Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application
Abstract
:1. Introduction
2. Extraction Methods Used to Extract Anthocyanins
2.1. Conventional Extraction Techniques
2.2. Non-Conventional Extraction Techniques
2.2.1. Ultrasound-Assisted Extraction (UAE)
2.2.2. Microwave-Assisted Extraction (MAE)
2.2.3. Pressure Fluids Extraction Techniques
Supercritical Fluid Extraction (SFE)
Pressurized Liquid Extraction (PLE)
High-Pressure Liquid Extraction (HPLE)
2.2.4. Pulsed Electric Field Extraction (PEFE)
2.2.5. Enzyme-Assisted Aqueous Extraction (EAE)
3. Advantages and Disadvantages of the Promising Green Extraction Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jezek, M.; Zorb, C.; Merkt, N.; Geilfus, C.-M. Anthocyanin Management in Fruits by Fertilization. J. Agric. Food Chem. 2018, 66, 753–764. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant Capacity of Anthocyanin Pigments. In Flavonoids—From Biosynthesis to Human Health; Justino, J., Ed.; Science, Technology and Medicine Open Access Publisher: Rijeka, Croatia, 2017; Chapter 11; pp. 205–255. [Google Scholar]
- Martín Bueno, J.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jímenez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Navas, M.J.; Jiménez-Moreno, A.M.; Martín Bueno, J.; Sáez-Plaza, P.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; López, C.; Palomero, F.; González, C. Emerging Non-Thermal Technologies for the Extraction of Grape Anthocyanins. Antioxidants 2021, 10, 1863. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, E.; Prieto, M.A.; Calhelha, R.C.; Ciric, A.; Sokovic, M.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C.F.R. Optimization of the Extraction Process to Obtain a Colorant Ingredient from Leaves of Ocimum basilicum var. Purpurascens. Molecules 2019, 24, 686. [Google Scholar] [CrossRef] [Green Version]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Fincan, M. Potential Application of Pulsed Electric Fields for Improving Extraction of Plant Pigments. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Germany, 2017; Chapter 9; pp. 2171–2192. [Google Scholar]
- EFSA. Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Kumara, K.; Srivastava, S.; Sharanagatb, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochemistry 2021, 70, 105325. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, Y.; Wang, F.; Li, W.; Zhang, X.; Niu, Z.; Wang, Z. Optimization the extraction of anthocyanins from blueberry residue by dual-aqueous phase method and cell damage protection study. Food Sci. Biotechnol. 2021, 30, 1709–1719. [Google Scholar] [CrossRef]
- Moirangthem, K.; Ramakrishna, P.; Amer, M.H.; Tucker, G.A. Bioactivity and anthocyanin content of microwave-assisted subcritical water extracts of Manipur black rice (Chakhao) bran and straw. Future Foods 2021, 3, 100030. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Carrera, C.; Aliaño-González, M.J.; Valaityte, M.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. A Novel Ultrasound-Assisted Extraction Method for the Analysis of Anthocyanins in Potatoes (Solanum tuberosum L.). Antioxidants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, T.; Li, J.; Ye, H.; Liu, R.; Li, H.; Deng, Z. Response surface methodology for optimization of microwave-assisted extraction and antioxidant activity of anthocyanins from red rice. J. Chin. Inst. Food Sci. Technol. 2015, 15, 74–81. [Google Scholar]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Perino-Issartier, S.; Zill-e-Huma; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Lončarić, A.; Jozinović, A.; Kovač, T.; Kojić, N.; Babić, J.; Šubarić, D. High Voltage Electrical Discharges and Ultrasound-Assisted Extraction of Phenolics from Indigenous Fungus-Resistant Grape By-Product. Pol. J. Food Nutr. Sci. 2020, 70, 101–111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Huang, H. Effects of Pulsed Electric Fields on Anthocyanin Extraction Yield of Blueberry Processing By-Products. J. Food Process. Preserv. 2015, 39, 1898–1904. [Google Scholar] [CrossRef]
- Varadharajan, V.; Shanmugam, S.; Ramaswamy, A. Model generation and process optimization of microwave assisted aqueous extraction of anthocyanins from grape juice waste. J. Food Process. Eng. 2017, 40, e12486. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.I.; Bhatt, D.; Daglia, M.; Baldi, A.; Prasad Devkota, H.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Martín, J.; Asuero, A.G. High hydrostatic pressure for recovery of anthocyanins: Effects, performance, and applications. Sep. Purif. Rev. 2021, 50, 159–176. [Google Scholar] [CrossRef]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Paludo, M.; Colombo, R.; Teixeira, J.; Hermosín-Gutiérrez, I.; Ballus, C.; Godoy, H. Optimizing the Extraction of Anthocyanins from the Skin and Phenolic Compounds from the Seed of Jabuticaba Fruits (Myrciaria jabuticaba (Vell.) O. Berg) with Ternary Mixture Experimental Designs. J. Braz. Chem. Soc. 2019, 30, 1506–1514. [Google Scholar] [CrossRef]
