Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Design
- CTL (control)—plants were irrigated with dH2O;
- SALT—plants were irrigated every two days with a 100 mM NaCl solution (60 mL per pot);
- HEAT—plants were irrigated with dH2O and daily exposed to 42 °C for 4 h in a twin growth chamber (temperature scaled up to 42 °C);
- COMBINED—plants were irrigated every two days with a 100 mM NaCl solution (60 mL per pot) and exposed to 42 °C for 4 h daily in a twin growth chamber (temperature scaled up to 42 °C).
2.3. Plant Harvest and Biometric Analysis
2.4. Element Quantification—Na+, K+, Ca2+, and Mg2+
2.5. Determination of ROS Content—Superoxide Anion (O2.−) and Hydrogen Peroxide (H2O2)
2.6. Estimation of the Lipid Peroxidation (LP) Degree
2.7. Quantification of Proline, Ascorbate (AsA), and Reduced Glutathione (GSH)
2.8. Quantification of Total Thiols and Non-Protein/Protein-Bound Thiols Ratio
2.9. Enzymatic Activity-Superoxide Dismutase (SOD; EC 1.15.1.1), Catalase (CAT; EC 1.11.1.6), Ascorbate Peroxidase (APX; EC 1.1.11.1), Glutathione Reductase (GR; EC 1.6.4.2), and Dehydroascorbate Reductase (DHAR; EC 1.8.5.1)
2.10. Statistical Analyses
3. Results
3.1. Biometric Analysis—Organ Length, Dry Biomass, and Water Content
3.2. Element Quantification—Na+, K+, Ca2+, and Mg2+
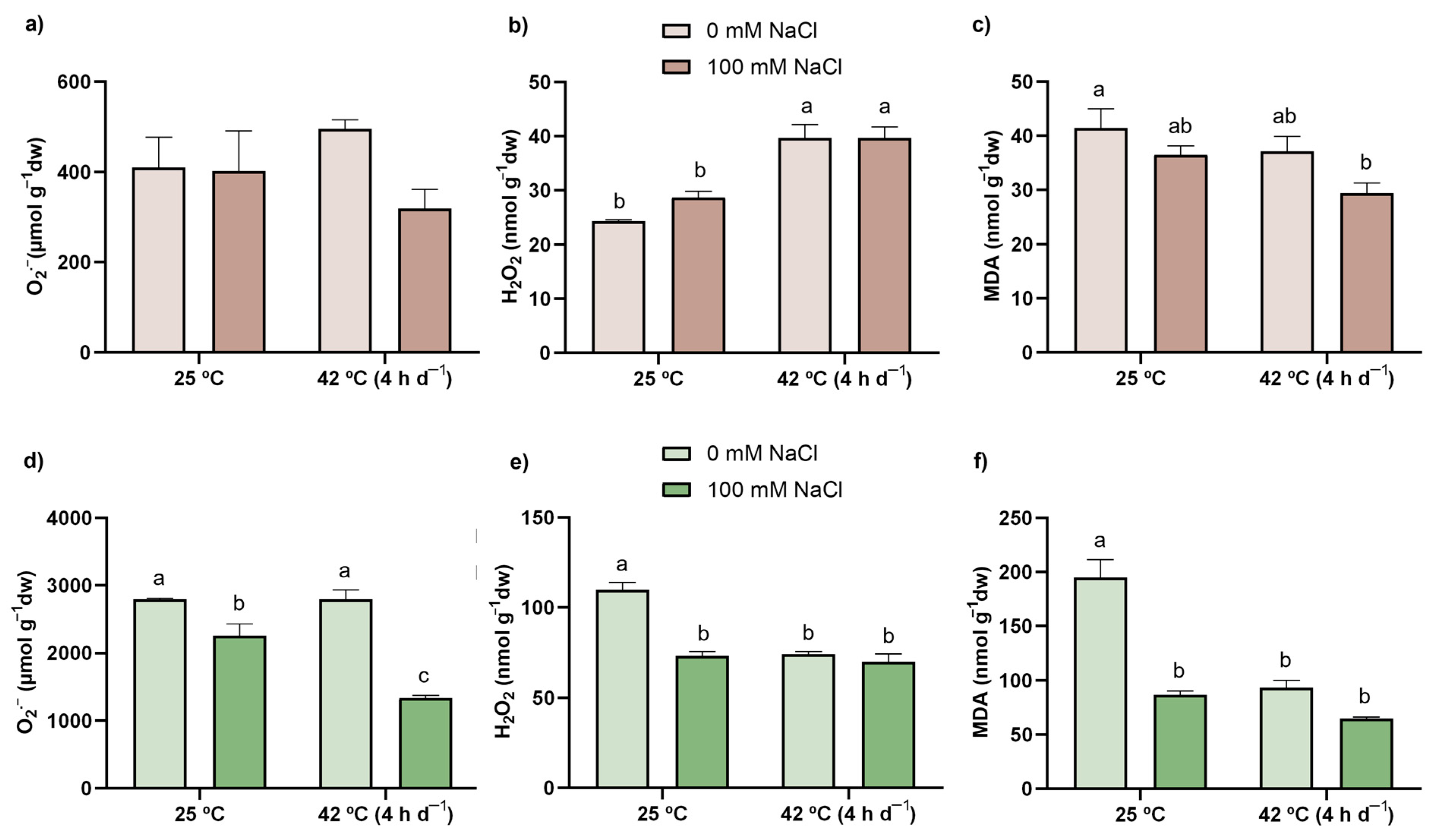
3.3. ROS Content
3.4. LP
3.5. Proline, AsA, and GSH
3.6. Thiols
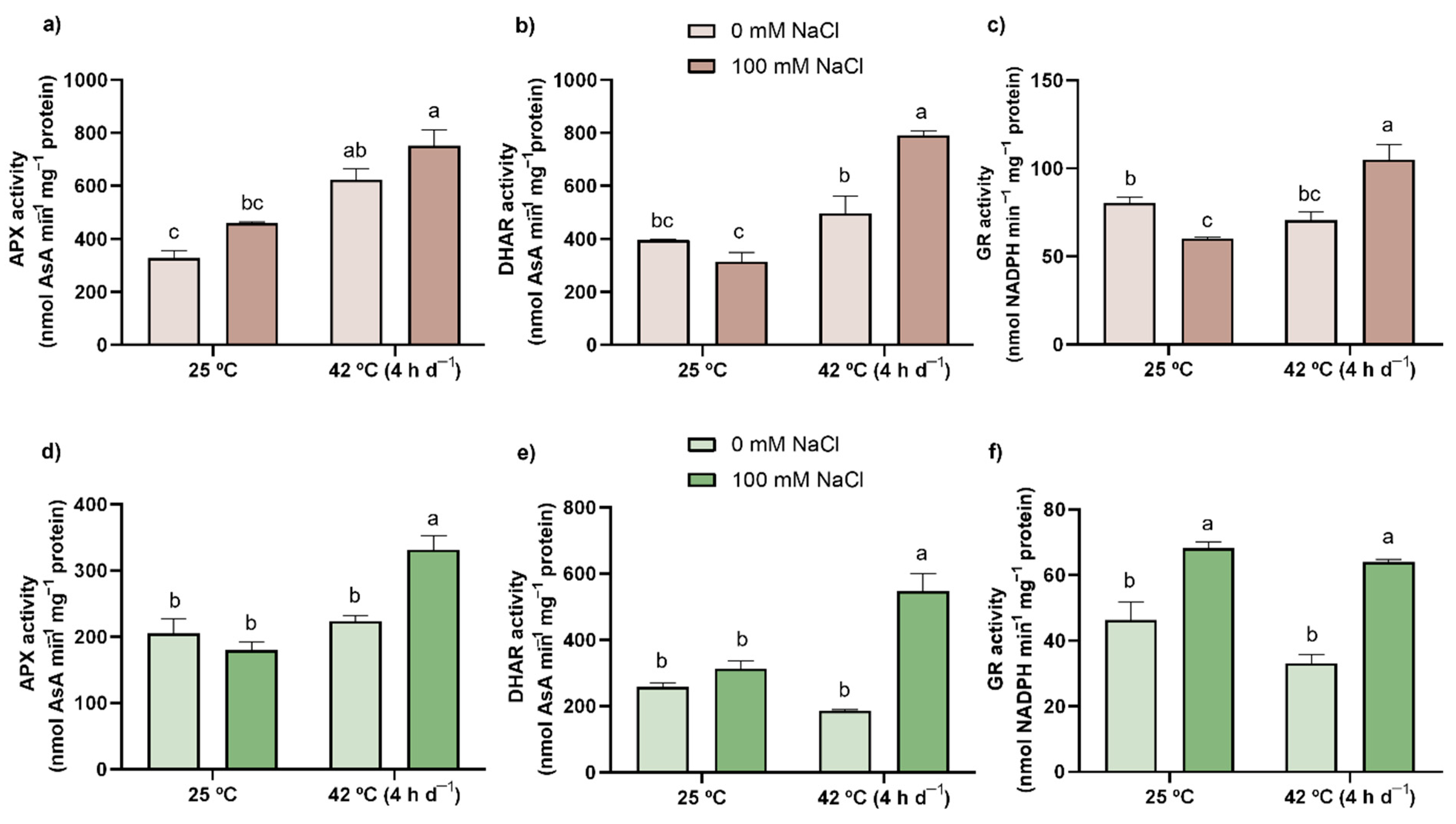
3.7. Enzymatic Activity (SOD, CAT, APX, DHAR, and GR)
3.8. Principal Component Analysis (PCA)
4. Discussion
4.1. The Combination of Heat and Salt Led to a Harsher Effect on Growth-Related Parameters
4.2. The Co-Exposure of Tomato Plants to Heat and Salinity, Individually or in Combination, Did Not Result in a Severe Oxidative Stress Condition
4.3. The Simultaneous Effect of Heat and Salinity on Tomato Plants Results in Differential Activation Patterns of AOX Metabolites
4.4. Combined Exposure to the Stressors Resulted in a Prompter Activation of the Enzymatic AOX Response, Especially the AsA-GSH Cycle Enzymes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- FAO How to Feed the World in 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 29 June 2021).
- Qafoku, N.P. Chapter Two-Climate-Change Effects on Soils: Accelerated Weathering, Soil Carbon, and Elemental Cycling. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 131, pp. 111–172. [Google Scholar]
