Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Reagents, Chemicals, Spectral Analyses, Extraction, and Fractionation of V. vinifera Seeds
2.2. In Vitro Antioxidant Activity
2.3. In Vivo Wound Healing Activity
2.4. Preparation of the Fatty Acid Methyl Esters with GC-MS Analysis, Isolation, and Purification of Compounds
2.5. Molecular Docking Studies
3. Results
3.1. In Vitro Antioxidant Potential of V. vinifera Seed Extract
3.1.1. Hydrogen Peroxide Scavenging Power
3.1.2. Superoxide Radical Scavenging Power
3.2. Wound Healing Activity
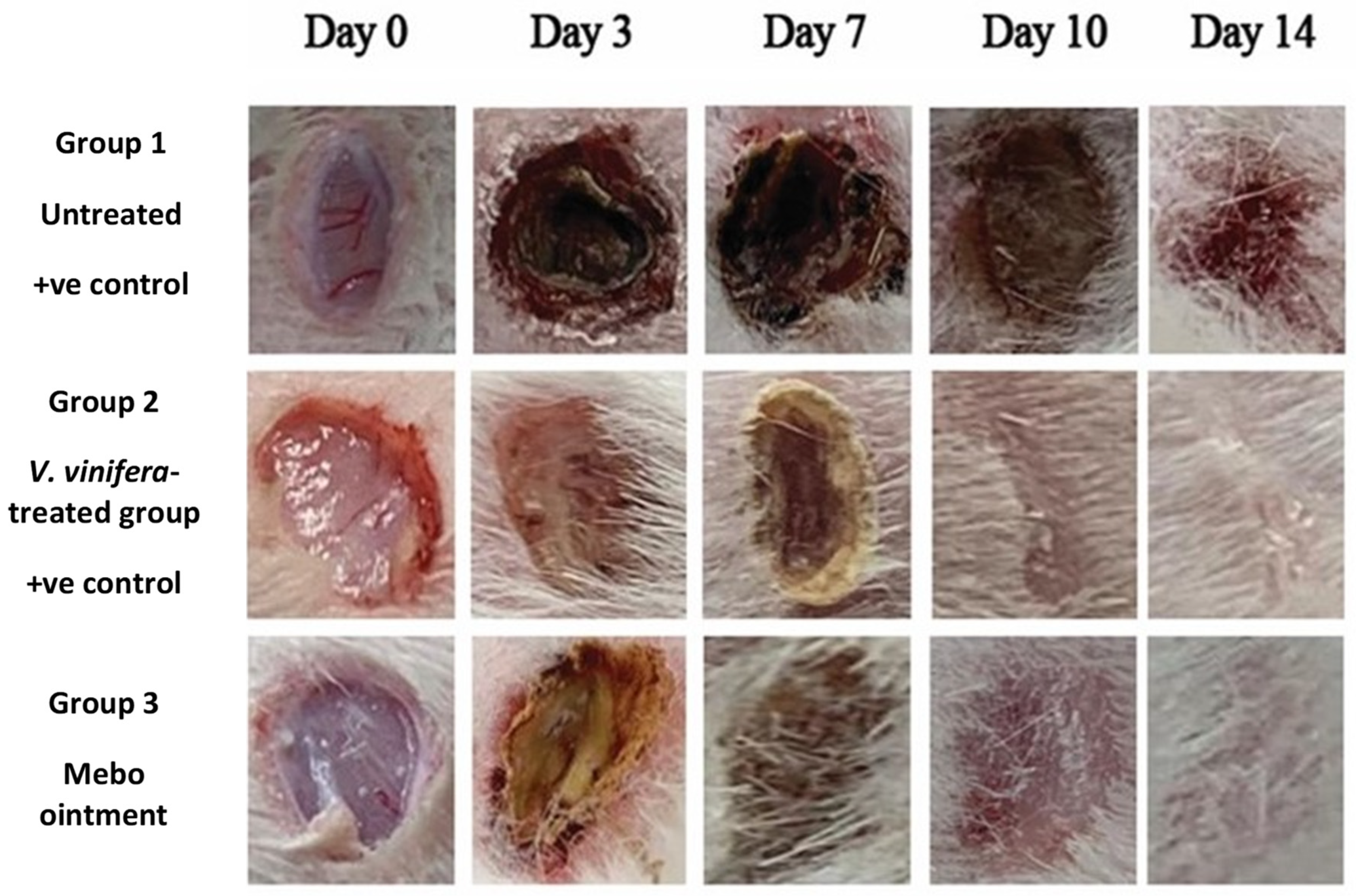
3.2.1. Estimation of Wound Closure Rate
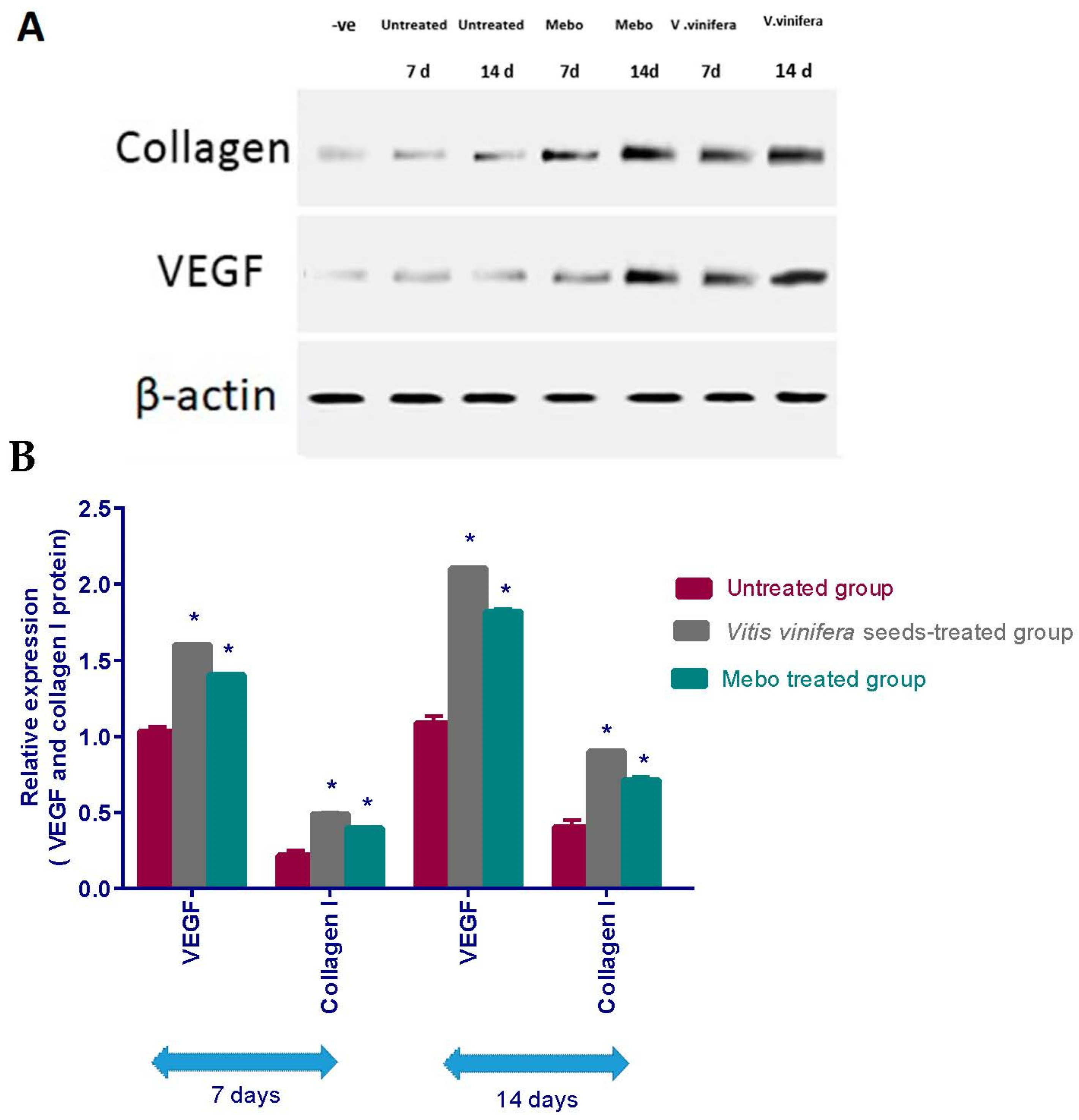
3.2.2. Effect of Seed Extract of V. vinifera on Expression of TGF-β, TNF-α, IL-1β, Collagen Type I and VEGF
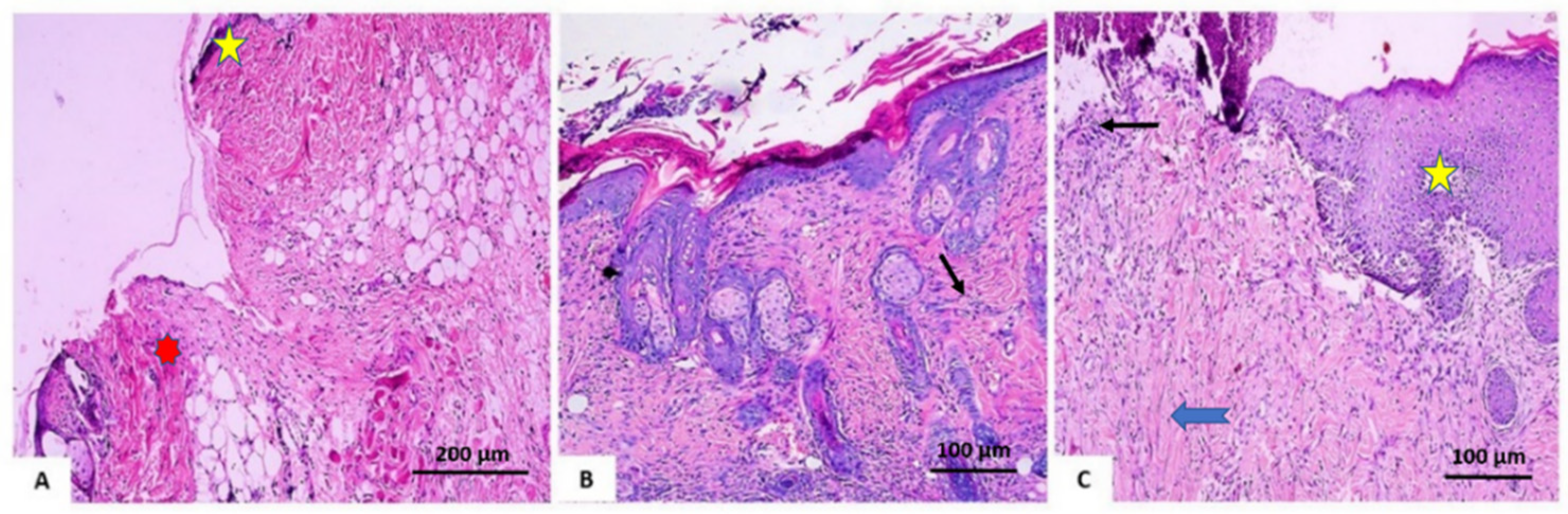
3.2.3. Histopathological Investigation
3.3. Phytochemical Investigation of Vitis vinifera Crude Seed Extract
3.3.1. GC/MS Analysis for Oil Content in Vitis vinifera Crude Extract
3.3.2. Phytochemical Investigation of V. vinifera Seed Extract
3.4. Molecular (In Silico) Docking Studies
3.5. In Vitro Antioxidant Potential of the Two Compounds Isolated from V. vinifera Seed Extract
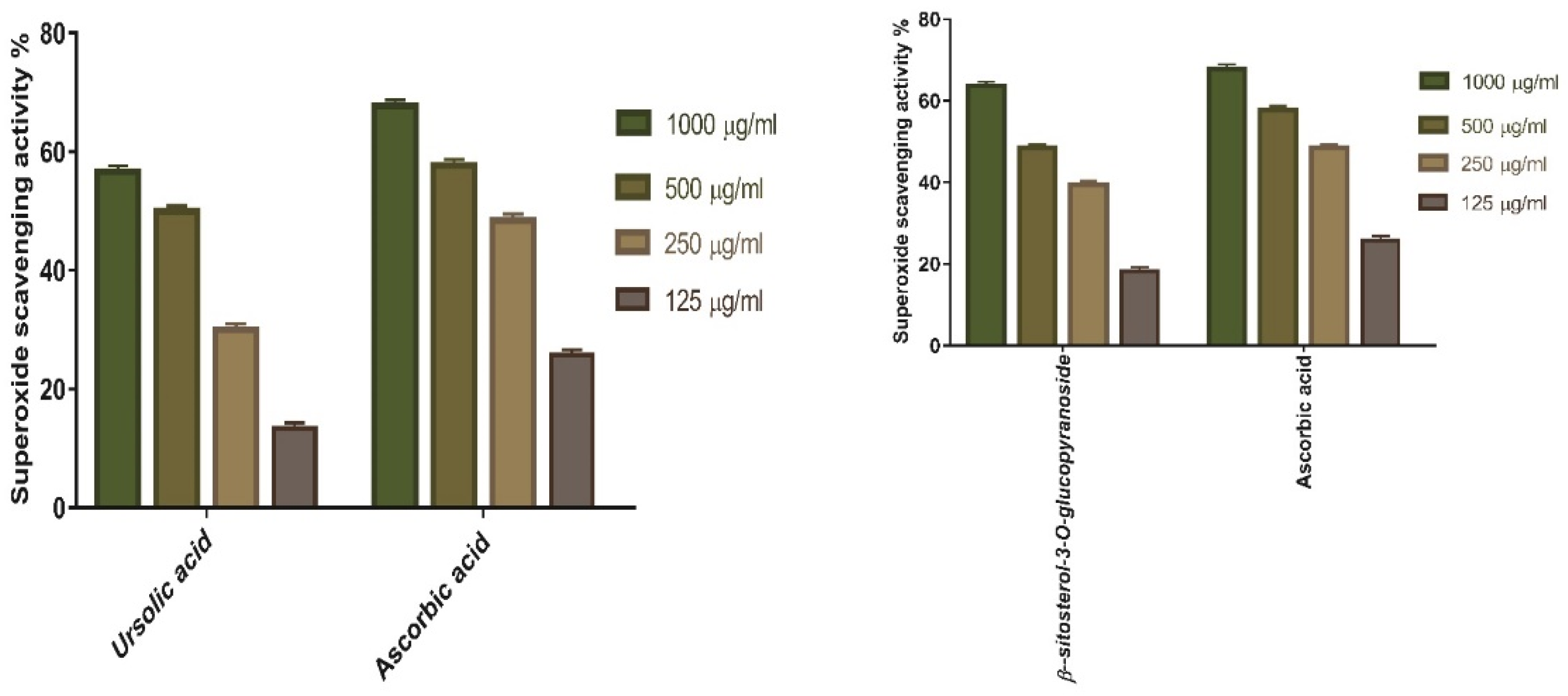
3.5.1. Hydrogen Peroxide Scavenging Activity of Ursolic Acid and β-Sitosterol-3-O-glucopyranoside
3.5.2. Superoxide Radical Scavenging Activity of Ursolic Acid, and β-Sitosterol-3-O-glucopyranoside
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benbow, M. Using Debrisoft [R] for wound debridement: Maureen Benbow briefly considers different methods of wound debridement and focuses on the advantages associated with a novel, alternative method of debridement. J. Community Nurs. 2011, 25, 17–19. [Google Scholar]
- Boakye, Y.D.; Agyare, C.; Ayande, G.P.; Titiloye, N.; Asiamah, E.A.; Danquah, K.O. Assessment of wound-healing properties of medicinal plants: The case of Phyllanthus muellerianus. Front. Pharmacol. 2018, 9, 945. