Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria
Abstract
:1. Introduction
2. Components of Fruit Extracts as Antimicrobial Agents
2.1. Organic Acids
2.2. Terpenes
2.3. Polyphenols
2.3.1. Flavonoids
2.3.2. Non-Flavonoid Polyphenols
Phenolic Acids
Stilbenes
Lignans
Xanthones
3. Mechanisms of Action of Fruit Extracts as Antimicrobials
3.1. Interaction with the Bacterial Cell Envelope
3.2. Effects on Cell–Cell Communication and Gene Regulation
3.3. Inhibition of Metabolic and Enzyme Activities
3.4. Fruit Extracts: Natural Antibiotics against Multi-Resistant Bacteria
3.5. Fruit Extracts and Gut Microbiota
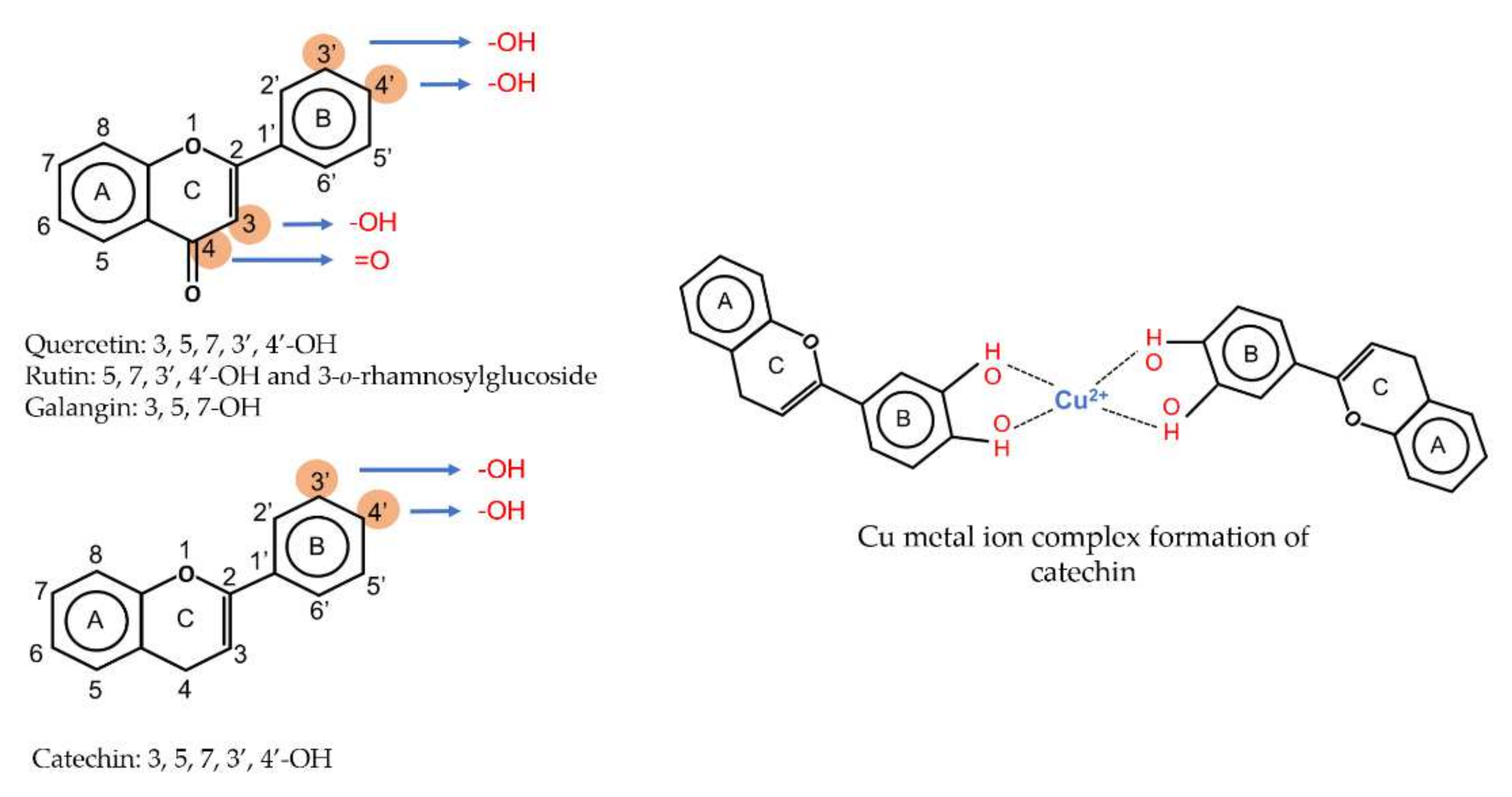
4. Antioxidant Properties of Fruit Extracts
4.1. Free Radical Scavenging Activity
4.2. Complexation of Metal Ions
4.3. Lipoxygenase Inhibition
5. Product Development from Fruit Extracts
6. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 December 2020).