- St.Clair, S.B.; Lynch, J.P. The opening of Pandora’s Box: Climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 2010, 335, 101–115. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Koutroulis, A.G.; Tsanis, I.K.; Daliakopoulos, I.N.; Jacob, D. Impact of climate change on water resources status: A case study for Crete Island, Greece. J. Hydrol. 2013, 479, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Haddeland, I.; Heinke, J.; Biemans, H.; Eisner, S.; Flörke, M.; Hanasaki, N.; Konzmann, M.; Ludwig, F.; Masaki, Y.; Schewe, J. Global water resources affected by human interventions and climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3251–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, D.; Cardoso Pereira, S.; Rocha, A. Future surface temperature changes for the Iberian Peninsula according to EURO-CORDEX climate projections. Clim. Dyn. 2021, 56, 123–138. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Gao, X.; Fu, J.; Zhou, J.; Wu, X. Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 2015, 388, 99–117. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and Metabolic Changes of Purslane (Portulaca oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, C.; Lu, Q.; Wen, X.; Lu, C. The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J. Plant Physiol. 2011, 168, 1743–1752. [Google Scholar] [CrossRef]
- Lopez-Delacalle, M.; Silva, C.J.; Mestre, T.C.; Martinez, V.; Blanco-Ulate, B.; Rivero, R.M. Synchronization of proline, ascorbate and oxidative stress pathways under the combination of salinity and heat in tomato plants. Environ. Exp. Bot. 2021, 183, 104351. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant. Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Liu, T.; Xu, Z.J. Modified responses of root growth and reactive oxygen species-scavenging system to combined salt and heat stress in transgenic rice. Russ. J. Plant Physiol. 2010, 57, 518–525. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Khavari-Nejad, R.A.; Mostofi, Y. Effects of NaCl on photosynthetic pigments, saccharides, and chloroplast ultrastructure in leaves of tomato cultivars. Photosynthetica 1998, 35, 151–154. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Debouba, M.; Gouia, H.; Suzuki, A.; Ghorbel, M.H. NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato “Lycopersicon esculentum” seedlings. J. Plant Physiol. 2006, 163, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations Rome: Rome, Italy, 1985; Volume 29, ISBN 9251022631. [Google Scholar]
- Sharma, A.; Thakur, S.; Kumar, V.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R. 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 2017, 17, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sousa, A.; Teixeira, J.; Regueiras, M.T.; Azenha, M.; Silva, F.; Fidalgo, F. Metalaxyl-induced changes in the antioxidant metabolism of Solanum nigrum L. suspension cells. Pestic. Biochem. Physiol. 2013, 107, 235–243. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Pereira, R.; Spormann, S.; Fidalgo, F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants?–Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019, 247, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lian, C.; Shen, Z. Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 2009, 103, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, F.; Freitas, R.; Ferreira, R.; Pessoa, A.M.; Teixeira, J. Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ. Exp. Bot. 2011, 72, 312–319. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Donahue, J.L.; Okpodu, C.M.; Cramer, C.L.; Grabau, E.A.; Alscher, R.G. Responses of Antioxidants to Paraquat in Pea Leaves (Relationships to Resistance). Plant Physiol. 1997, 113, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Cheng, L. Exposure of the shaded side of apple fruit to full sun leads to up-regulation of both the xanthophyll cycle and the ascorbate–glutathione cycle. Plant Sci. 2004, 166, 1479–1486. [Google Scholar] [CrossRef]
- Hernández, J.A. Salinity Tolerance in Plants: Trends and Perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst.- Int. J. Deal. Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarres Sanavy, S.A.M.; Ghanati, F. Effect of Salinity on Growth, Xylem Structure and Anatomical Characteristics of Soybean. Not. Sci. Biol. 2011, 3, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Fujita, M.; Islam, M.N.; Ahamed, K.U.; Nahar, K. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2009, 6, 85–90. [Google Scholar]
- Ahmad, P.; Kumar, A.; Ashraf, M.; Akram, N.A. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 2012, 11, 2694–2703. [Google Scholar] [CrossRef]
- Mittova, V.; Guy, M.; Tal, M.; Volokita, M. Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot. 2004, 55, 1105–1113. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Chaichi, M.R.; Keshavarz-Afshar, R.; Lu, B.; Rostamza, M. Growth and nutrient uptake of tomato in response to application of saline water, biological fertilizer, and surfactant. J. Plant Nutr. 2017, 40, 457–466. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015; ISBN 1605353531. [Google Scholar]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis Transporter MGT6 Mediates Magnesium Uptake and Is Required for Growth under Magnesium Limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [Green Version]
- Sigel, H.; Sigel, A. Metal Ions in Biological Systems: Volume 26: Compendium on Magnesium and Its Role in Biology: Nutrition and Physiology; CRC Press: Boca Raton, FL, USA, 1990; Volume 26, ISBN 0824783158. [Google Scholar]
- Amirjani, M.R. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 2011, 7, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Nagesh Babu, R.; Devaraj, V.R. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop. Sci. 2008, 2, 40–48. [Google Scholar]