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Lodhi, M.; Rehan, Z.A.; Tayyab, H.; Javed, T.; Shabbir, R.; Mukhtar, A.; El Sabagh, A.; Adamski, R.; Sakran, M.I.; et al. Sustainable development of chitosan/Calotropis procera-based hydrogels to stimulate formation of granulation tissue and angiogenesis in wound healing applications. Molecules 2021, 26, 3284. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of hydrogels with the incorporation of Raphanus sativus L. seed extract in sodium alginate for wound-healing application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Agyare, C.; Asase, A.; Lechtenberg, M.; Niehues, M.; Deters, A.; Hensel, A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009, 125, 393–403. [Google Scholar] [CrossRef]
- Farzaei, H.; Abbasabadi, Z.; Abdollahi, M.; Rahimi, R. A comprehensive review of plants and their active constituents with wound healing activity in traditional Iranian medicine. Wounds A Compend. Clin. Res. Pract. 2014, 26, 197–206. [Google Scholar]
- Bungau, S.G.; Popa, V.-C. Between religion and science: Some aspects: Concerning illness and healing in antiquity. Transylv. Rev. 2015, 26, 3–19. [Google Scholar]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional uses of some medicinal plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef]
- Hayta, S.; Polat, R.; Selvi, S. Traditional uses of medicinal plants in Elazığ (Turkey). J. Ethnopharmacol. 2014, 154, 613–623. [Google Scholar] [CrossRef]
- Adnan, M.; Ullah, I.; Tariq, A.; Murad, W.; Azizullah, A.; Khan, A.L.; Ali, N. Ethnomedicine use in the war affected region of northwest Pakistan. J. Ethnobiol. Ethnomed. 2014, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egea, T.; Signorini, M.A.; Bruschi, P.; Rivera, D.; Obón, C.; Alcaraz, F.; Palazón, J.A. Spirits and liqueurs in European traditional medicine: Their history and ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 2015, 175, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Gharib Naseri, M.K.; Ehsani, P. Spasmolytic effect of Vitis vinifera hydroalcoholic leaf extract on the isolated rat uterus. Physiol. Pharmacol. 2003, 7, 107–114. [Google Scholar]