- Demirdöven, A.; Özdoğan, K.; Erdoğan-Tokatli, K. Extraction of Anthocyanins from Red Cabbage by Ultrasonic and Conventional Methods: Optimization and Evaluation. J. Food Biochem. 2015, 39, 491–500. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, K.M.; Jafari, S.M.; Ghorbani, M.; Kakhki, A.H.; Sarfarazi, M. Optimization of Anthocyanin Extraction from Saffron Petals with Response Surface Methodology. Food Anal. Methods 2016, 9, 1993–2001. [Google Scholar] [CrossRef]
- Sang, J.; Ma, Q.; Li, B.; Li, C.-Q. An approach for extraction, purification, characterization and quantitation of acylated-anthocyanins from Nitraria tangutorun Bobr. fruit. J. Food Meas. Charact. 2018, 12, 45–55. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- de Sousa Sabino, L.B.; Alves Filho, E.G.; Fernandes, F.A.N.; de Brito, E.S.; da Silva Júnior, I.J. Optimization of pressurized liquid extraction and ultrasound methods for recovery of anthocyanins present in jambolan fruit (Syzygium cumini L.). Food Bioprod. Process. 2021, 127, 77–89. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamadon, A.M.; Niakousari, M. Optimization of ultrasound assisted extraction of anthocyanins from red cabbage using Taguchi design method. J. Food Sci. Technol. 2015, 52, 8140–8147. [Google Scholar] [CrossRef] [Green Version]
- Agcam, E.; Akyıldız, A.; Balasubramaniam, V.M. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017, 237, 461–470. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.-L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using Response Surface Methodology. Ultrason. Sonochemistry 2015, 27, 449–455. [Google Scholar] [CrossRef]
- Dibazar, R.; Celli, G.B.; Brooks, M.S.-L.; Ghanem, A. Optimization of ultrasound-assisted extraction of anthocyanins from lowbush blueberries (Vaccinium Angustifolium Aiton). J. Berry Res. 2015, 5, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Barreiro, M.F.; Baraldi, I.J.; Barros, L.; Ferreira, I.C.F.R. Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit epicarp: Comparative study with conventional heat-based extraction. Molecules 2019, 24, 573. [Google Scholar] [CrossRef] [Green Version]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochemistry 2015, 27, 509–514. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochemistry 2011, 18, 1249–1257. [Google Scholar] [CrossRef]
- Xu, W.J.; Zhai, J.W.; Cui, Q.; Liu, J.Z.; Luo, M.; Fu, Y.J.; Zu, Y.G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Tan, Z.J.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Wang, C.Y. Extraction, preconcentration and isolation of flavonoids from Apocynum venetum L. Leaves using ionic liquid-based ultrasonic-assisted extraction coupled with an aqueous biphasic system. Molecules 2016, 21, 262. [Google Scholar] [CrossRef] [Green Version]
- Ardestani, S.B.; Sahari, M.A.; Barzegar, M. Effect of Extraction and Processing Conditions on Organic Acids of Barberry Fruits. J. Food Biochem. 2015, 39, 554–565. [Google Scholar] [CrossRef]
- Xue, H.; Xu, H.; Wang, X.; Shen, L.; Liu, H.; Liu, C.; Qin, Q.; Zheng, X.; Li, Q. Effects of Microwave Power on Extraction Kinetic of Anthocyanin from Blueberry Powder considering Absorption of Microwave Energy. J. Food Qual. 2018, 2018, 9680184. [Google Scholar] [CrossRef] [Green Version]
- Farzaneh, V.; Carvalho, I.S. Modelling of microwave assisted extraction (MAE) of anthocyanins (TMA). J. Appl. Res. Med. Aromat. Plants 2017, 6, 92–100. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, H.K.; Liu, C.H.; Liu, C.; Su, X.L.; Zheng, X.Z. Comparison of microwave assisted extraction with hot reflux extraction in acquirement and degradation of anthocyanin from powdered blueberry. Int. J. Agric. Biol. Eng. 2016, 9, 186–199. [Google Scholar]
- Xue, H.; Tan, J.; Fan, L.; Li, Q.; Cai, X.J. Optimization microwave-assisted extraction of anthocyanins from cranberry using response surface methodology coupled with genetic algorithm and kinetics model análisis. Food Process Eng. 2021, 44, e13688. [Google Scholar]
- Yiğit, Ü.; Yolaçaner, E.T.; Hamzalıoğlu, A.; Gökmen, V. Optimization of microwave-assisted extraction of anthocyanins in red cabbage by response surface methodology. J. Food Process. Preserv. 2021, 46, e16120. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Zhou, C.; Wen, Z.; Dong, X. An improved microwave-assisted extraction of anthocyanins from purple sweet potato in favor of subsequent comprehensive utilization of pomace. Food Bioprod. Process. 2019, 115, 1–9. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Yang, D. Comparison of extraction methods for anthocyanins from fruit of rubus coreanus maq. and optimization of microwave-assisted extraction process. J. Food Sci. Technol. China 2019, 37, 91–97. [Google Scholar]