- Aly, R. Microbial infections of skin and nails. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 2014. [Google Scholar]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Schurr, M.J.; LeBlanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of bacterial pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.T.; Kafkas, E. Phytochemicals in fruits and vegetables. In Superfood and Functional Food; Waisundara, V., Ed.; IntechOpen: London, UK, 2017; pp. 175–184. [Google Scholar]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Polyphenols and intestinal health. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 191–210. [Google Scholar]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Jepson, R.G.; Craig, J.C. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol. Nutr. Food Res. 2007, 51, 738–745. [Google Scholar] [CrossRef]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics 2012, 67, 661–668. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Fruit extracts to control pathogenic Escherichia coli: A sweet solution. Heliyon 2020, 6, e03410. [Google Scholar] [CrossRef]
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. Crit. Rev. Plant Sci. 2006, 25, 267–278. [Google Scholar] [CrossRef]
- Pascual-Teresa, D.; Moreno, D.A.; García-Viguera, C. Flavanols and anthocyanins in cardiovascular health: A review of current evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasawa, M.M.G.; Mohan, C. Fruits as prospective reserves of bioactive compounds: A review. Nat. Prod. Bioprospect. 2018, 8, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintie, C.A.; Singh, C.K.; Ahmad, N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl. Oncol. 2020, 13, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Wu, V.C. The potential of berries to serve as selective inhibitors of pathogens and promoters of beneficial microorganisms. Food Qual. Saf. 2017, 1, 3–12. [Google Scholar] [CrossRef]
- Oulkheir, S.; Ounine, K.; Haloui, N.E.E.; Attarassi, B. Antimicrobial effect of citric, acetic, lactic acids and sodium nitrite against Escherichia coli in tryptic soy broth. J. Biol. Agric. Healthc. 2015, 5, 12–19. [Google Scholar]
- Lieleg, O.; Caldara, M.; Baumgärtel, R.; Ribbeck, K. Mechanical robustness of Pseudomonas aeruginosa biofilms. Soft Matter. 2011, 7, 3307–3314. [Google Scholar] [CrossRef] [Green Version]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahya, A.; Belmehdi, O.; Kounnoun, A.; Zahli, R.; Abrini, J. Evaluation of antimicrobial activity of four organic acids used in chicks feed to control Salmonella typhimurium: Suggestion of amendment in the search standard. Int. J. Microbiol. 2018, 2018, 7352593. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šmejkal, K.; Chudík, S.; Kloucek, P.; Marek, R.; Cvacka, J.; Urbanová, M.; Julínek, O.; Kokoška, L.; Šlapetová, T.; Holubová, P.; et al. Antibacterial C-geranylflavonoids from Paulownia tomentosa fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Park, Y.J.; Biswas, R.; Phillips, R.D.; Chen, J. Antibacterial activities of blueberry and muscadine phenolic extracts. J. Food Sci. 2011, 76, M101–M105. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.; Sanz, M.; Herrera, D. New evidences of antibacterial effects of cranberry against periodontal pathogens. Foods 2020, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widsten, P.; Cruz, C.D.; Fletcher, G.C.; Pajak, M.A.; McGhie, T.K. Tannins and extracts of fruit byproducts: Antibacterial activity against foodborne bacteria and antioxidant capacity. J. Agric. Food Chem. 2014, 62, 11146–11156. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef] [PubMed]
- Man, N.Y.; Knight, D.R.; Stewart, S.G.; McKinley, A.J.; Riley, T.V.; Hammer, K.A. Spectrum of antibacterial activity and mode of action of a novel tris-stilbene bacteriostatic compound. Sci. Rep. 2018, 8, 6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ani, W.M.; Aziz, F.M. Antimicrobial activity of hydroxymatairesinol (HMR) lignan. Iraqi J. Pharm. Sci. 2013, 22, 30–34. [Google Scholar]
- Ragasa, C.Y.; Crisostomo, C.J.J.; Garcia, K.D.C.; Shen, C.C. Antimicrobial xanthones from Garcinia mangostana L. Philipp. Sci. 2010, 47, 63–75. [Google Scholar]
- Narasimhan, S.; Maheshwaran, S.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Rethavathi, J.; Das, P.E.; Poltronieri, P. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana. Molecules 2017, 22, 275. [Google Scholar] [CrossRef] [Green Version]
- Dharmaratne, H.R.W.; Sakagami, Y.; Piyasena, K.G.P.; Thevanesam, V. Antibacterial activity of xanthones from Garcinia mangostana (L.) and their structure–activity relationship studies. Nat. Prod. Res. 2013, 27, 938–941. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: Metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of organic acids and soluble sugars in juices from Nordic berries. Acta Agric. Scand. B Soil Plant Sci. 2002, 52, 101–109. [Google Scholar] [CrossRef]
- Sabra, W.; Dietz, D.; Zeng, A.P. Substrate-limited co-culture for efficient production of propionic acid from flour hydrolysate. Appl. Microbiol. Biotechnol. 2013, 97, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Buchwald, W.; Kozlowski, J. Variation in the content of flavonols and main organic acids in the fruit of European cranberry (Oxycoccus palustris Pers.) growing in peatlands in peatlands of North-Western Poland. Herba Pol. 2011, 57, 5–15. [Google Scholar]
- Takita, M.A.; Berger, I.J.; Basílio-Palmieri, A.C.; Borges, K.M.; Souza, J.M.D.; Targon, M.L. Terpene production in the peel of sweet orange fruits. Genet. Mol. 2007, 30, 841–847. [Google Scholar] [CrossRef]