- Oprea, O.B.; Apostol, L.; Bungau, S.; Cioca, G.; Samuel, A.D.; Badea, M.; Gaceu, L. Research on the chemical composition and the rheological properties of wheat and grape epicarp flour mixes. Rev. Chim. 2018, 69, 70–75. [Google Scholar] [CrossRef]
- Nayak, B.S.; Ramdath, D.D.; Marshall, J.R.; Isitor, G.N.; Eversley, M.; Xue, S.; Shi, J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phytother. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef]
- Jadeja, R.; Devkar, R. Polyphenols in chronic diseases and their mechanisms of action. Polyphen. Hum. Health Dis. 2014, 1, 615–623. [Google Scholar]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Shivananda Nayak, B.; Dan Ramdath, D.; Marshall, J.R.; Isitor, G.; Xue, S.; Shi, J. Wound-healing properties of the oils of Vitis vinifera and Vaccinium macrocarpon. Phytother. Res. 2011, 25, 1201–1208. [Google Scholar] [CrossRef]
- de Lampasona, M.E.; Catalán, C.A.; Gedris, T.E.; Herz, W. Benzofurans, benzofuran dimers and other constituents from Ophryosporus charua. Phytochemistry 1997, 46, 1077–1080. [Google Scholar] [CrossRef]
- Loevgren, K.; Hedberg, A.; Nilsson, J.L.G. Adrenergic receptor agonists. J. Med. Chem. 1980, 23, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Jedinák, A.; Mučková, M.; Košt’álová, D.; Maliar, T.; Mašterová, I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z. Für Nat. C 2006, 61, 777–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Shin, D.-S.; Oh, M.-N.; Chung, S.-C.; Lee, J.-S.; Chang, I.-M.; Oh, K.-B. Inhibition of sortase, a bacterial surface protein anchoring transpeptidase, by β-sitosterol-3-O-glucopyranoside from Fritillaria verticillata. Biosci. Biotechnol. Biochem. 2003, 67, 2477–2479. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, K.; Yaoita, Y.; Kikuchi, M. Three New Dimeric Benzofuran Derivatives from the Roots of Ligularia stenocephala M ATSUM. et K OIDZ. Chem. Pharm. Bull. 2005, 53, 1555–1558. [Google Scholar] [CrossRef] [Green Version]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Suguna, L.; Singh, S.; Sivakumar, P.; Sampath, P.; Chandrakasan, G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother. Res. 