- Zheng, S.; Deng, Z.; Jiang, H.; Li, H. Comparison of Different Extraction Methods and Antioxidant Activity of Anthocyanins from Eggplant Peel. J. Chin. Inst. Food Sci. Technol. 2017, 17, 92–99. [Google Scholar]
- Li, X.; Chen, F.; Li, S.; Jia, J.; Gu, H.; Yang, L. An efficient homogenate-microwave-assisted extraction of flavonolsand anthocyanins from blackcurrant marc: Optimization usingcombination of Plackett-Burman design and Box-Behnken design. Ind. Crops Prod. 2016, 94, 834–847. [Google Scholar] [CrossRef]
- Odabas, H.I.; Koca, I. Simultaneous separation and preliminary purification of anthocyanins from Rosa pimpinellifolia L. fruits by microwave assisted aqueous two-phase extraction. Food Bioprod. Process. 2021, 125, 170–180. [Google Scholar] [CrossRef]
- Zill-e-Huma, M.A.V.; Maingonnat, J.F.; Chemat, F. Clean recovery of antioxidant flavonoids from onions: Optimising solvent free microwave extraction method. J. Chromatogr. A 2009, 1216, 7700–7707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, S. A novel technology for extraction of phenolic antioxidants from mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean J. Chem. Eng. 2015, 32, 950–957. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. Evaluation of a simple and promising method for extraction of antioxidants from sea buckthorn (Hippophae rhamnoides L.) berries: Pressurised solvent-free microwave assisted extraction. Food Chem. 2011, 126, 1380–1386. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Jiao, G.; Kermanshahi pour, A. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Woz´niak, Ł.; Marszałek, K.; Skapska, S.; Jedrzejczak, R. The Application of Supercritical Carbon Dioxide and Ethanol for the Extraction of Phenolic Compounds from Chokeberry Pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. Supercritical fluids and ultrasound assisted extractions applied to spruce bark conversion. Environ. Eng. Manag. J. 2015, 14, 615–623. [Google Scholar]
- López-Padilla, A.; Ruiz-Rodriguez, A.; Flórez, C.E.R.; Barrios, D.M.R.; Reglero, G.; Fornari, T. Vaccinium meridionale Swartz supercritical CO2 extraction: Effect of process conditions and scaling up. Materials 2016, 9, 519. [Google Scholar] [CrossRef] [Green Version]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Idham, Z.; Nasir, H.M.; Yunus, M.A.C.; Yian, L.N.; Peng, W.L.; Hassan, H.; Setapar, S.H.M. Optimisation of supercritical CO2 extraction of red colour from roselle (Hibiscus Sabdariffa Linn.) calyces. Chem. Eng. Trans. 2017, 56, 871–876. [Google Scholar]
- Pazir, F.; Kocak, E.; Turan, F.; Ova, G. Extraction of anthocyanins from grape pomace by using supercritical carbon dioxide. J. Food Process. Preserv. 2021, 45, e14950. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S.H. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Reategui, J.L.P.; da Fonseca Machado, A.P.; Barbero, G.F.; Rezende, C.A.; Martinez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Solana, M.; Mirofci, S.; Bertucco, A. Production of phenolic and glucosinolate extracts from rocket salad by supercritical fluid extraction: Process design and cost benefits analysis. J. Food Eng. 2016, 168, 35–41. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Wang, L.; Yin, W.; Liang, J. Antioxidant activity and subcritical water extraction of anthocyanin from raspberry process optimization by response surface methodology. Food Biosci. 2021, 44, 101394. [Google Scholar] [CrossRef]
- Kang, H.J.; Ko, M.J.; Chung, M.S. Anthocyanin Structure and pH Dependent Extraction Characteristics from Blueberries (Vaccinium corymbosum) and Chokeberries (Aronia melanocarpa) in SubcriticalWater State. Foods 2021, 10, 527. [Google Scholar] [CrossRef]
- Sharifi, A.; Mortazav, S.A.; Maskooki, A.; Niakousari, M.; Elhamirad, A.H. Optimization of Subcritical Water Extraction of Bioactive Compounds from Barberry Fruit (Berberis vulgaris) by Using Response Surface Methodology. Int. J. Agri Crop. Sci. 2013, 6, 89–96. [Google Scholar]
- Teixeira, R.F.; Benvenutti, L.; Burin, V.M.; Gomes, T.M.; Ferreira, S.R.S.; Ferreira-Zielinski, A.A. An eco-friendly pressure liquid extraction method to recover anthocyanins from broken black bean hulls. Innov. Food Sci. Emerg. Technol. 2021, 67, 102587. [Google Scholar] [CrossRef]
- Andrade, T.A.; Hamerski, F.; Fetzer, D.E.L.; Roda-Serrat, M.C.; Corazza, M.L.; Norddahl, B.; Errico, M. Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep. Purif. Technol. 2021, 276, 119290. [Google Scholar] [CrossRef]