- Javed, S.; Javaid, A.; Mahmood, Z.; Javaid, A.; Nasim, F. Biocidal activity of citrus peel essential oils against some food spoilage bacteria. J. Med. Plant Res. 2011, 5, 3697–3701. [Google Scholar]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.L.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [Green Version]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Chanthaphon, S.; Chanthachum, S.; Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin J. Sci. Technol. 2008, 30, 125–131. [Google Scholar]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Hong, H.; Landauer, M.R.; Foriska, M.A.; Ledney, G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Mukne, A.P.; Viswanathan, V.; Phadatare, A.G. Structure pre-requisites for isoflavones as effective antibacterial agents. Pharmacogn. Rev. 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Harmand, M.F.; Blanquet, P. The fate of total Flavanolic Oligomers (OFT) extracted from “Vitis vinifera L.” in the rat. Eur. J. Drug Metab. Pharmacokinet. 1978, 3, 15–30. [Google Scholar] [CrossRef]
- Howell, A.B. Cranberry proanthocyanidins and the maintenance of urinary tract health. Crit. Rev. Food Sci. Nutr. 2002, 42, 273–278. [Google Scholar] [CrossRef]
- Lamy, E.; Pinheiro, C.; Rodrigues, L.; Capela-Silva, F.; Lopes, O.; Tavares, S.; Gaspar, R. Determinants of tannin-rich food and beverage consumption: Oral perception vs. psychosocial aspects. In Tannins: Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Nova Publishers: New York, NY, USA, 2016; pp. 29–58. [Google Scholar]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.; Falk, M. Polyphenols in the prevention and treatment of vascular and cardiac disease, and cancer. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1049–1065. [Google Scholar]
- Mazur, W.M.; Uehara, M.; Wähälä, K.; Adlercreutz, H. Phyto-oestrogen content of berries, and plasma concentrationsand urinary excretion of enterolactone after asingle strawberry-meal in human subjects. Br. J. Nutr. 2000, 83, 381–387. [Google Scholar]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Sir Elkhatim, K.A.; Elagib, R.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Gonthier, M.P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef]
- Määttä, K.R.; Kamal-Eldin, A.; Törrönen, A.R. High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes species. J. Agric. Food Chem. 2003, 51, 6736–6744. [Google Scholar] [CrossRef]
- Tomiyama, K.; Mukai, Y.; Saito, M.; Watanabe, K.; Kumada, H.; Nihei, T.; Hamada, N.; Teranaka, T. Antibacterial action of a condensed tannin extracted from astringent persimmon as a component of food addictive pancil PS-M on oral polymicrobial biofilms. BioMed Res. Int. 2016, 2016, 5730748. [Google Scholar] [CrossRef] [Green Version]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef]
- Rane, H.S.; Bernardo, S.M.; Howell, A.B.; Lee, S.A. Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm-and adherence-specific mechanisms. J. Antimicrob. Chemother. 2014, 69, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vostalova, J.; Vidlar, A.; Simanek, V.; Galandakova, A.; Kosina, P.; Vacek, J.; Vrbkova, J.; Zimmermann, B.F.; Ulrichova, J.; Student, V. Are high proanthocyanidins key to cranberry efficacy in the prevention of recurrent urinary tract infection? Phytother. Res. 2015, 29, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective anti-adhesives of uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdulis, D.; Sarkinas, A.; Jasutiene, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) Fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 1934578X1100600136. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Costa, E.M.; Pereira, M.F.; Costa, M.R.; Pintado, M.E. Evaluation of the antimicrobial activity of aqueous extracts from dry Vaccinium corymbosum extracts upon food microorganism. Food Control 2013, 34, 645–650. [Google Scholar] [CrossRef]
- Lian, P.Y.; Maseko, T.; Rhee, M.; Ng, K. The antimicrobial effects of cranberry against Staphylococcus aureus. Food Sci. Technol. Int. 2012, 18, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventos, R.M. Phenolic compounds: Chemistry and occurrence in fruits and vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability, 1st ed.; de la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 53–88. [Google Scholar]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Salama, Z.A.; Askar, M.S.; El-Hariri, D.M.; Bakry, B.A. In Vitro antioxidant and antimicrobial activities of Lignan flax seed extract (Linum usitatissimum, L.). Int. J. Pharm. Sci. Rev. Res 2013, 23, 291–297. [Google Scholar]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial effects and mechanism of Mandarin (Citrus reticulata L.) essential oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ordóñez, A.; Carvajal, A.; Arguello, H.; Martínez-Lobo, F.J.; Naharro, G.; Rubio, P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013, 115, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.A.; Moon, S.H.; Kim, K.T.; Mendonca, A.F.; Paik, H.D. Antimicrobial effects of various flavonoids on Escherichia coli O157: H7 cell growth and lipopolysaccharide production. Food Sci. Biotechnol. 2010, 19, 257–261. [Google Scholar] [CrossRef]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and mode of action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid.-Based Complement. Alternat. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef] [Green Version]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, B.S.; Patil, B.S. Grapefruit bioactive limonoids modulate E. coli O157:H7 TTSS and biofilm. Int. J. Food Microbiol. 2010, 140, 109–116. [Google Scholar] [CrossRef]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC “top-six” non-O157 Shiga toxin producing E. coli. Int. J. Food Microbiol. 2016, 229, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Porras, G.; Brown, M.M.; Chassagne, F.; Lyles, J.T.; Bacsa, J.; Bacsa, J.; Horswill, A.R.; Quave, C.L. Triterpenoid acids isolated from Schinus terebinthifolia fruits reduce Staphylococcus aureus virulence and abate dermonecrosis. Sci. Rep. 2020, 10, 8046. [Google Scholar] [CrossRef]