2002, 16, 227–231. [Google Scholar] [CrossRef]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Wankell, M.; Munz, B.; Hübner, G.; Hans, W.; Wolf, E.; Goppelt, A.; Werner, S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 2001, 20, 5361–5372. [Google Scholar] [CrossRef] [Green Version]
- Beer, H.-D.; Gassmann, M.G.; Munz, B.; Steiling, H.; Engelhardt, F.; Bleuel, K.; Werner, S. Expression and function of keratinocyte growth factor and activin in skin morphogenesis and cutaneous wound repair. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2000; pp. 34–39. [Google Scholar]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Haroon, Z.A.; Amin, K.; Saito, W.; Wilson, W.; Greenberg, C.S.; Dewhirst, M.W. SU5416 delays wound healing through inhibition of TGF-β activation. Cancer Biol. Ther. 2002, 1, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, R.A.; Kim, I.S.; Hokama, L.; De Ruyter, K.; Keen, C. Operational determinants of caller satisfaction in the call center. Int. J. Serv. Ind. Manag. 2000, 11, 34–36. [Google Scholar] [CrossRef]
- Schultz, G.S.; Ladwig, G.; Wysocki, A. Extracellular matrix: Review of its roles in acute and chronic wounds. World Wounds 2005, 2005, 1–18. [Google Scholar]
- Sasaki, M.; Kashima, M.; Ito, T.; Watanabe, A.; Izumiyama, N.; Sano, M.; Kagaya, M.; Shioya, T.; Miura, M. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1β and TNF-α. Mediat. Inflamm. 2000, 9, 155–160. [Google Scholar] [CrossRef]
- Sano, C.; Shimizu, T.; Tomioka, H. Effects of secretory leukocyte protease inhibitor on the tumor necrosis factor-alpha production and NF-κB activation of lipopolysaccharide-stimulated macrophages. Cytokine 2003, 21, 38–42. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Hochstein, A.; Bhatia, A. Collagen: Its role in wound healing. Wound Manag. 2014, 4, 104–109. [Google Scholar]
- Brett, D. A review of collagen and collagen-based wound dressings. Wounds 2008, 20, 347–356. [Google Scholar] [PubMed]