- Altuner, E.M.; Tokusoglu, O. The effect of high hydrostatic pressure processing on the extraction, retention and stability of anthocyanins and flavonols contents of berry fruits and berry juices. Int. J. Food Sci. Technol. 2013, 48, 1991–1997. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Kouba, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.I.P.; Pereira, J.A.; Ramalhosa, E.; Saraiva, J.A. Optimization of high pressure bioactive compounds extraction from pansies (Viola × wittrockiana) by response surface methodology. High Press. Res. 2017, 37, 415–429. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Chacana-Ojeda, M. High pressure extraction increases the antioxidant potential and in vitro bio-accessibility of bioactive compounds from discarded blueberries. CyTA J. Food 2019, 17, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xu, Q.; Li, X.; Wang, Y.; Zhu, J.; Ning, C.; Chang, X.; Meng, X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov. Food Sci. Emerg. Technol. 2016, 36, 48–58. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium Spp.) puree. Food Chem. 2021, 342, 128564. [Google Scholar] [CrossRef]
- Morata, A.; Guamis, B. Use of UHPH to obtain juices with better nutritional quality and healthier wines with low levels of SO2. Front. Nutr. 2020, 241. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Puig-Pujol, A.; Guamis, B.; González, C.; Suárez-Lepe, J.A. Use of Ultra-High Pressure Homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innov. Food Sci. Emerg. Technol. 2018, 50, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Li, B.; Shu, C.; Tian, J.; Zhang, Y.; Gao, N.; Cheng, Z.; Xie, X.; Wang, J. Combined effect of thermosonication and high hydrostatic pressure on bioactive compounds, microbial load, and enzyme activities of blueberry juice. Food Sci. Technol. Int. 2021, 25, 33765872. [Google Scholar] [CrossRef]
- Zderic, A.; Zondervan, E. Polyphenol extraction from fresh tea leaves by pulsed electric field: A study of mechanisms. Chem. Eng. Res. Des. 2016, 109, 586–592. [Google Scholar] [CrossRef]
- Buchmann, L.; Mathys, A. Perspective on Pulsed Electric Field Treatment in the Bio-based Industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of Pulsed Electric Field Treatment for the Extraction of Bioactive Compounds from Blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Lamanauskas, N.; Bobinaitė, R.; Šatkauskas, S.; Viškelis, P.; Pataro, G.; Ferrari, G. Pulsed electric field-assisted juice extraction of frozen/thawed blueberries. Zemdirb. Agric. 2015, 102, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Taiebirad, F.; Bakhshabadi, H.; Rashidzadeh, S.H. Optimization of extraction of bioactive compounds from seedless barberry fruit using pulsed electric field pretreatment. Iran. J. Food Sci. Technol. 2021, 18, 305–317. [Google Scholar]
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.-W.; Zhang, Z.-H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Teusdea, A.C.; Bandici, L.; Kordiaka, R.; Bandici, G.E.; Vicas, S.I. The Effect of Different Pulsed Electric Field Treatments on Producing High Quality Red Wines. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sahar, A.; Khalil, A.A.; Rahman, U.U.; Khan, M.; Mehmood, T. Combined effects of pulsed electric field and ultrasound on bioactive compounds and microbial quality of grapefruit juice. J. Food Process. Preserv. 2018, 42, e13507. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Changes in Quality Characteristics of Strawberry Juice After Equivalent High Pressure, Ultrasound, and Pulsed Electric Fields Processes. Food Eng. Rev. 2021, 13, 601–612. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Aadil, R.M.; Al-Farga, A.; Waheed Iqbal, M.; Rahaman, A.; Zeng, X.A. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar] [CrossRef]
- Barba, F.J.; Galanakis, C.M.; Esteve, M.J.; Frigola, A.; Vorobiev, E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J. Food Eng. 2015, 167, 38–44. [Google Scholar] [CrossRef]
- Siddeeg, A.; Manzoor, M.F.; Ahmad, M.H.; Ahmad, N.; Ahmed, Z.; Khan, M.K.I.; Maan, A.A.; Mahr-Un-Nisa; Zeng, X.-A.; Ammar, A.-F. Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties. Processes 2019, 7, 585. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, K.A.G.; Hamid, N.; Oey, I.; Pook, C.; Gutierrez-Maddox, N.; Ma, Q.; Leong, S.Y.; Lu, J. Red cherries (Prunus avium var. Stella) processed by pulsed electric field–Physical, chemical and microbiological analyses. Food Chem. 2018, 240, 926–934. [Google Scholar] [CrossRef]
- Bandici, L.; Vicas, S.; Bandici, G.E.; Teusdean, A.C.; Popa, D. The Effect of Pulsed Electric Field (PEF) Treatment on the Quality of Wine. In Proceedings of the 14th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 1–2 June 2017; IEEE Institute of Electrical and Electronics Engineers: Manhattan, NY, USA, 2017. [Google Scholar]