- Hayat, S.; Masood, A.; Yusuf, M.; Fariduddin, Q.; Ahmad, A. Growth of Indian mustard (Brassica juncea L.) in response to salicylic acid under high-temperature stress. Braz. J. Plant Physiol. 2009, 21, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat Stress Decreases Levels of Nutrient-Uptake and -Assimilation Proteins in Tomato Roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat stress lowers yields, alters nutrient uptake and changes seed quality in quinoa grown under Mediterranean field conditions. J. Agron. Crop Sci. 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sterling, T.M. Transpiration: Water Movement through Plants. J. Nat. Resour. Life Sci. Educ. 2005, 34, 123. [Google Scholar] [CrossRef]
- Margalha, L.; Confraria, A.; Baena-González, E. SnRK1 and TOR: Modulating growth–defense trade-offs in plant stress responses. J. Exp. Bot. 2019, 70, 2261–2274. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Yazici, I.; Türkan, I.; Sekmen, A.H.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Ashraf, M.; Shahbaz, M. Growth stage-based modulation in antioxidant defense system and proline accumulation in two hexaploid wheat (Triticum aestivum L.) cultivars differing in salinity tolerance. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 388–397. [Google Scholar] [CrossRef]
- Fidalgo, F.; Santos, A.; Santos, I.; Salema, R. Effects of long-term salt stress on antioxidant defence systems, leaf water relations and chloroplast ultrastructure of potato plants. Ann. Appl. Biol. 2004, 145, 185–192. [Google Scholar] [CrossRef]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef] [Green Version]
- Al Hassan, M.; Martínez Fuertes, M.; Ramos Sánchez, F.J.; Vicente, O.; Boscaiu, M. Effects of Salt and Water Stress on Plant Growth and on Accumulation of Osmolytes and Antioxidant Compounds in Cherry Tomato. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Khan, M.H.; Panda, S.K. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant. 2007, 30, 81. [Google Scholar] [CrossRef]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium-Induced Up-Regulation of the Antioxidant Defense and Methylglyoxal Detoxification System Reduces Salinity-Induced Damage in Rapeseed Seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- de la Torre-González, A.; Navarro-León, E.; Albacete, A.; Blasco, B.; Ruiz, J.M. Study of phytohormone profile and oxidative metabolism as key process to identification of salinity response in tomato commercial genotypes. J. Plant Physiol. 