- Pizzimenti, S.; Toaldo, C.; Pettazzoni, P.; Dianzani, M.U.; Barrera, G. The “two-faced” effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers 2010, 2, 338–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol.-Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Menocal, L.; Shareef, S.; Salgado, M.; Shabbir, A.; Van Badiavas, E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res. Ther. 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.; Agrawal, N.K.; Gupta, S.K.; Sinha, P.; Singh, K. Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J. Diabetes Its Complicat. 2016, 30, 99–108. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Bolliger, H.R.; Brenner, M.; Gänshirt, H.; Mangold, H.K.; Seiler, H.; Stahl, E.; Waldi, D. Thin-Layer Chromatography: A Laboratory Handbook; Springer: Berlin/Heidelberg, Germany, 1965. [Google Scholar]
- Harikrishnan, L.S.; Warrier, J.; Tebben, A.J.; Tonukunuru, G.; Madduri, S.R.; Baligar, V.; Mannoori, R.; Seshadri, B.; Rahaman, H.; Arunachalam, P. Heterobicyclic inhibitors of transforming growth factor beta receptor I (TGFβRI). Bioorg. Med. Chem. 2018, 26, 1026–1034. [Google Scholar] [CrossRef]
- Tramontina, V.A.; Machado, M.A.N.; Filho, G.d.R.N.; Kim, S.H.; Vizzioli, M.R.; Toledo, S. Effect of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings. Braz. Dent. J. 2002, 13, 11–16. [Google Scholar]
- Hummon, A.B.; Lim, S.R.; Difilippantonio, M.J.; Ried, T. Isolation and solubilization of proteins after TRIzol® extraction of RNA and DNA from patient material following prolonged storage. Biotechniques 2007, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Alsenani, F.; Ashour, A.M.; Alzubaidi, M.A.; Azmy, A.F.; Hetta, M.H.; Abu-Baih, D.H.; Elrehany, M.A.; Zayed, A.; Sayed, A.M.; Abdelmohsen, U.R. Wound Healing Metabolites from Peters’ Elephant-Nose Fish Oil: An In Vivo Investigation Supported by In Vitro and In Silico Studies. Mar. Drugs 2021, 19, 605. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, S.I.; Elmaidomy, A.H.; Saber, H.; Musa, A.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Youssif, K.A.; Alanazi, A.S.; Alharbi, M. Potential anticancer lipoxygenase inhibitors from the red sea-derived brown algae sargassum cinereum: An in-silico-supported In-Vitro Study. Antibiotics 2021, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Hassan, H.; Abdel-Aziz, A. Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem. Toxicol. 2010, 48, 1999–2004. [Google Scholar] [CrossRef]