- Konishi, K.; Adachi, H.; Ishigaki, N.; Kanamura, Y.; Adachi, I.; Tanaka, T.; Nishioka, I.; Nonaka, G.; Horikoshi, I. Inhibitory effects of tannins on NADH dehydrogenases of various organisms. Biol. Pharm. Bull. 1993, 16, 716–718. [Google Scholar] [CrossRef] [Green Version]
- Konishi, K.; Tanaka, T. Inhibitory effects of tannins on the NADH dehydrogenase activity of bovine heart mitochondrial complex I. Biol. Pharm. Bull. 1999, 22, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Chang, Y.S.; Wu, S.L.; Chao, D.C.; Chang, C.S.; Li, C.C.; Ho, T.Y.; Hsiang, C.Y. Inhibition of Escherichia coli heat-labile enterotoxin-induced diarrhea by Chaenomeles speciosa. J. Ethnopharmacol. 2007, 113, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Taylor, M.; Zhao, M.; Cherubin, P.; Geden, S.; Ray, S.; Francis, D.; Teter, K. Grape extracts inhibit multiple events in the cell biology of cholera intoxication. PLoS ONE 2013, 8, e73390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. Int. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peabody, M.A.; Laird, M.R.; Vlasschaert, C.; Lo, R.; Brinkman, F.S. PSORTdb: Expanding the bacteria and archaea protein subcellular localization database to better reflect diversity in cell envelope structures. Nucleic Acids Res. 2016, 44, D663–D668. [Google Scholar] [CrossRef] [Green Version]
- Fitriyanto, N.A.; Lewa, N.; Prasetyo, R.A.; Kurniawati, A.; Erwanto, Y.; Bachruddin, Z. Antibacterial activity of Maja fruit extract against Escherichia coli and its potential as urease inhibitor for reducing ammonia emission in poultry excreta. IOP Conf. Ser. Earth Environ. Sci. 2020, 465, 012006. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Cox, S.D.; Gustafson, J.E.; Mann, C.M.; Markham, J.L.; Liew, Y.C.; Hartland, R.P.; Bell, H.C.; Warmington, J.R.; Wyllie, S.G. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 355–358. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture–in vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Das, M.C.; Das, A.; Samaddar, S.; Daware, A.V.; Ghosh, C.; Acharjee, S.; Sandhu, P.; Jawed, J.J.; De, U.C.; Majumdar, S.; et al. Vitexin alters Staphylococcus aureus surface hydrophobicity to interfere with biofilm formation. bioRxiv 2018, 301473. [Google Scholar] [CrossRef] [Green Version]
- Booth, I.R. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 1985, 49, 359. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Lu, H.J.; Breidt, F., Jr.; Pérez-Díaz, I.M.; Osborne, J.A. Antimicrobial effects of weak acids on the survival of Escherichia coli O157:H7 under anaerobic conditions. J. Food Prot. 2011, 74, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The effect of food preservatives on pH homeostasis in Escherichia coli. Microbiology 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, A.; Gutknecht, J. Monocarboxylic acid permeation through lipid bilayer membranes. J. Membr. Biol. 1984, 77, 255–264. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Mason, P.K. Phlorotannin-protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.A.; Wu, V.C. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control. 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella typhimurium. LWT Food Sci. Technol. 2016, 66, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Singla, R.; Goel, H.; Ganguli, A. Novel synergistic approach to exploit the bactericidal efficacy of commercial disinfectants on the biofilms of Salmonella enterica serovar Typhimurium. J. Biosci. Bioeng. 2014, 118, 34–40. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Laird, K.; Armitage, D.; Phillips, C. Reduction of surface contamination and biofilms of Enterococcus sp. and Staphylococcus aureus using a citrus-based vapour. J. Hosp. Infect. 2012, 80, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Pagán, R.; López, D.; García-Gonzalo, D. Individual constituents from essential oils inhibit biofilm mass production by multi-drug resistant Staphylococcus aureus. Molecules 2015, 20, 11357–11372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef]
- Zhong, Z.; Yu, X.; Zhu, J. Red bayberry extract inhibits growth and virulence gene expression of the human pathogen Vibrio cholerae. J. Antimicrob. Chemother. 2008, 61, 753–754. [Google Scholar] [CrossRef]
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2002, 99, 3129–3134. [Google Scholar] [CrossRef] [Green Version]
- Opoku-Temeng, C.; Sintim, H.O. Inhibition of cyclic diadenylate cyclase, DisA, by polyphenols. Sci. Rep. 2016, 6, 25445. [Google Scholar] [CrossRef] [Green Version]
- Schuier, M.; Sies, H.; Illek, B.; Fischer, H. Cocoa-related flavonoids inhibit CFTR-mediated chloride transport across T84 human colon epithelia. J. Nutr. 2005, 135, 2320–2325. [Google Scholar] [CrossRef]
- Lescano, C.H.; de Oliveira, I.P.; Zaminelli, T.; Baldivia, D.D.S.; da Silva, L.R.; Napolitano, M.; Silvério, C.B.M.; Lincopan, N.; Sanjinez-Argandona, E.J. Campomanesia adamantium peel extract in antidiarrheal activity: The ability of inhibition of heat-stable enterotoxin by polyphenols. PLoS ONE 2016, 11, e0165208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellarin, M.G.; Albrecht, C.; Rojas, M.J.; Aguilar, J.J.; Konigheim, B.S.; Paraje, M.G.; Albesa, I.; Eraso, A.J. Inhibition of cytotoxicity of Shiga toxin of Escherichia coli O157: H7 on vero cells by Prosopis alba Griseb (Fabaceae) and Ziziphus mistol Griseb (Rhamnaceae) extracts. J. Food Prot. 2013, 76, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.; Craft, J.W., Jr.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of cholera toxin and other AB toxins by polyphenolic compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar]
- World Health Organization (WHO). Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 13 October 2020).
