint of dose assessments. J. Radioanal Nucl. Chem. 2021, 330, 1437–1444. [Google Scholar] [CrossRef]
- Gorobets, M.G.; Wasserman, L.A.; Vasilyeva, A.D.; Bychkova, A.V.; Pronkin, P.G.; Bugrova, A.E.; Indeykina, M.I.; Shilkina, N.G.; Konstantinova, M.L.; Kononikhin, A.S.; et al. Modification of human serum albumin under induced oxidation. Dokl. Biochem. Biophys. 2017, 474, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Kubota, K.; Yamada, N.; Tagami, U.; Takehana, K.; Sonaka, I.; Suzuki, E.; Hirayama, K. Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions. FEBS J. 2006, 273, 3346–3357. [Google Scholar] [CrossRef]
- Sukadana, I.G.; Harijoko, A.; Setijadji, L.D. Tectonic setting of adang volcanic complex in Mamuju Region, West Sulawesi Province. Eksplorium 2015, 36, 2769. [Google Scholar] [CrossRef]
- Rosianna, I.; Nugraha, E.D.; Syaeful, H.; Putra, S.; Hosoda, M.; Akata, N.; Tokonami, S. Natural radioactivity of laterite and volcanic rock sample for radioactive mineral exploration in Mamuju, Indonesia. Geosciences 2020, 10, 376. [Google Scholar] [CrossRef]
- Bai, J.; He, A.; Huang, C.; Yang, J.; Zhang, W.; Wang, J.; Yang, Y.; Zhang, P.; Zhang, Y.; Zhou, F. Serum peptidome based biomarkers searching for monitoring minimal residual disease in adult acute lymphocytic leukemia. Proteome Sci. 2014, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Z.; Piao, Z.; Jin, S.Y.; Kwak, Y.G. Differential expression of serum proteins in multiple myeloma. Exp. Ther. Med. 2019, 17, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Gong, B.; Wang, P.; Huang, H.; Luo, L.; Liu, F. Novel prognostic biomarkers of gastric cancer based on gene expression microarray: COL12A1, GSTA3, FGA and FGG. Mol. Med. Rep. 2018, 18, 3727–3736. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Luo, C.; Zhu, S.; Fang, H.; Gao, Q.; Ge, S.; Qu, H.; Ma, Q.; Ren, H.; Wang, Y.; et al. Serum peptidome profiling for the diagnosis of colorectal cancer: Discovery and validation in two independent cohorts. Oncotarget 2017, 8, 59376–59386. [Google Scholar] [CrossRef] [PubMed]
- Ferrín, G.; Rodríguez-Perálvarez, M.; Aguilar-Melero, P.; Ranchal, I.; Llamoza, C.; Linares, C.I.; González-Rubio, S.; Muntané, J.; Briceño, J.; López-Cillero, P.; et al. Plasma protein biomarkers of hepatocellular carcinoma in HCV-infected alcoholic patients with cirrhosis. PLoS ONE 2015, 10, e0118527. [Google Scholar] [CrossRef] [PubMed]
- Rithidech, K.N.; Honikel, L.; Rieger, R.; Xie, W.; Fischer, T.; Simon, S.R. Protein-expression profiles in mouse blood-plasma following acute whole-body exposure to 137Cs gamma rays. Int. J. Radiat. Biol. 2009, 85, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Feurgard, C.; Bayle, D.; Guézingar, F.; Sérougne, C.; Mazur, A.; Lutton, C.; Aigueperse, J.; Gourmelon, P.; Mathé, D. Effects of ionizing radiation (neutrons/gamma rays) on plasma lipids and lipoproteins in rats. Radiat. Res. 1998, 150, 43–51. [Google Scholar] [CrossRef]