- Jin, T.Z.; Yub, Y.; Gurtlera, J.B. Processing on microbial survival, quality change and nutritional characteristics of blueberries. LWT Food Sci. Technol. 2017, 77, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Leong, S.Y.; Oey, I.; Burritt, D.J. Pulsed Electric Field Technology Enhances Release of Anthocyanins from Grapes and Bioprotective Potential against Oxidative Stress. In Proceedings of the 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Portorož, Slovenia, 6–10 September 2015; Springer: Singapore, 2015; pp. 47–50. [Google Scholar]
- Aganovic, K.; Smetana, S.; Grauwet, T.; Toepfl, S.; Mathys, A.; Van Loey, A.; Heinz, V. Pilot scale thermal and alternative pasteurization of tomato and watermelon juice: An energy comparison and life cycle assessment. J. Clean. Prod. 2017, 141, 514–525. [Google Scholar] [CrossRef]
- Reineke, K.; Schottroff, F.; Meneses, N.; Knorr, D. Sterilization of liquid foods by pulsed electric fields—An innovative ultra-high temperature process. Front. Microbiol. 2015, 6, 400. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, H.; Knorr, D. Pulsed Electric Fields Treatment in Food Technology: Challenges and Opportunities. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Germany, 2017; pp. 2657–2680. [Google Scholar]
- Lotfi, M.; Hamdami, N.; Dalvi-Isfahan, M.; Fallah-Joshaqani, S. Effects of high voltage electric field on storage life and antioxidant capacity of whole pomegranate fruit. Innov. Food Sci. Emerg. Technol. 2022, 75, 102888. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Sun, D.-W.; Zhu, Z. Enhancing Food Processing by Pulsed and High Voltage Electric Fields: Principles and Applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 2285–2298. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus. Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Electrical, mechanical, and chemical effects of high-voltage electrical discharges on the polyphenol extraction from vine shoots. Innov. Food Sci. Emerg. Technol. 2015, 31, 60–66. [Google Scholar] [CrossRef]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, L.; Kalbasi-Ashtari, A.; Hamedi, M.; Ghorbani, F. Effects of enzymatic extraction on anthocyanins yield of saffron tepals (Crocos sativus) along with its color properties and structural stability. J. Food Drug Anal. 2015, 23, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binaschi, M.; Duserm Garrido, G.; Cirelli, C.; Spigno, G. Biotechnological strategies to valorise grape pomace for food applications. Chem. Eng. Trans. 2018, 64, 367–372. [Google Scholar]
- Vardakas, A.T.; Shikov, V.T.; Dinkova, R.H.; Mihalev, K.M. Optimisation of the enzyme-assisted extraction of polyphenols from saffron (Crocus sativus L.) tepals. Acta Sci. Pol. Technol. Aliment. 2021, 20, 359–367. [Google Scholar]
- Serea, D.; Râpeanu, G.; Constantin, O.E.; Bahrim, G.E.; Stănciuc, N.; Croitoru, C. Ultrasound and enzymatic assisted extractions of bioactive compounds found in red grape skins băbească neagră (vitis vinifera) variety. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2021, 45, 9–25. [Google Scholar] [CrossRef]
- Li, Y.; Tao, F.; Wang, Y.; Cui, K.; Cao, J.; Cui, C.; Nan, L.; Li, Y.; Yang, J.; Wang, Z. Process optimization for enzymatic assisted extraction of anthocyanins from the mulberry wine residue. IOP Conf. Series Earth Environ. Sci. 2020, 559, 012011. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Silva, T.O.M.; Barros, R.G.C.; de Oliveira, C.S.; Cunha, G.C.; Gualberto, N.C.; Rajan, M.; Narain, N. Enzymatic and ultrasonic-assisted pretreatment in the extraction of bioactive compounds from Monguba (Pachira aquatic Aubl) leaf, bark and seed. Food Res. Int. 2021, 140, 109869. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Pereira, G.A.; Arruda, H.S.; Prado, L.G.; Ruiz, A.L.T.G.; Eberlin, M.N.; de Castro, R.; Pastore, G.M. Enzymatic treatment improves the antioxidant and antiproliferative activities of Adenanthera pavonina L. seeds. Biocatal. Agric. Biotechnol. 2019, 18, 101002. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Zeng, Z. Effects of different extraction methods on antioxidant properties of blueberry anthocyanins. Open Chem. 2021, 19, 138–148. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oancea, S.; Perju, M. Influence of enzymatic and ultrasonic extraction on phenolics content and antioxidant activity of Hibiscus Sabdariffa, L. flowers. Bulg. Chem. Commun. 2020, 52, 25–29. [Google Scholar]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Raspberry Wine Residues: Process Optimization, Isolation, Purification, and Bioactivity Determination. Food Anal. Methods 2021, 14, 1369–1386. [Google Scholar] [CrossRef]

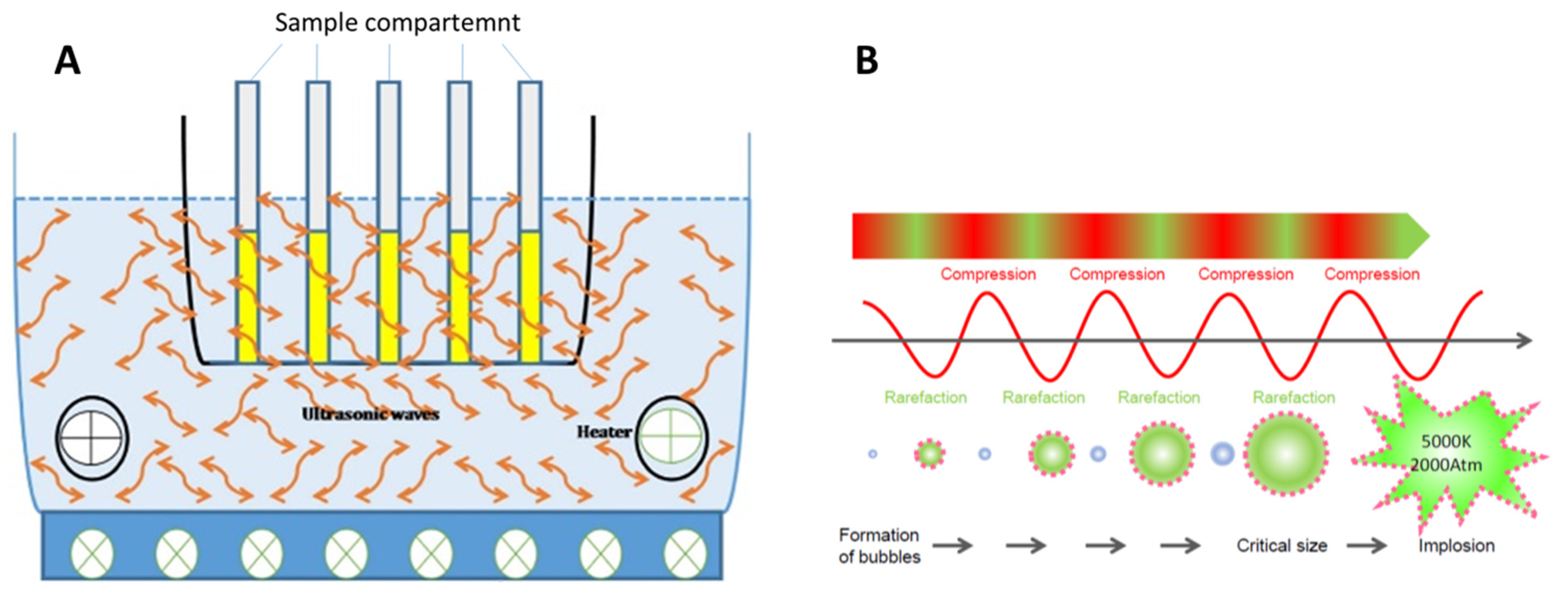


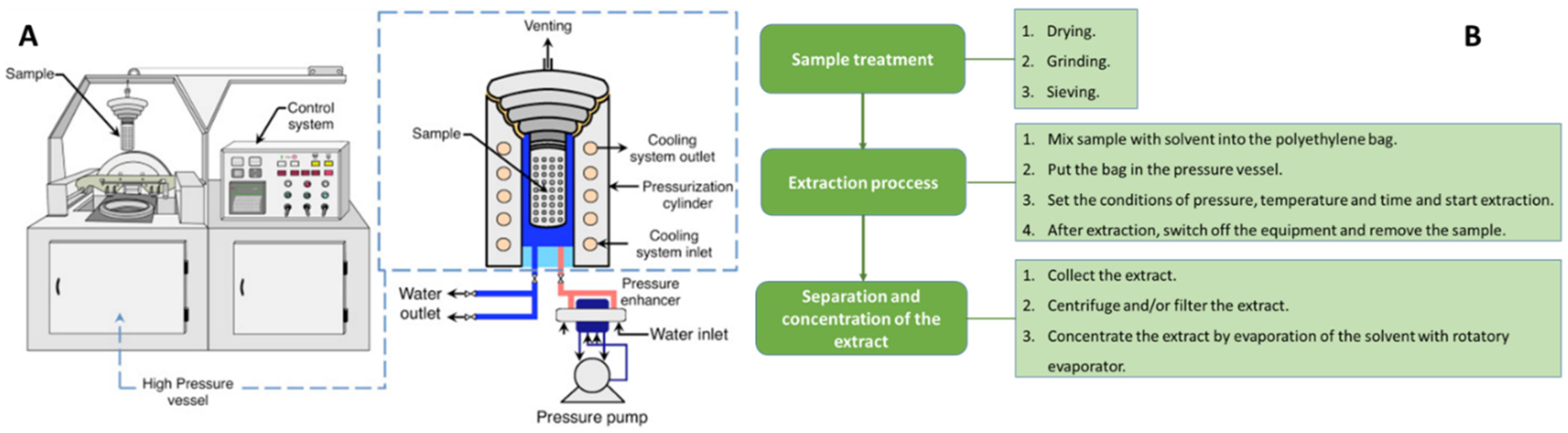
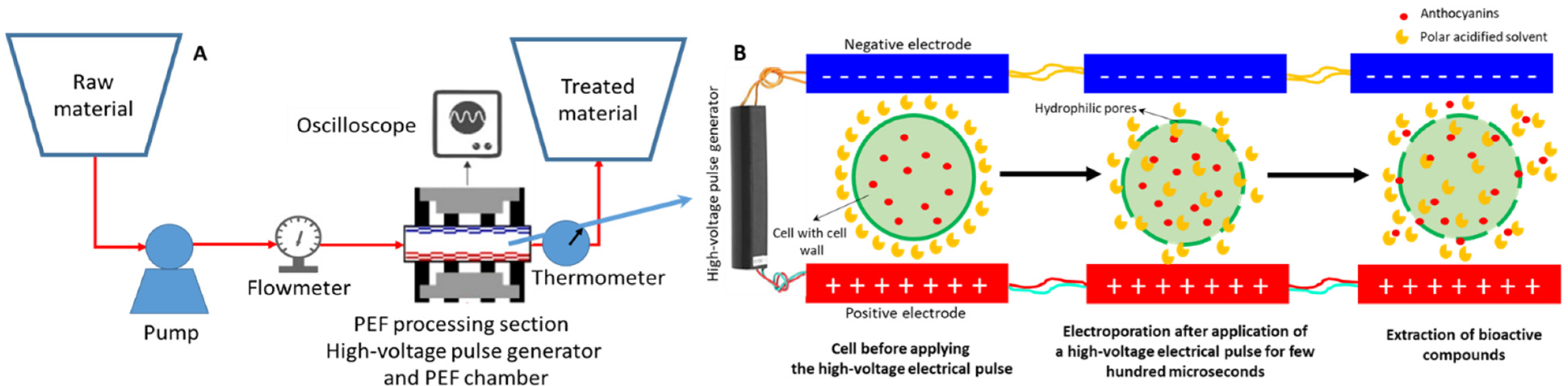
| Natural Matrices | Tª (°C) | Solvent (%) | Time (min) | L/S Ratio (mL/g) | Power (W)/ Frequency (kHz)/Solid Amount (g) |
| Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|
| Jabuticaba epicarp. | 30–35 | Ethanol 34.47% | 24.44 | 100:5 | 500/20/2.5 |