2017, 216, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Razavi, S.M.; Omran, V.O.G.; Pirdashti, H. Piriformospora indica Alleviates Salinity by Boosting Redox Poise and Antioxidative Potential of Tomato. Russ. J. Plant Physiol. 2018, 65, 898–907. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Hjernø, K.; Larsen, M.; Wingsle, G.; Larsen, P.; Fey, S.; Roepstorff, P.; Salomé Pais, M. Proteome Profiling of Populus euphratica Oliv. Upon Heat Stress. Ann. Bot. 2006, 98, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G.; Ahsan, N.; Lee, S.H.; Kang, K.Y.; Bahk, J.D.; Lee, I.J.; Lee, B.H. A proteomic approach in analyzing heat-responsive proteins in rice leaves. Proteomics 2007, 7, 3369–3383. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Response of osmotic adjustment and ascorbate-glutathione cycle to heat stress in a heat-sensitive and a heat-tolerant genotype of wucai (Brassica campestris L.). Sci. Hortic. 2016, 211, 87–94. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Romero, L. Oxidative metabolism in tomato plants subjected to heat stress. J. Hortic. Sci. Biotechnol. 2004, 79, 560–564. [Google Scholar] [CrossRef]
- Shalata, A.; Tal, M. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol. Plant. 1998, 104, 169–174. [Google Scholar] [CrossRef]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd_Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat Stress Injury in Relation to Membrane Lipid Peroxidation in Creeping Bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Goyal, M.; Asthir, B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2010, 60, 13–25. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Zhu, S.; Chen, G.; Liu, F.; Zou, M.; Wang, C. Comparative response of two wucai (Brassica campestris L.) genotypes to heat stress on antioxidative system and cell ultrastructure in root. Acta Physiol. Plant. 2016, 38, 223. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | CTL | SALT | HEAT | COMBINED |
|---|---|---|---|---|
| Root Na+ (mg g−1dw) | 1.793 ± 0.003 d | 15.920 ± 0.021 b | 1.990 ± 0.026 c | 17.693 ± 0.015 a |
| Shoot Na+ (mg g−1dw) | 5.163 ± 0.5003 c | 25.380 ± 0.044 b | 4.180 ± 0.012 d | 40.050 ± 0.015 a |
| Root K+ (mg g−1dw) | 3.833 ± 0.019 b | 2.607 ± 0.015 c | 4.800 ± 0.012 a | 2.332 ± 0.002 d |
| Shoot K+ (mg g−1dw) | 11.583 ± 0.019 a | 8.050 ± 0.015 c | 9.917 ± 0.018 b | 7.570 ± 0.025 d |
| Root Ca2+ (mg g−1dw) | 0.487 ± 0.004 b | 0.323 ± 0.001 c | 0.557 ± 0.009 a | 0.181 ± 0.001 d |
| Shoot Ca2+ (mg g−1dw) | 2.000 ± 0.015 c | 2.343 ± 0.018 b | 2.757 ± 0.007 a | 1.767 ± 0.012 d |
| Root Mg2+ (mg g−1dw) | 2.547 ± 0.009 b | 3.193 ± 0.037 a | 2.527 ± 0.007 b | 2.397 ± 0.012 c |
| Shoot Mg2+ (mg g−1dw) | 6.097 ± 0.054 b | 6.737 ± 0.038 a | 5.730 ± 0.052 c | 5.473 ± 0.026 d |