- Sonboli, A.; Mojarrad, M.; Ebrahimi, S.N.; Enayat, S. Free Radical Scavenging Activity and Total Phenolic Content of Methanolic Extracts from Male Inflorescence of Salix aegyptiaca Grown in Iran. Iran. J. Pharm. Res. IJPR 2010, 9, 293–296. [Google Scholar]


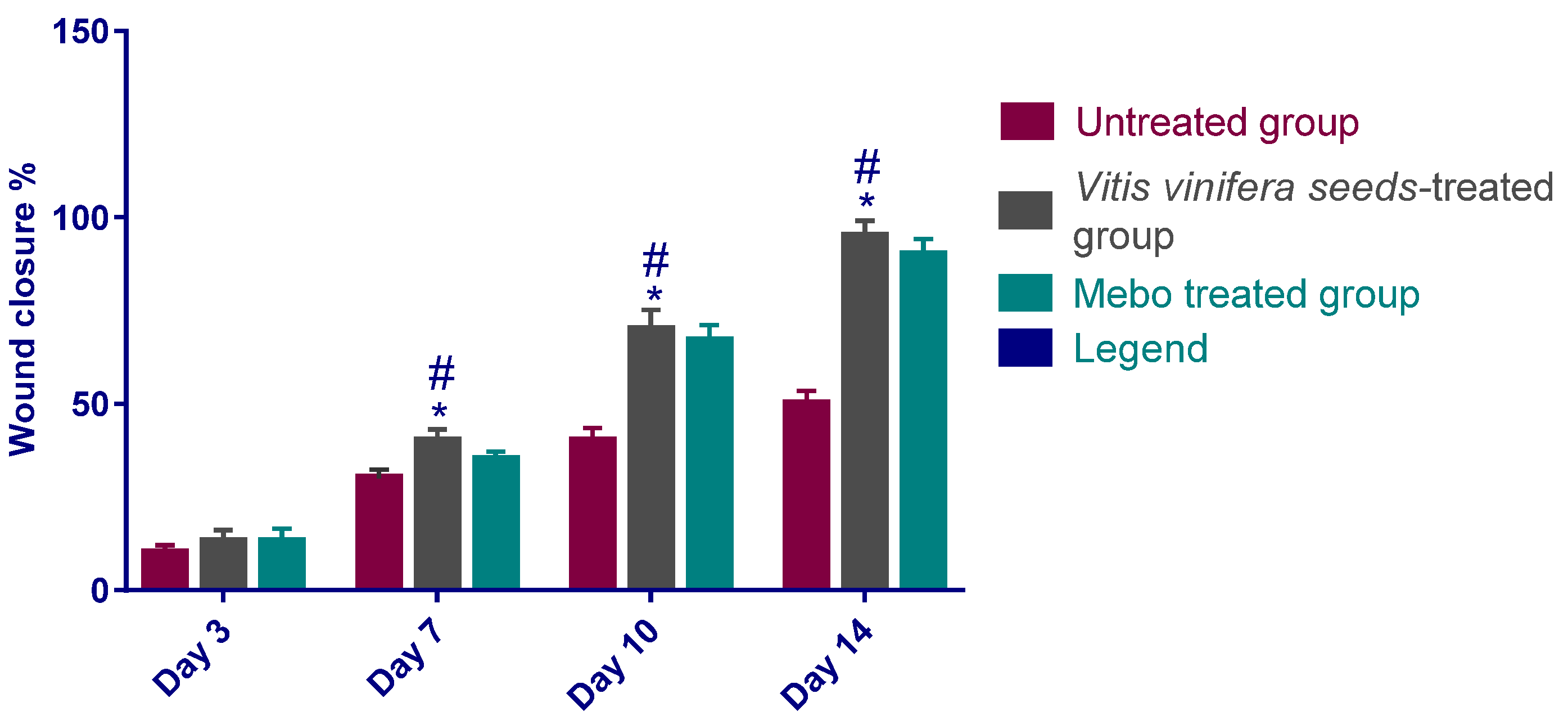




 )correlations of compound 28.
)correlations of compound 28.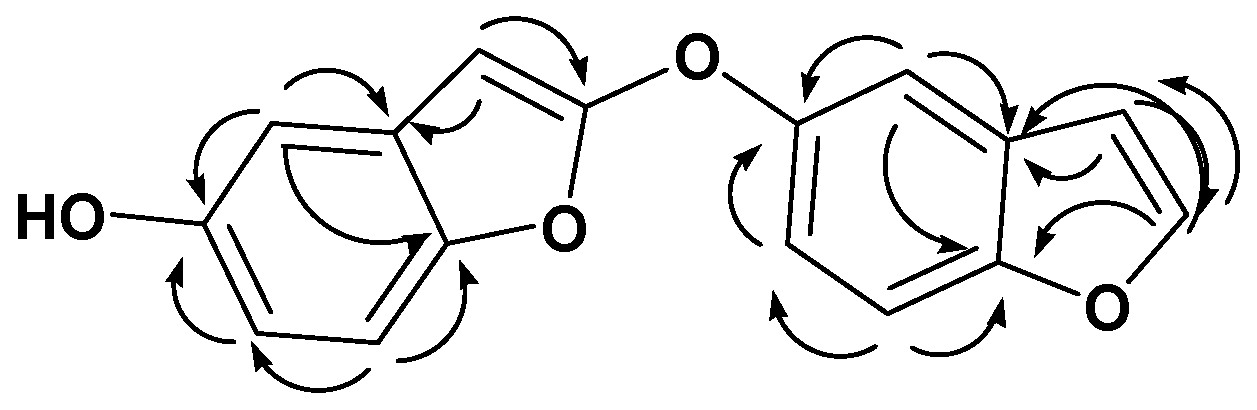


| No. | Compound | C:D | Type | Area % | RT | RI |
|---|---|---|---|---|---|---|
| 1 | Tetradecane | C14:0 | SHC | 1.06 | 5.94 | 920 |
| 2 | Nonanoic acid, 9-oxo- | C9:0 | SFA | 2.31 | 10.60 | 887 |
| 3 | Octanedioic acid (Suberic acid) | C8:0 | SFA | 0.55 | 10.81 | 904 |
| 4 | Octanoic acid, 6,6-dimethoxy- | C10:0 | SFA | 0.80 | 11.81 | 827 |
| 5 | Undecanoic acid, 10-methyl- | C12:0 | SFA | 0.34 | 12.30 | 864 |
| 6 | Nonanedioic acid (Azelaic acid) | C9:0 | SFA | 3.85 | 12.87 | 912 |
| 7 | 1-Hexadecanol | C16:0 | SFO | 2.43 | 13.64 | 943 |
| 8 | Decanedioic acid (Sebacic acid) | C10:0 | SFA | 0.99 | 14.71 | 902 |
| 9 | Tetradecanoic acid (Myristic acid) | C14:0 | SFA | 1.54 | 16.09 | 923 |
| 10 | Undecanedioic acid | C11:0 | SFA | 0.34 | 16.54 | 863 |
| 11 | Pentadecanoic acid | C15:0 | SFA | 1.30 | 17.87 | 784 |
| 12 | 9-Hexadecenoic acid | C16:1 (9) | MUFA | 2.57 | 19.21 | 915 |
| 13 | Hexadecanoic acid (Palmitic acid) | C16:0 | SFA | 8.90 * | 19.61 | 939 |
| 14 | 1-Docosene | C22:1 (1) | MUHC | 11.55 * | 20.76 | 962 |
| 15 | Heptadecanoic acid (Margaric acid) | C17:0 | SFA | 1.04 | 21.21 | 893 |
| 16 | 9,12-Octadecadienoic acid | C18:2 (9,12) | PUFA | 6.91 * | 22.31 | 923 |
| 17 | 12,15-Octadecadienoic acid | C18:2 (12,15) | PUFA | 5.72 * | 23.10 | 885 |
| 18 | 9-Octadecenoic acid | C18:1 (9) | MUFA | 2.42 | 23.16 | 921 |