| 32 mg of D3G + C3G/g of extract. | [11] |
| Purple sweet potato. | 60 | Ethanol 90% (0.1% HCl) | 60 | 100:5 | 200/-/10 |
| 214.92 mg of C3GE/100 g of potato DW. | [22] |
| Red cabbage. | 40 | Ethanol 42.39% | 75 | 3:1 | -/37/- |
| 58.67 mg of C3G/L of extract. | [37] |
| Fig (Ficus carica L.) peel. | 30–35 | Ethanol 100% | 21 | 100:15 | 310/-/2.5 |
| 4.32 mg C3R/g of fig peel DW. | [38] |
| Jambolan (Syzygium cumini L.) fruit. | 30 | Ethanol 79.6% | 7.5 | 15:1 | Power density: 112.5 W/L/40/4 |
| (BUE) 54.2 mg C3GE/g of fruit DW. | [42] |
| Jambolan (Syzygium cumini L.) fruit. | 30 | Ethanol 79.6% | 7.5 | 15:1 | Power density: 5000 W/L/20/4 |
| (PUE) 60.5 mg C3GE/g of fruit DW. | [42] |
| Red cabbage. | 30 | Water | 15 | 100:2 | 100/30/2 |
| 20.9 mg of P3G/L of extract. | [43] |
| Black carrot pomace. | 50 | Water | 20 | 3:1 | 102/24/75 |
| 12.4 mg of C3XGG/L of extract. 69.7 mg of C3XG/L of extract. 16.0 mg of C3XGGS/L of extract. 73.4 mg of C3XGGF/L of extract. 34.2 mg of C3XGGC/L of extract. | [44] |
| Mulberry (Morus nigra) pulps. | 48 | Methanol 76% pH = 3 | 10 | 12:1.5 | 200/24/1.5 |
| 149.95 μg of C3G + C3R + C3MG + C3DG/g of mulberry FW. | [45] |
| Haskap (Lonicera caerulea L.) berries. | 35 | Ethanol 80%, (0.5% formic acid) | 20 | 25:1 | 100/40/- |
| 22.45 mg C3GE/g of berries DW. | [46] |
| Blueberries (V. Angustifolium Aiton). | 65 | Ethanol 60% acidified | 11.5 | 50:1 | 100/40/- |
| 13.22 mg C3GE/g of blueberries DW. | [47] |
| Blackthorn (Prunus spinosa L.) Fruit Epicarp. | Room Tª | Ethanol 47.98% acidified (citric acid, pH = 3). | 5 | 100:5 | 400/40/2.5 |
| 11.76 mg of C3R+P3R/g of fruit epicarp DW. | [48] |
| Purple Majesty potato. | 33 | Ethanol 70% | 5 | 200:5 | 35/20/5 |
| 364.3 mg C3G/kg of potato FW. | [49] |
| Natural Matrices | Tª (°C) | Solvent (%) | Time (s) | L/S Ratio (mL/g) | Irradiation Power (W)/Solid Amount (g) |
| Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fig (Ficus carica L.) peel. | 62.4 | Ethanol 100% (pH 3) | 300 | 100:5 | 400/0.5 |
| 411 mg of C3R/100 g of fig peel DW. | [38] |
| Lavender (Lavandula pedunculata L.) fresh plants. | - | Water | 114.3 | 30.32:1 | 464.9/1 |
| 273.3 mg of C3G/L. | [55] |
| Cranberry. | 50 | Ethanol 52% (pH = 3) | 8 | 28:1 | -/2 |
| 306 mg of C3G/100 g of cranberry. | [57] |
| Red cabbage. | 100 | Water (pH = 3–3.3) | 300 | 30:1 | 200/5 |
| 110.0 mg of C3G/L. | [58] |
| Red cabbage. | 90 | Ethanol 50% (pH = 3–3.3) | 600 | 20:1 | 600/5 |
| 220.2 mg of C3G/L. | [58] |
| Purple sweet potato. | - | Ethanol 30% (citric acid pH = 2) | 500 | 3:1 | 320/10 |
| 31 mg of C3GE/100 g of potato. | [59] |
| Black raspberry Korean. | - | Ethanol 74% (pH = 2) | 66 | 30:1 | 148/5 |
| 372 mg of C3G/100 g of fruit. | [60] |
| Eggplant Peel. | - | Ethanol 80% | 40 | 50:1 | 480/- |
| 881 mg of C3G/100 g of peel. | [61] |
| Grape juice waste. | 55 | Double distilled water | 138.6 | 19.2:1 | 435/1 |
| 132 mg of M3G/100 g of grape juice waste DW. | [28] |
| Blackcurrant. | - | Ethanol 60% (pH = 2.5) | 984 | 28.3:1 | 551/- |
| 47.37 mg of C3G + D3R + C3R + D3DG/100 g of blackcurrant. | [62] |
| Red rice. | - | Ethanol 85% acidified | 100 | 22:1 | 400/- |
| 3.82 mg of C3G/100 g of rice. | [23] |
| Rosa pimpinellifolia L. fruits. | 60 | Ethanol 26.85% (NH₄)₂SO₄ 19.15% | 1037.4 | 40:1 | 400/20 |
| 1373.04 mg C3GE/g of fruit DW. | [63] |
| Natural Matrices | Tª (°C) | P. (bar) | Co-Solvent | Flow Rate |
| Recovery | Ref. |
|---|---|---|---|---|---|---|---|
| Haskap (Lonicera caerulea L.) berry pulp paste. | 65 | 450 | Water L/S ratio 5.4/3.2 (w/w) | 15 min static time 20 min dynamic time at 10 mL/min. |
| 25 mg of C3G/g of paste DW. | [68] |
| Indian blackberry (Syzygium Cumini) fruit pulp. | 50 | 162 | Ethanol (10 g of sample) | 2 g/min. |
| 231.28 mg C3G/100 g of fruit. | [69] |
| Colombian blueberry (Vaccinium meridionale) fresh and mature fruit. | 40 | 300 | None (160 g of sample and 800 g of sample) | 32 g/min. |
| 26.7 mg of extract/g of sample. | [72] |
| Bilberry (Vaccinium myrtillus L.) dried fruits. | 45 | 250 | (1) 6% of 30% distilled water, 70% ethanol. (2) 6% of 50% distilled water, 50% ethanol at 6 mL/min. (3) 9% of 90% distilled water, 10% ethanol. (430 g of sample) | Multistage supercritical/subcritical extraction: (1) SC-CO2 8 kg/h (2) SubC-CO2 6 kg/h (3) SubC-CO2 6 kg/h. |
| 60 mg of C3G/100 g of fruit DW. | [73] |
| Roselle (Hibiscus Sabdariffa L.) dry calyces. | 70 | 89 | Ethanol 75% (1.5 g of sample) | 6 mL/min (modifier flow rates 9.5%) |
| 26.7 g of dried extract/100 g of sample. | [74] |
| Merlot red grape (Vitis vinifera) pomace. | 95 | 100 | Ethanol 10 mL/min (30 g of sample) | 32 g/min |
| 700 mg of M3G/kg of grape DW. | [75] |
| Natural Matrices | Tª (°C) | P. (bar) | Time (min) | Solvent/Flow Rate (mL/min) (Amount of Sample) |
| Recovery | Ref. |