- Jedidi, I.; Thérond, P.; Zarev, S.; Cosson, C.; Couturier, M.; Massot, C.; Jore, D.; Gardès-Albert, M.; Legrand, A.; Bonnefont-Rousselot, D. Paradoxical protective effect of aminoguanidine toward low-density lipoprotein oxidation: Inhibition of apolipoprotein B fragmentation without preventing its carbonylation. Mechanism of action of aminoguanidine. Biochemistry 2003, 42, 11356–11365. [Google Scholar] [CrossRef] [PubMed]
- Zarev, S.; Bonnefont-Rousselot, D.; Cosson, C.; Beaudeux, J.L.; Delattre, J.; Gardès-Albert, M.; Legrand, A.; Thérond, P. In vitro low-density lipoprotein oxidation by copper or *OH/O*(2)(-): New features on carbonylation and fragmentation of apolipoprotein B during the lag phase. Arch. Biochem. Biophys. 2002, 404, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Huang, S. PRDM5 is silenced in human cancers and has growth suppressive activities. Oncogene 2004, 23, 4903–4910. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Liu, D.; Xing, R.; Song, H.; Tian, X.; Yan, C.; Han, Y. Orosomucoid 1 Attenuates Doxorubicin-Induced Oxidative Stress and Apoptosis in Cardiomyocytes via Nrf2 Signaling. Biomed. Res. Int. 2020, 2020, 5923572. [Google Scholar] [CrossRef]
- Zaias, J.; Mineau, M.; Cray, C.; Yoon, D.; Altman, N.H. Reference values for serum proteins of common laboratory rodent strains. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 387–390. [Google Scholar]
- Watanabe, H.; Imafuku, T.; Otagiri, M.; Maruyama, T. Clinical implications associated with the posttranslational modification-induced functional impairment of albumin in oxidative stress-related diseases. J. Pharm. Sci. 2017, 106, 2195–2203. [Google Scholar] [CrossRef] [Green Version]
- Tabata, F.; Wada, Y.; Kawakami, S.; Miyaji, K. Serum albumin redox states: More than oxidative stress biomarker. Antioxidants 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Komatsu, Y.; Izumi, H.; Shimizu, T.; Takeda, Y.; Kuwahata, M. Increased ratio of non-mercaptalbumin-1 among total plasma albumin demonstrates potential protein undernutrition in adult rats. Front. Nutr. 2018, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Izumi, H.; Shimizu, T.; Takeda, Y. A more oxidized plasma albumin redox state and lower plasma hdl particle number reflect low-protein diet ingestion in adult rats. J. Nutr. 2020, 150, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kodera, Y.; Saito, T.; Fujimoto, K.; Momozono, A.; Hayashi, A.; Kamata, Y.; Shichiri, M. Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci. Rep. 2016, 6, 38299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momozono, A.; Kodera, Y.; Sasaki, S.; Nakagawa, Y.; Konno, R.; Shichiri, M. Oxidised Met147 of human serum albumin is a biomarker of oxidative stress, reflecting glycaemic fluctuations and hypoglycaemia in diabetes. Sci. Rep. 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrzyński, L.; Fornalski, K.W.; Feinendegen, L.E. Cancer mortality among people living in areas with various levels of natural background radiation. Dose Response 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Boice, J.D., Jr.; Wei, L.X.; Beebe, G.W.; Zha, Y.R.; Kaplan, M.M.; Tao, Z.F.; Maxon, H.R., 3rd; Zhang, S.Z.; Schneider, A.B. Thyroid nodularity and chromosome aberrations among women in areas of high background radiation in China. J. Natl. Cancer Inst. 1990, 82, 478–485. [Google Scholar] [CrossRef]
- Ghiassi-nejad, M.; Mortazavi, S.M.J.; Cameron, J.R.; Niroomand-rad, A.; Karam, P.A. Very high background radiation areas of Ramsar, Iran: Preliminary biological studies. Health Phys. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Aliyu, A.S.; Ramli, A.T. The world’s high background natural radiation areas (HBNRAs) revisited: A broad overview of the dosimetric, epidemiological and radiobiological issues. Radiat Meas 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Hayashi, T.; Morishita, Y.; Khattree, R.; Misumi, M.; Sasaki, K.; Hayashi, I.; Yoshida, K.; Kajimura, J.; Kyoizumi, S.; Imai, K. Evaluation of systemic markers of inflammation in atomic-bomb survivors with special reference to radiation and age effects. FASEB J. 2012, 26, 4765–4773. [Google Scholar] [CrossRef] [Green Version]
- Pornnumpa, C.; Oyama, Y.; Iwaoka, K.; Hosoda, M.; Tokonami, S. Development of Radon and Toron Exposure Systems at Hirosaki University. Radiat Environ. Med. 2018, 7, 13–20. [Google Scholar]
- Lecomte, J.F.; Solomon, S.; Takala, J.; Jung, T.; Strand, P.; Murith, C.; Kiselev, S.; Zhuo, W. ICRP Publication 126: Radiological Protection against Radon Exposure. Ann. ICRP 2014, 43, 5–73. [Google Scholar] [CrossRef]
- ICRP. Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–335. [Google Scholar]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1116. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; et al. JPOSTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar] [CrossRef]
| No | Sex | Age (Years) | External Dose | Internal Dose-Inhalation- | Internal Dose-Ingestion- | Total Dose Effective Dose (mSv) | |||
|---|---|---|---|---|---|---|---|---|---|
| OSLD (mSv) | Radon Dose (mSv) | Thoron Dose (mSv) | Radon Water (mSv) | Radium Water (mSv) | Food (mSv) | ||||
| 1 | M | 43 | 0.58 | 1.5 | 1.2 | 0.006 | 0.003 | 0.3 | 3.6 |
| 2 | F | 41 | 0.61 | 1.5 | 1.2 | 0.006 | 0.003 | 0.3 | 3.6 |
| 3 | M | 70 | 0.51 | 1.3 | 2.1 | 0.006 | 0.003 | 0.3 | 4.2 |