| 19 | Octadecanoic acid (Stearic acid) | C18:0 | SFA | 3.84 | 23.33 | 911 |
| 20 | 6-Cis,9-cis,11-trans-octadecatrienoic acid | C18:3 (6,9,11) | PUFA | 1.84 | 24.72 | 849 |
| 21 | Cis-11-eicosenoic acid | C20:1 (11) | MUFA | 2.00 | 25.37 | 848 |
| 22 | Eicosanoic acid | C20:0 | SFA | 1.48 | 25.72 | 882 |
| 23 | 9,12,15-Octadecatrienoic acid,2,3 dihydroxy propyl ester | C21:3 (9,12,15) | Lipid | 0.30 | 26.27 | 808 |
| 24 | 9,12,15-Octadecatrienoic acid,2-(acetyloxy)-1-[(acetyloxy)methyl] ethyl ester | C25:3 (9,12,15) | Lipid | 1.49 | 26.50 | 817 |
| 25 | Nonacos-1-ene | C29:1 (1) | MUHC | 4.00 | 26.61 | 920 |
| 26 | 13-Docosenoic acid | C22:1 (13) | MUFA | 1.33 | 28.04 | 894 |
| 27 | Docosanoic acid | C22:0 | SFA | 0.26 | 28.37 | 852 |
| SFA | 27.54 | |||||
| MUFA | 8.32 | |||||
| PUFA | 14.47 | |||||
| SHC | 1.06 | |||||
| MUHC | 15.55 | |||||
| SFO | 2.43 | |||||
| Lipid | 1.79 | |||||
| Total | 71.16 | |||||
| Position | δC | δH (J in Hz) |
|---|---|---|
| 2 | 145.9, CH | 7.68, d (7.0) |
| 3 | 104.0, CH | 7.09, dd (3.0, 7.0) |
| 4 | 108.8, CH | 7.40, d (2.5) |
| 5 | 148.4, qC | |
| 6 | 114.0, CH | 6.37, dd (2.5,8.0) |
| 7 | 123.8, CH | 7.30, d (8.0) |
| 8 | 116.8, qC | |
| 9 | 157.3, qC | |
| 2′ | 148.4, qC | |
| 3′ | 144.5, CH | 7.79, d (3.0) |
| 4′ | 108.8, CH | 7.40, d (2.5) |
| 5′ | 160.8, qC | |
| 6′ | 114.0, CH | 6.37, dd (2.5,8.0) |
| 7′ | 123.8, CH | 7.30, d (8.0) |
| 8′ | 113.5, qC | |
| 9′ | 157.3, qC |
| Compounds | Energy Score (S; kcal/mol) | RMSD (Å) |
|---|---|---|
| 29 | −3.7887 | 0.9620 |
| 31 | −4.4254 | 1.2060 |
| 33 | −6.3236 | 1.2519 |
| 34 | −5.2661 | 1.1197 |
| 2AZ5 co−crystallized ligand | −5.5254 | 1.3787 |
| 28 | −4.8903 | 1.5440 |
| 30 | −4.5148 | 1.5722 |
| 32 | −4.5435 | 1.8509 |
| 35 | −5.3554 | 1.6566 |
| 36 | −5.5049 | 1.8187 |
| Compounds | Energy Score (S; kcal/mol) | RMSD (Å) |
|---|---|---|
| 28 | −5.7708 | 0.9288 |
| 29 | −4.847 | 0.5381 |
| 30 | −6.777 | 0.9806 |
| 31 | −5.5238 | 1.0294 |
| 32 | −7.019 | 0.9637 |
| 34 | 6.5779 | 1.0290 |
| 6B8Y co-crystallized ligand | −5.102 | 1.1231 |
| 33 | −7.3066 | 1.6720 |
| 35 | −5.4909 | 1.2527 |