- Cheesman, M.J.; White, A.; Matthews, B.; Cock, I.E. Terminalia ferdinandiana fruit and leaf extracts inhibit methicillin-resistant Staphylococcus aureus growth. Planta Med. 2019, 85, 1253–1262. [Google Scholar] [CrossRef]
- Dharmaratne, M.P.J.; Manoraj, A.; Thevanesam, V.; Ekanayake, A.; Kumar, N.S.; Liyanapathirana, V.; Abeyratne, E.; Bandara, B.R. Terminalia bellirica fruit extracts: In-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complement. Altern. Med. 2018, 18, 325. [Google Scholar] [CrossRef]
- Chaves-López, C.; Usai, D.; Donadu, M.G.; Serio, A.; González-Mina, R.T.; Simeoni, M.C.; Molicotti, P.; Zanetti, S.; Pinna, A.; Paparella, A. Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018, 9, 2725–2734. [Google Scholar] [CrossRef]
- Viktorová, J.; Kumar, R.; Řehořová, K.; Hoang, L.; Ruml, T.; Figueroa, C.R.; Valdenegro, M.; Fuentes, L. Antimicrobial activity of extracts of two native fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics 2020, 9, 444. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of food antioxidants in modulating gut microbial communities: Novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Pérez-Jiménez, J. What contribution is beer to the intake of antioxidants in the diet? In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 441–448. [Google Scholar]
- Rojas, J.; Buitrago, A. Antioxidant activity of phenolic compounds biosynthesized by plants and its relationship with prevention of neurodegenerative diseases. In Bioactive Compounds; Segura-Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–31. [Google Scholar]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Denardin, C.C.; Hirsch, G.E.; da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.; Guma, F.T.; Emanuelli, T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.D.O.; de Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; Dos Santos, A.L.S.; Moreira, D.D.L.; Amaral, B.S.D.; Jung, E.P.; Kunigami, C.N. Umbu fruit peel as source of antioxidant, antimicrobial and α-amylase inhibitor compounds. Molecules 2022, 27, 410. [Google Scholar] [CrossRef]
- Shan, S.; Huang, X.; Shah, M.H.; Abbasi, A.M. Evaluation of polyphenolics content and antioxidant activity in edible wild fruits. BioMed Res. Int. 2019, 2019, 1381989. [Google Scholar] [CrossRef] [Green Version]
- Batiston, W.P.; Maruyama, S.A.; Gomes, S.T.M.; Visentainer, J.V.; de Souza, N.E.; Matsushita, M. Total phenolic content and antioxidant capacity of methanolic extracts of ten fruits. Acta Sci. Technol. 2013, 35, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Gallardo, J.O.; Molina-Torres, J.; Angoa-Pérez, M.V.; Cárdenas-Valdovinos, J.G.; García-Ruíz, I.; Ceja-Díaz, J.A.; Mena-Violante, H.G. Phenolic compound content and the antioxidant and antimicrobial activity of wild blueberries (Vaccinium stenophyllum steud.) fruits extracts during ripening. Horticulturae 2021, 8, 15. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive compounds and antioxidant capacity of small berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Najafabad, A.M.; Jamei, R. Free radical scavenging capacity and antioxidant activity of methanolic and ethanolic extracts of plum (Prunus domestica L.) in both fresh and dried samples. Avicenna J. Phytomed. 2014, 4, 343. [Google Scholar]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy-and trihydroxyphenolic acids—A structure–activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhu, Z.Q.; Hu, T.X.; Zhu, D.Y. Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharmacol. Sin. 2002, 23, 667–672. [Google Scholar]
- Sukmaningsih, A.A.S.A.; Permana, S.; Santjojo, D.J.D.H.; Wardoyo, A.Y.P.; Sumitro, S.B. Investigating natural transition metal coordination anthocyanin complex in java plum (Syzygium cumini) fruit as free radical scavenging. Rasayan J. Chem. 2018, 11, 1193–1203. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serbian Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- De Souza, R.F.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysal, T.; Demirdöven, A. Lipoxygenase in fruits and vegetables: A review. Enzyme Microb. Technol. 2007, 40, 491–496. [Google Scholar] [CrossRef]
- Veldink, G.A.; Hilbers, M.P.; Nieuwenhuizen, W.F.; Vliegenthart, J.F.G. Plant lipoxygenase: Structure and mechanism. In Eicosanoids and Related Compounds in Plants and Animals; Princeton University Press: New Jersey, NJ, USA, 1999; pp. 69–96. [Google Scholar]