| Parameter (Roots) | CTL | SALT | HEAT | COMBINED |
|---|---|---|---|---|
| Proline (mg g−1dw) | 0.099 ± 0.02 c | 5.820 ± 0.114 a | 0.088 ± 0.039 c | 4.320 ± 0.357 b |
| Total AsA (µg g−1dw) | 7.273 ± 0.500 | 7.803 ± 0.444 | 8.607 ± 0.406 | 8.240 ± 0.633 |
| AsA (µg g−1dw) | 1.980 ± 0.665 | 1.867 ± 0.044 | 1.933 ± 0.079 | 1.960 ± 0.269 |
| DHA (µg g−1dw) | 5.917 ± 0.173 | 6.023 ± 0.3868 | 6.723 ± 0.3480 | 6.280 ± 0.6201 |
| AsA/DHA | 0.338 ± 0.047 | 0.297 ± 0.012 | 0.280 ± 0.012 | 0.263 ± 0.019 |
| GSH (nmol g−1dw) | 252.5 ± 13.5 b | 233.2 ± 1.95 b | 295.0 ± 18.2 ab | 374.5 ± 36.4 a |
| Total thiols (µmol g−1dw) | 1.306 ± 0.023 b | 1.116 ± 0.038 b | 1.785 ± 0.111 a | 1.739 ± 0.032 a |
| Non-protein/Protein thiols | 0.232 ± 0.045 | 0.300 ± 0.050 | 0.179 ± 0.018 | 0.146 ± 0.021 |
| Parameter (Shoots) | CTL | SALT | HEAT | COMBINED |
|---|---|---|---|---|
| Proline (mg g−1dw) | 1.112 ± 0.154 c | 29.930 ± 2.265 a | 0.593 ± 0.023 c | 18.540 ± 1.117 b |
| Total AsA (µg g−1dw) | 18.84 ± 0.93 a | 13.20 ± 1.65 b | 14.19 ± 1.03 ab | 12.86 ± 0.47 b |
| AsA (µg g−1dw) | 6.497 ± 0.224 | 5.340 ± 0.932 | 4.173 ± 0.376 | 4.397 ± 0.095 |
| DHA (µg g−1dw) | 12.34 ± 0.80 a | 7.87 ± 0.73 b | 10.01 ± 0.67 ab | 8.46 ± 0.38 b |
| AsA/DHA | 0.53 ± 0.03 ab | 0.67 ± 0.06 a | 0.42 ± 0.02 b | 0.52 ± 0.01 ab |
| GSH (nmol g−1dw) | 1039.0 ± 9.8 a | 733.1 ± 18.6 c | 904.6 ± 41.6 b | 870.0 ± 28.2 b |
| Total thiols (µmol g−1dw) | 7.862 ± 0.720 a | 7.292 ± 0.350 ab | 5.324 ± 0.438 b | 8.809 ± 0.198 a |
| Non-protein/Protein thiols | 0.110 ± 0.004 b | 0.094 ± 0.009 b | 0.194 ± 0.007 a | 0.113 ± 0.006 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, B.; Rodrigues, F.; Soares, C.; Martins, M.; Azenha, M.; Lino-Neto, T.; Santos, C.; Cunha, A.; Fidalgo, F. Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants 2022, 11, 478. https://doi.org/10.3390/antiox11030478
Sousa B, Rodrigues F, Soares C, Martins M, Azenha M, Lino-Neto T, Santos C, Cunha A, Fidalgo F. Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants. 2022; 11(3):478. https://doi.org/10.3390/antiox11030478
Chicago/Turabian StyleSousa, Bruno, Francisca Rodrigues, Cristiano Soares, Maria Martins, Manuel Azenha, Teresa Lino-Neto, Conceição Santos, Ana Cunha, and Fernanda Fidalgo. 2022. "Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis" Antioxidants 11, no. 3: 478. https://doi.org/10.3390/antiox11030478
APA StyleSousa, B., Rodrigues, F., Soares, C., Martins, M., Azenha, M., Lino-Neto, T., Santos, C., Cunha, A., & Fidalgo, F. (2022). Impact of Combined Heat and Salt Stresses on Tomato Plants—Insights into Nutrient Uptake and Redox Homeostasis. Antioxidants, 11(3), 478. https://doi.org/10.3390/antiox11030478