| 36 | 2.7322 | 2.2620 |
| Compounds | Energy Score (S; kcal/mol) | RMSD (Å) |
|---|---|---|
| 29 | −3.1641 | 1.0862 |
| 30 | –3.6842 | 1.0402 |
| 32 | −3.7588 | 1.0871 |
| 34 | −4.3905 | 1.0397 |
| 35 | −4.6213 | 0.8752 |
| 6Y8M co-crystallized ligand | −4.2536 | 1.0950 |
| 28 | −3.9952 | 1.3281 |
| 31 | −4.2309 | 1.1256 |
| 33 | −4.2061 | 1.8351 |
| 36 | −5.2578 | 2.1526 |
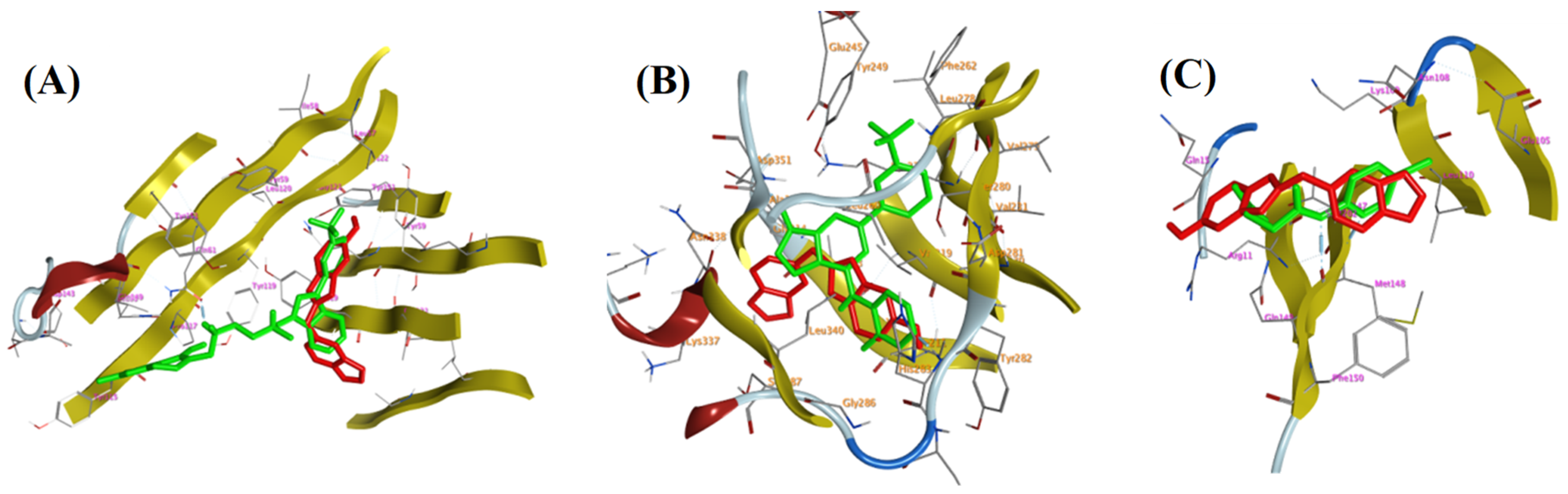
| Active Site | Ligand | Binding Energy Score (S; kcal/mol) | Ligand—Active Site Interactions | ||
|---|---|---|---|---|---|
| a. a. Residue | Bond Type | Bond Length (Å) | |||
| TNF-α (PDB ID: 2AZ5) | Co-crystallized ligand | −5.5254 | GLN 61 | H-donor | 2.97 |
| TYR 119 | H-pi | 4.08 | |||
| Compound 28 | −4.8903 | GLY 121 | H-donor | 3.11 | |
| TGFBR1 kinase (PDB ID: 6B8Y) | Co-crystallized ligand | −5.102 | ASP 351 | H-donor | 2.72 |
| HIS 283 | H-acceptor | 2.89 | |||
| LYS 232 | pi-H | 3.94 | |||
| Compound 28 | −5.7708 | VAL 219 | pi-H | 4.27 | |
| VAL 219 | pi-H | 4.15 | |||
| IL-1β (PDB ID: 6Y8M) | Co-crystallized ligand | −4.2536 | MET 148 | H-donor | 2.73 |
| MET 148 | H-acceptor | 2.94 | |||
| THR 147 | H-acceptor | 2.62 | |||
| GLN 149 | H-acceptor | 2.46 | |||
| Compound 28 | −3.9952 | MET 148 | pi-H | 4.51 | |
| MET 148 | pi-H | 4.15 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S.; et al. Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies. Antioxidants 2022, 11, 881. https://doi.org/10.3390/antiox11050881
Al-Warhi T, Zahran EM, Selim S, Al-Sanea MM, Ghoneim MM, Maher SA, Mostafa YA, Alsenani F, Elrehany MA, Almuhayawi MS, et al. Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies. Antioxidants. 2022; 11(5):881. https://doi.org/10.3390/antiox11050881
Chicago/Turabian StyleAl-Warhi, Tarfah, Eman Maher Zahran, Samy Selim, Mohammad M. Al-Sanea, Mohammed M. Ghoneim, Sherif A. Maher, Yaser A. Mostafa, Faisal Alsenani, Mahmoud A. Elrehany, Mohammed S. Almuhayawi, and et al. 2022. "Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies" Antioxidants 11, no. 5: 881. https://doi.org/10.3390/antiox11050881