| 4 | M | 51 | 0.50 | 2.1 | 1.7 | 0.006 | 0.003 | 0.3 | 4.7 |
| 5 | M | 27 | 0.32 | 1.5 | 2.0 | 0.006 | 0.003 | 0.3 | 4.2 |
| 6 | F | 49 | 0.60 | 2.9 | 0.7 | 0.006 | 0.003 | 0.3 | 4.4 |
| 7 | M | 32 | 0.70 | 1.2 | 1.6 | 0.006 | 0.003 | 0.3 | 3.8 |
| 8 | M | 33 | 0.68 | 1.2 | 2.5 | 0.006 | 0.003 | 0.3 | 4.8 |
| 9 | M | 50 | 0.57 | 1.8 | 0.7 | 0.006 | 0.003 | 0.3 | 3.3 |
| 10 | F | 30 | 0.72 | 1.5 | 2.5 | 0.006 | 0.003 | 0.3 | 5.1 |
| 11 | M | 48 | 0.53 | 1.1 | 1.5 | 0.006 | 0.003 | 0.3 | 3.4 |
| 12 | F | 40 | 0.53 | 2.1 | 1.7 | 0.006 | 0.003 | 0.3 | 4.7 |
| 13 | F | 34 | 0.49 | 1.5 | 1.6 | 0.006 | 0.003 | 0.3 | 3.9 |
| 14 | M | 42 | 0.54 | 2.9 | 1.6 | 0.006 | 0.003 | 0.3 | 5.3 |
| 15 | M | 41 | 0.56 | 2.1 | 1.7 | 0.006 | 0.003 | 0.3 | 4.7 |
| 16 | F | 25 | 0.56 | 2.1 | 1.7 | 0.006 | 0.003 | 0.3 | 4.7 |
| 17 | M | 43 | 0.42 | 1.7 | 1.6 | 0.006 | 0.003 | 0.3 | 4.0 |
| 18 | M | 37 | 0.47 | 1.5 | 1.6 | 0.006 | 0.003 | 0.3 | 3.9 |
| 19 | M | 42 | 0.66 | 1.7 | 1.8 | 0.006 | 0.003 | 0.3 | 4.4 |
| 20 | F | 42 | 0.70 | 1.7 | 1.8 | 0.006 | 0.003 | 0.3 | 4.5 |
| 21 | F | 45 | 0.53 | 1.1 | 1.5 | 0.006 | 0.003 | 0.3 | 3.4 |
| 22 | M | 54 | 0.44 | 1.6 | 1.0 | 0.006 | 0.003 | 0.3 | 3.4 |
| 23 | F | 20 | 0.39 | 1.1 | 1.9 | 0.006 | 0.003 | 0.3 | 3.7 |
| No | Sex | Age (Years) | External Dose | Internal Dose-Inhalation- | Internal Dose-Ingestion- | Total Dose Effective Dose (mSv) | |||
|---|---|---|---|---|---|---|---|---|---|
| OSLD (mSv) | Radon Dose (mSv) | Thoron Dose (mSv) | Radon Water (mSv) | Radium Water (mSv) | Food (mSv) | ||||
| 1 | M | 38 | 7.8 | 78.2544 | 28.462 | 0.00228 | 0.00222 | 0.5132 | 115 |
| 2 | F | 29 | 8.0 | 78.2544 | 28.462 | 0.00228 | 0.00222 | 0.5132 | 115 |
| 3 | M | 37 | 9.1 | 44.1252 | 15.729 | 0.00222 | 0.00229 | 0.5130 | 69 |
| 4 | M | 55 | 10.1 | 26.0848 | 10.486 | 0.00222 | 0.00229 | 0.5130 | 47 |
| 5 | F | 42 | 10.2 | 44.1252 | 15.729 | 0.00222 | 0.00229 | 0.5130 | 71 |
| 6 | F | 41 | 7.8 | 44.3156 | 14.98 | 0.00222 | 0.00228 | 0.5130 | 68 |
| 7 | F | 28 | 11.3 | 44.1252 | 15.729 | 0.00222 | 0.00228 | 0.5130 | 72 |
| 8 | M | 74 | 8.6 | 44.3156 | 11.984 | 0.00228 | 0.00229 | 0.5132 | 65 |
| 9 | M | 74 | 7.8 | 36.414 | 20.972 | 0.00228 | 0.00229 | 0.5130 | 66 |
| 10 | F | 63 | 8.5 | 36.414 | 20.972 | 0.00228 | 0.00229 | 0.5128 | 66 |
| 11 | M | 75 | 9.4 | 26.9892 | 10.486 | 0.00228 | 0.00229 | 0.5130 | 47 |
| 12 | F | 49 | 10.7 | 33.7008 | 29.96 | 0.00220 | 0.00229 | 0.5132 | 75 |
| 13 | M | 29 | 9.5 | 33.7008 | 29.96 | 0.00220 | 0.00228 | 0.5128 | 74 |
| 14 | F | 26 | 10.5 | 46.886 | 23.219 | 0.00228 | 0.00229 | 0.5130 | 81 |
| 15 | F | 27 | 9.9 | 42.9352 | 9.737 | 0.00220 | 0.00229 | 0.5132 | 63 |
| 16 | M | 33 | 8.8 | 42.9352 | 9.737 | 0.00228 | 0.00229 | 0.5130 | 62 |
| 17 | M | 73 | 8.7 | 45.3152 | 17.227 | 0.00228 | 0.00229 | 0.5130 | 72 |