|---|---|---|---|---|---|---|---|
| SWE | |||||||
| Raspberry. | 130 | 70 | 90 | Double distilled water/3 (20 g FW). |
| 815 mg of C3GE/100 g of Raspberry FW. | [79] |
| Blueberries. | 130 | 100 | 3 | Water 1% citric acid)/- (1 g DW). |
| 50 mg of anthocyanin pigment/100 g of blueberries FW. 18 mg of M3G/100 g of blueberries FW. | [80] |
| Chokeberries. | 190 | 100 | 1 | Water 1% citric acid)/- (1 g DW). |
| 66 mg of anthocyanin pigment/100 g of chokeberries FW. 134 mg of C3Ga/100 g of chokeberries FW. | [80] |
| Barberry (Berberis vulgaris) Fruit. | 157.5 | 29.64 | 170 | Water. |
| 9.84 mg C3G/mL of sample. | [81] |
| PLE | |||||||
| Purple sweet potatoes. | 90 | - | 15 (2 cycles) | Ethanol 80% (acidified 0.1% HCl)/- (10 g). |
| 252.34 mg of C3GE/100 g potatoes DW. | [22] |
| Jambolan (Syzygium cumini L.) fruit. | 90 | 117.2 | 5 rinsing time; 10 extraction time/cycle (2 cycles) | Ethanol 80% (acidified 0.1% TFA)/- (4 g). |
| 47.05 mg C3GE/g of fruit DW. | [42] |
| Broken black bean (Phaseolus vulgaris L.) hulls. | 60 | 100 | 26 | Ethanol: citric acid 30:70 (pH = 3.4)/5 (5 g DW). |
| 3.96 mg C3GE/g of sample DW. | [82] |
| Natural Matrices | Tª (°C) | P. (Mpa) | Time (min) | Solvent (Amount of Sample) | Solid/Liquid Ratio |
| Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pansies (Viola x wittrockiana). | Room Tª | 384 | 15 | Ethanol 35% (0.8 g DW) | 1:30 |
| 6.09 mg of C3G/g of flower DW | [86] |
| Blueberries (O’Neal variety). | 20 | 500 | 15 | Acetone/water/acetic acid 70:29.5:0.5 (2g) | - |
| 117.1 mg C3GE/100 g of blueberry extract. | [87] |
| Haskap (Lonicera caerulea) berry. | 18–22 | 200 | 10 | Ethanol 60% (acidified HCl0.1%) (1 g) | 1:20 |
| 336 mg C3G/100 g of sample | [88] |
| Natural Matrix | Pulses/Pulses Width/Frequency (Hz) | Electric Field Intensity (kV/cm) | Tª (°C) |
| Recovery | Ref. |
|---|---|---|---|---|---|---|
| By-products of Blueberry. | 10/2 μs/- | 20 | Room Tª |
| 223 mg of C3GE/L of sample. | [27] |
| Blueberry pomace. | 100/2 μs/- | 20 | Room Tª |
| 175 mg of/100 g of sample DW. | [95] |
| Grape peels. | 25/6 μs/10 | 25 | 25 |
| 78 mg of C3G/mL of sample. | [96] |
| Blackcurrant. | 315/100 ms/- | 1.32 | 22 |
| 1.38 mg of C3G/g of the extract. | [97] |
| Frozen/thawed European blueberry (Vaccinium myrtillus L.). | -/20 µs/20 | 1 | 20–25 |
| 1750 mg of C3G/L of juice. | [98] |
| Pinot Noir (PN) and Merlot (M) grapes. | -/300 s/344 | 8 | Room Tª |
| 81.5 mg of M3GE/L of pinot noir wine or must. 76.92 mg of M3GE/of merlot wine or must. | [101] |
| Grapefruit juice. | -/600 μs/1000 | 20 | 40 |
| 1.58 mg of C3GE/L of juice obtained after PEF treatment. 1.68 mg of C3GE/L of juice obtained after PEF treatment followed by UAE. | [102] |
| Strawberry juice (SJ). | 13/2 μs/155 | 35 | 22–46 |
| 179.21 mg of Pl3G/L of juice. | [103] |
| Spinach juice. | 4/80 μs/1000 | 9 | 30 |
| 38.12 mg of M3G/L of juice. 41.31 mg of M3G/L of juice when UAE is applied before PEF treatment. | [104] |
| Valuable compounds from blackberries. | 100/10 μs/- | 13.3 | 20–35 |
| 100 mg of C3G/100 g of sample when W50 was applied. 90 mg of C3G/100 g of sample when EE was applied. 40 mg of C3G/100 g of sample when W20 was applied. | [105] |
| Date palm fruit. | 30/30 μs/10 | 3 | Room Tª |
| 2.08 mg of C3GE/L of sample. | [106] |
| Red cherry samples. | -/20 μs/100 | 2.5 | 20 |
| 0.23 mg of C3G/100 g of sample FW. 0.20 mg of C3R/100 g of sample FW. 0.02 mg of Pl3G/100 g of sample FW. 0.10 mg of P3G/100 g of sample FW. | [107] |
| Red grapes (Pinot Noir (PN) and Merlot (M). | -/150 s/178 | 7 | 20 |
| Maximum absorbance = 0.67 u.a. for PN wine. Maximum absorbance = 1.45 u.a. for M wine. | [108] |
| Fresh blueberries. | 24,000/1 μs/- | 2 | 26 |
| 110 mg of C3GE/g of sample FW. | [109] |
| Merlot grapes (Vitis vinifera) | 1033/20 μs/50 | 1.4 | Room Tª |
| 2.07 mg of M3G+MAG+MCG/100 mL of J48. 0.87 mg of M3G+MAG+MCG+CEG/100 mL of PEFJ. 9.88 mg of M3G+D3G+Pt3G+C3G+MA+DAG+PtAG+MCG+PtCG/100 mL of PEFJ48. | [110] |
| Natural Matrix | Enzymatic Mixture | pH/Tª (°C) | L:S Ratio/Enzymes:Mixture Ratio/Hydrolysis Time (min). |
| Recovery | Ref. |
|---|---|---|---|---|---|---|
| Saffron tepals | Pectinex (containing cellulase, hemicellulase, and pectinase). | 3.5/4 5 | 10:1/5:100/120 |
| 675 mg of C3G/100 g of saffron tepals extracted. | [119] |
| Saffron (Crocus sativus L.) tepals. | Cellulolytic preparation Celluclast BG and hemicellulolytic preparation Xylanase AN (1:1). | 4/50 | 10:1/10:100/145-185 |
| 2.0 g of C3GE/kg of saffron tepals DW. | [121] |
| Skin of the Băbească neagră grapes. | Zymorouge pectolytic enzyme EG from Aspergillus niger. | 5.0/40 | 28:1/2:100/60 |
| 2.54 mg of C3G/g of sample DW. | [122] |
| Mulberry wine residue. | Pectinase. | 5.9/45 | 20:1/-/58 |
| 6.04 mg of C3G/g of sample. | [123] |
| Leaf of monguba. | α-Amylase and protease. | 6.0/50 | 10:1/-/160 |