- Salas, J.J.; Sánchez, C.; García-González, D.L.; Aparicio, R. Impact of the suppression of lipoxygenase and hydroperoxide lyase on the quality of the green odor in green leaves. J. Agric. Food Chem. 2005, 53, 1648–1655. [Google Scholar] [CrossRef]
- Alam, F.; us Saqib, Q.N.; Ashraf, M. Gaultheria trichophylla (Royle): A source of minerals and biologically active molecules, its antioxidant and anti-lipoxygenase activities. BMC Complement Altern. Med. 2017, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Berkoz, M. Antioxidant and anti-lipoxygenase activities of Cydonia oblonga. Medicine 2020, 9, 251–254. [Google Scholar] [CrossRef]
- Kohyama, N.; Nagata, T.; Fujimoto, S.I.; Sekiya, K. Inhibition of arachidonate lipoxygenase activities by 2-(3, 4-dihydroxyphenyl) ethanol, a phenolic compound from olives. Biosci. Biotechnol. Biochem. 1997, 61, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [Green Version]
- Purkiewicz, A.; Pietrzak-Fiećko, R. Antioxidant properties of fruit and vegetable whey beverages and fruit and vegetable mousses. Molecules 2021, 26, 3126. [Google Scholar] [CrossRef]
- Vundela, S.R.; Kalagatur, N.K.; Nagaraj, A.; Krishna, K.; Chandranayak, S.; Kondapalli, K.; Hashem, A.; Abd_Allah, E.F.; Poda, S. Multi-biofunctional properties of phytofabricated selenium nanoparticles from Carica papaya fruit extract: Antioxidant, antimicrobial, antimycotoxin, anticancer, and biocompatibility. Front. Microbiol. 2021, 12, 76981–769891. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naeem, H.H.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pașcalău, R.; Sallam, K.I. Antioxidant and antibacterial effect of fruit peel powders in chicken patties. Foods 2022, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; Van de Wiele, T.; Schüller, S.; Juge, N.; et al. Experimental models to study intestinal microbes–mucus interactions in health and disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, E.; Roussel, C.; Dominicis, A.; Ley, D.; Peyron, M.A.; Collado, V.; Mercier-Bonin, M.; Lacroix, C.; Alric, M.; Van de Wiele, T.; et al. In vitro models of gut digestion across childhood: Current developments, challenges and future trends. Biotechnol. Adv. 2021, 54, 107796. [Google Scholar] [CrossRef] [PubMed]
| Extract/Compound | Major Fruit Source | Antimicrobial Action | Inhibitory Concentration | References |
|---|---|---|---|---|
| Organic acids | ||||
| Citric acid | Citrus fruits | S. aureus, S. dysenteriae, E. coli and P. aeruginosa | Plate count method, 1–6% | [20,21] |
| Malic acid | Apples, blackberries, cherries, apricots, peaches, mangos, and plums | L. monocytogenes, E. coli O157:H7 and S. Gaminara | Antibacterial effectiveness on film disc, 2.6% | [22] |
| Propionic acid | Apples and strawberries | S. aureus, S. lutea, L. monocytogenes and P. vulgaris | Growth inhibition, 0.3% | [23] |
| Tartaric acid | Grapes | L. monocytogenes, S. Gaminara and E. coli O157:H7 | Antibacterial effectiveness on film disc, 1.8–2.6% | [22] |
| Tartaric acid, citric acid and acetic acid | Cranberries, bilberries, blueberries, blackberries, raspberries, black chokeberries, red currants, and blackcurrants | S. Typhimurium | MIC, 0.312–1.25% | [24] |
| Salicylic acid | Berry fruits | S. aureus, E. coli, P. aeruginosa and E. faecalis | MIC, 250–500 µg/mL | [25] |
| Terpenes | ||||
| Eugenol | Strawberries, blackberries, bananas, and citrus | S. Typhimurium | MIC, 0.07 mg/mL | [26] |
| Terpineol | Apples, blueberries, and limes | S. aureus | MIC, 0.12 mg/mL | [26] |
| Carveol, citronellol and geraniol | Citrus fruits | E. coli | MIC, 0.25 mg/mL | [26] |
| Thymol, (+) menthol, and linalyl acetate | Bergamot | S. aureus and E. coli | MIC, 0.31–1.25 mg/mL | [27] |
| Flavonoid polyphenols | ||||
| Flavones | Oranges, grapefruits, and lemons | E. faecalis, E. coli, and P. aeruginosa | MIC, 500 µg/mL | [25] |
| Flavonols | Berries, black grapes, apricots and apples | Gram-positive (S. aureus, MRSA, S. epidermidis, S. haemolyticus, and S. pyogenes) Gram-negative (E. coli, S. Typhimurium and K. pneumoniae) | MIC, 0.25 mg/mL | [28] |
| Flavan-3-ols (3′-O-methyldiplacol) | Apples, grapes, blackberries, and cranberries | Gram-positive bacteria (B. subtilis, E. faecalis, L. monocytogenes, S. aureus, and S. epidermidis) | MIC, 2–4 μg/mL | [29] |