| 18 | F | 59 | 8.8 | 17.5644 | 20.223 | 0.00216 | 0.00229 | 0.5130 | 47 |
| 19 | F | 63 | 9.8 | 45.3152 | 17.227 | 0.00228 | 0.00229 | 0.5128 | 73 |
| 20 | M | 52 | 8.7 | 44.3156 | 14.98 | 0.00220 | 0.00229 | 0.5130 | 69 |
| 21 | M | 31 | 9.6 | 44.3156 | 11.984 | 0.00228 | 0.00228 | 0.5130 | 66 |
| 22 | M | 29 | 9.3 | 46.886 | 23.219 | 0.00228 | 0.00229 | 0.5130 | 80 |
| 23 | F | 59 | 10.5 | 26.9892 | 10.486 | 0.00216 | 0.00229 | 0.5132 | 49 |
| 24 | F | 26 | 7.8 | 44.3156 | 11.235 | 0.00228 | 0.00229 | 0.5130 | 64 |
| 25 | F | 44 | 11.4 | 38.8416 | 17.227 | 0.00228 | 0.00228 | 0.5128 | 68 |
| 26 | M | 20 | 10.1 | 38.8416 | 17.227 | 0.00216 | 0.00229 | 0.5128 | 67 |
| hOMSA ID | Peptide Sequence and Modification Site | Amino Acid Residue | Modification Type |
|---|---|---|---|
| 1 | DLGEENFKALVLIAFAQY[Oxi]LQQC[CAM]PFEDHVK | 54th tyrosine | Oxidation |
| 3 | SLHTLFGDKLC[CAM]TVATLRETYGEM[Oxi]ADC[CAM]C[CAM]AK | 111th methionine | Oxidation |
| 9 | Y[Oxi]LYEIAR | 162nd tyrosine | Oxidation |
| 10 | RHP[Oxi]YFYAPELLFFAK | 171th proline | Oxidation |
| 11 | RHPYFY[Oxi]APELLFFAK | 174th tyrosine | Oxidation |
| 12 | RHPYFYAPELLFFAK[LAA] | 183th lysine | Lys -> Allysine |
| 15 | LVTDLTK[LAA]VHTEC[CAM]C[CAM]HGDLLEC[CAM]ADDRADLAK | 264th lysine | Lys -> Allysine |
| 24 | DVFLGMFLY[Oxi]EYAR | 356th tyrosine | Oxidation |
| 29 | QNC[CAM]ELF[Oxi]EQLGEYK | 419th phenylalanine | Oxidation |
| 32 | RM[Oxi]PC[CAM]AEDYLSVVLNQLC[CAM]VLHEK | 470th methionine | Oxidation |
| 33 | RM[Oxi]PC[CAM]AEDY[Oxi]LSVVLNQLC[CAM]VLHEK | 476th tyrosine | Oxidation |
| 34 | M[Oxi]PC[CAM]AEDYLSVVLNQLC[CAM]VLHEK | 470th methionine | Oxidation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, M.; Tatara, Y.; Nugraha, E.D.; Tamakuma, Y.; Sato, Y.; Miura, T.; Hosoda, M.; Yoshinaga, S.; Syaifudin, M.; Tokonami, S.; et al. Oxidative Modification Status of Human Serum Albumin Caused by Chronic Low-Dose Radiation Exposure in Mamuju, Sulawesi, Indonesia. Antioxidants 2022, 11, 2384. https://doi.org/10.3390/antiox11122384
Yamaguchi M, Tatara Y, Nugraha ED, Tamakuma Y, Sato Y, Miura T, Hosoda M, Yoshinaga S, Syaifudin M, Tokonami S, et al. Oxidative Modification Status of Human Serum Albumin Caused by Chronic Low-Dose Radiation Exposure in Mamuju, Sulawesi, Indonesia. Antioxidants. 2022; 11(12):2384. https://doi.org/10.3390/antiox11122384
Chicago/Turabian StyleYamaguchi, Masaru, Yota Tatara, Eka Djatnika Nugraha, Yuki Tamakuma, Yoshiaki Sato, Tomisato Miura, Masahiro Hosoda, Shinji Yoshinaga, Mukh Syaifudin, Shinji Tokonami, and et al. 2022. "Oxidative Modification Status of Human Serum Albumin Caused by Chronic Low-Dose Radiation Exposure in Mamuju, Sulawesi, Indonesia" Antioxidants 11, no. 12: 2384. https://doi.org/10.3390/antiox11122384