| 30.59 mg of TA/100 g of sample DW. | [124] |
| Seeds (Adenanthera pavonina L.) | Protease and cellulose. | 7.0/50 | 45:5/-/160 |
| 14.71 μg of total phenols/g of sample DW. | [125] |
| Blueberry. | Pectinase. | 4.5/45 | 8:1/-/60 |
| 2.346 mg of TA/mL of extract. | [126] |
| Raspberry (Rubus idaeus L.) pomace. | Ultrazym AFP-L. | -/45 | 100:15/1:100/60 |
| 0.32 mg of C3S+C3G+C3R/g of sample FW. | [127] |
| Roselle samples. | Cellulase solution with exo- and endo-β-1,4-D-glucanases. | 4.8/40 | 40:1/16:100/60 |
| 676.03 mg of C3G/100 g of sample DW. | [128] |
| Raspberry wine residues. | Pectinase | 3/40 | 30:1/0.16:100/30 |
| 0.853 mg of C3G/g of sample. | [129] |
| Raspberry wine residues. | Pectic enzyme | -/52 | 100:1/0.2:100/66 |
| 0.75 mg of C3G/g of sample. | [129] |
| Technique | Strengths | Weaknesses | Suitability |
|---|---|---|---|
| UAE | Versatile, flexible, low cost, and very easy to use; fast energy transfers; low solvent usage; extraction time (5–60 min); can be combined with heating to improve the yield or with enzymatic treatment to improve the anthocyanin yield and the bioactivity of the extract; available on a large scale. | Lack of homogeneity in the process improved by probe system (PUE); the large-scale application could be limited by the higher cost and nonlinearity of process; after the extraction, a filtration and clean-up step is required; the process can lead to operator fatigue. |  |
| MAE | Quick and homogeneous heating; low solvent usage; extraction time (1–40 min); currently, vacuum microwave extraction has been developed to provide a MAE method with a lower reactor temperature; possible application on a large scale. | The solvent must absorb microwaves; the heating could damage the structure and the activity of some compounds; after the extraction, a filtration and clean-up step is required. |  |
| SFE | CO2 as a solvent; easy to remove after extraction; reduced the thermal degradation. Extraction time (up to 1 h); it does not require an alternative energy source; it is available on a large scale. | Needs a co-solvent to extract polar compounds. The amount and type of co-solvent need to be optimize together with other parameters. SWE present the limitation of need high temperature to reach the subcritical condition, ethanol could be used instead of water. |  |
| PLE | Low solvent consumption; protection for oxygen and light sensitive compounds; it needs temperature; possible application on a large scale. | Expensive equipment required; after the extraction, a clean-up step is required; extraction time (1–2 h). |  |
| HHPE | Short extraction time (~ 5 min); performed at room temperature; higher repeatability; smaller amount of solvents; possible application at large scale. | High investment cost and cost maintenance and service; high pressure could affect the structure or activity of some compounds. The parameter should be optimized to avoid it. |  |
| PEFE | Short extraction time (less than 1 s); performed at room temperature; low energy and monetary costs; possible application on a large scale. | Some compounds could be affected by high electric fields; it is desirable to reduce the electrical conductivity of the matrix before the extraction. For industrial application there are some problems related to: non-uniform distribution of the electric pulses, the suitable solvents are very limited and cooling system is necessary to control the temperature when extracting thermolabile compounds if high electrical pulses are applied. |  |
| HVED | Low temperature; short extraction time and energy input; possible application on a large scale. | High cost maintenance and service; high voltage electrical discharges may generate chemical products and free reactive radicals, which can react with antioxidant compounds decreasing their bioactive activity. |  |
| EAE | Moderate extraction conditions; eco-friendly; selectivity due to the specificity of enzymes; can be combined with ultrasonic extraction to improve the yield and the bioactivity of the extract. | Expensive cost of enzymes; activity of enzymes varying with the pH, temperature and nutrients of the matrix; after the extraction, a filtration and clean-up step is required. Difficulties to be applied on a large scale; extraction time (1–12 h); low availability of commercial enzyme types; sometimes they have low selectivity and variability. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. https://doi.org/10.3390/antiox11020286
Tena N, Asuero AG. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants. 2022; 11(2):286. https://doi.org/10.3390/antiox11020286
Chicago/Turabian StyleTena, Noelia, and Agustin G. Asuero. 2022. "Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application" Antioxidants 11, no. 2: 286. https://doi.org/10.3390/antiox11020286