| Flavan-3-ols (Quercetin 3-O-methyl ether) | Apples, grapes, blackberries, and cranberries | H. pylori | MIC, 3.9 µg/mL | [28] |
| Flavanones | Citrus fruits | Gram-positive bacteria (B. cereus, B. subtilis, E. faecalis, L. monocytogenes, and S. aureus) | MIC, 2–4 μg/mL | [29] |
| Flavanones (Sophoraflavanone G) | Citrus fruits | S. epidermidis, S. aureus, and B. subtilis | MIC, 3.1–12.5 μg/mL | [28] |
| Anthocyanins | Grapes, cherries, plums, raspberries, strawberries, blackberries, apples, peaches, red and blackcurrants, blueberries, and cranberries | E. coli and Salmonella sp. | MIC, 10–400 mg/mL | [30] |
| Anthocyanins | Blueberry extract | S. aureus and E. coli | Biofilm formation, 500 µg/mL | [31] |
| Non-flavonoid polyphenols | ||||
| Phenolic acids | Blueberries and muscadines | S. Enteritidis | Growth inhibition, 24–46 µg/mL | [32] |
| Phenolic acids | Cranberries | L. monocytogenes | Plate count method, 10 mg/mL | [10] |
| Phenolic acids | Cranberry extract | S. oralis, A. naeslundii, V. parvula, F. nucleatum, P. gingivalis and A. actinomycetemcomitans | Antibiofilm activity, 0.1–1.0 mg/mL | [33] |
| Phenolic acids (4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid) | Blueberries, cloudberries and bilberries | Gram-positive (S. aureus, S. epidermidis, and B. subtilis) Gram-negative (E. coli, S. Typhimurium, P. aeruginosa, and P. syringae) | Disc diffusion method, 100–170 µg/mL | [34] |
| Phenolic acids (Tannic acids) | Berries, pomegranates, grapes, persimmons, pears, and apples | S. Typhimurium, S. Enteritidis, E. coli and S. aureus | MIC, 0.3–3 mg/mL | [35,36,37] |
| Phenolic acids (Ellagitannins) | Bilberries, lingonberries, cranberries, red raspberries, cloudberries, strawberries, blackcurrants and sea buckthorn berries | Staphylococcus sp. | Plate count method, 2 mg/mL | [10] |
| Stilbenes (Triacid derivative 135 C) | Grapes, pomegranates, bilberries, blueberries and mulberries | S. epidermidis, S. pneumoniae, S. pyogenes, Micrococcus spp., and MRSA | MIC, 1–32 μg/mL | [38] |
| Lignans (Hydroxymatairesinol (HMR) lignan) | Lingonberries, strawberries, apples, cranberries, prunes, and pears | S. epidermidis, Proteus sp., and Klebsiella sp. | Disc diffusion method, 25–100 mg/mL | [39] |
| Xanthones (α-mangostin) | Mangosteen | Gram-positive (B. subtilis and S. aureus) Gram-negative (E. coli and P. aeruginosa) | MIC, 30–100 µg/mL | [40,41] |
| Xanthones (γ-mangostin) | Mangosteen | MSSA, MRSA, VSE and VRE | MIC, 3.13–6.25 µg/mL | [42] |
| Polyphenols | Structure | Derivatives | Sources | References |
|---|---|---|---|---|
| Flavonoids | ||||
| Flavones |  | Apigenin Baicalein Chrysin Luteolin | Herbs (parsley, celery) Citrus fruits (oranges, grapefruits, and lemons) | [28,56] |
| Flavonols | 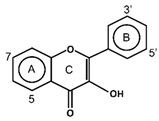 | Isohanmetin Kaemferol Myricetin Quercetin Rutin | Cranberries, black currants, lingonberries, blueberries, black grapes, apricots, and apples | [9,57] |
| Flavan-3-ols | Monomers Polymers 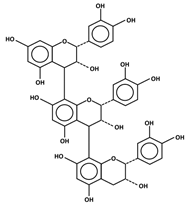 Proanthocyanidins (Condensed Tannins) | Catechin Epicatechin Gallocatechin | Apples, grapes, blackberries, and cranberries | [9,28] |
| Procyanidin A2 Procyanidin B1 Procyanidin B2 Procyanidin C1 | Grapes, apples, bilberries, cranberries, strawberries, and blueberries | [58] | ||
| Isoflavones | 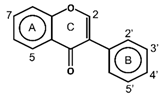 | Daidzin Equol Formononetin Genestein Glycitein | Soy products, legumes, currants, and raisins | [56,59,60,61] |
| Flavanones | 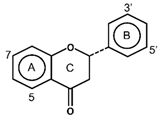 | Hesperidin Narinagin Sophoraflavanone G | Prunes and citrus fruits (lemons, oranges, and grapefruits) | [9,28] |
| Anthocyanins | 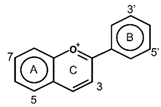 | Cyanidin Delphindin Malvidin Pelagonidin Peonidin | Families Vitaceae (grapes), Rosaceae (cherries, plums, raspberries, strawberries, blackberries, apples, peaches), Saxifragaceae (red and black currants), Ericaceae (blueberries and cranberries) | [62] |
| Non-Flavonoids | ||||
| Phenolic acids | Monomers Hydroxybenzoic acids Polymers 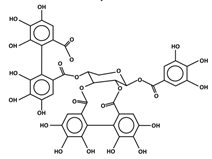 Hydrolyzable tannin (Ellagitannins) | Ellagic acid Gallic acid Protocatechuic acid Salicylic acid Syringic acid Vanillic acid | Tannic acid Genus Rubus (red raspberries, arctic brambles, and cloudberries), genus Fragaria (strawberries) | [9,10] |
| Ellagitannins Gallotannins | Berries, pomegranates, grapes, persimmons, pears, and apples | [9,10,63,64,65] | ||
| Hydroxycinnamic acids | 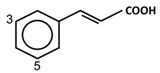 | Caffeic acid Caftaric acid Chlorogenic acid Cinnamic acid Coumaric acid Curcumin Ferulic acid | Blueberries, cloudberries, and bilberries | [9,66,67] |
| Stilbenes |  | Resveratrol Piceatannol | Grape skins, pomegranates, bilberries, blueberries, and mulberries | [9,68] |
| Lignans | 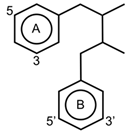 | Pinoresinol Matairesinol Secoisolaricire Sinol | Strawberries, apples, cranberries, prunes, and pears | [69] |
| Xanthone |  | γ-mangostin | Mangosteens (G. mangostana) | [70] |
| Antimicrobial Activity Mechanisms | Fruit Extracts or Individual Compounds | Target Bacteria | Consequences | References |
|---|---|---|---|---|
| Interaction with the bacterial cell envelope | Cloudberry and raspberry phenolic extracts | S. Typhimurium E-1151, S. Typhimurium SH-5014 | Outer membrane disintegration, LPS release, and an increase in cytoplasmic membrane permeability | [87] |
| Mandarin (C. reticulata L.) essential oil | S. aureus (ATCC 25923) | Collapse and pitting of bacterial surface cell Disruption of the cell membrane Leakage of cytoplasmic content, protein, and nucleic acid | [88] | |
| C. paradisi (grapefruit), C. reticulata (mandarin), C. aurantium subsp. bergamia (bergamot), C. sinensis (sweet orange), and BIOLL+® (commercial form) | S. Typhimurium (CECT443, CECT883, and DT104), S. Enteritidis CECT4300, S. Infancis CECT700, S. Cholerasuis CECT915, S. London, S. Derby, E. coli (haemolytic strain), and B. hyodysenteriae (ATCC27164 and ATCC31212) | Membrane disruption, membrane expansion, increase in membrane fluidity and permeability, loss of cytoplasmic material, and inhibition of respiration and alteration in ion transport processes | [27,89] | |
| Quercetin (flavonol) and hesperetin (flavanone) | E. coli O157:H7 S. pyogenes (DMST30653, DMST30654, and DMST30655) | Disruption of membrane integrity, increased cytoplasmic membrane permeability, and loss of electron dense cellular material | [90,91] | |
| Effects on cell-cell communication and gene regulation | Flavonoids from citrus fruits (naringenin, quercetin, sinensetin and apigenin) | E. coli O157:H7 ATCC 43895 | Inhibition of autoinducers (AI)-mediated cell–cell signaling | [92] |
| Furocumarins from grapefruit extracts | V. harveyi reporter strains BB886, BB170, and BB120 | Inhibition of AI-1 (oligopeptides and N-acylhomoserine lactones (AHL)) and AI-2, (boronated-diester molecules) | [93] | |
| Limonoids | EHEC O157:H7 ATCC 43895 | Inhibition of AHL and AI-2 mediated cell–cell signaling Repression of the expression of Type III secretion system (T3SS) of EHEC | [94] | |
| Grape seed extract | Non-O157 STEC (Shiga toxin-encoding E. coli) | Reduction of AI-2 production, production of flagellum protein FliC and regulator FliA in E. coli O103:H2 and E. coli O111:H2. | [95] | |
| Triterpenoid acids | S. aureus | Inhibition of accessory gene regulator, agr-type QS (quorum sensing), Decreased δ-toxin production and biofilm formation | [96] | |
| Inhibition of metabolic and enzyme activities | Tannins and polyphenolic compounds | P. denitrificans, B. subtilis, P. phosphoreum, and T. thermophilus | Inhibition of activity on NADH dehydrogenases (NDH) and NADH-ubiquinone-1 oxidoreductase | [97,98] |
| Chinese quince (C. speciosa) extract | E. coli BL21(DE3)pLysS strain | Inhibition of E. coli heat-labile toxin (LT)-induced diarrhea Elimination of the binding of the B subunit of LT (LTB) to GM1 | [99] | |
| Grape extract | V. cholerae | Inhibition of cholera toxin (CT) and E. coli heat-labile toxin | [100] | |
| Applephenon (polyphenol from apple) | V. cholerae | Inhibition of ADP-ribosyltransferase activity of cholera toxin (CT) | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants 2022, 11, 602. https://doi.org/10.3390/antiox11030602
Suriyaprom S, Mosoni P, Leroy S, Kaewkod T, Desvaux M, Tragoolpua Y. Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria. Antioxidants. 2022; 11(3):602. https://doi.org/10.3390/antiox11030602
Chicago/Turabian StyleSuriyaprom, Sureeporn, Pascale Mosoni, Sabine Leroy, Thida Kaewkod, Mickaël Desvaux, and Yingmanee Tragoolpua. 2022. "Antioxidants of Fruit Extracts as Antimicrobial Agents against Pathogenic Bacteria" Antioxidants 11, no. 3: 602. https://doi.org/10.3390/antiox11030602






