Valorization of Aloe vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Raw Materials
2.2. AVS Characterization
2.3. Microwave-Assisted Extraction (MAE)
Box-Behnken Experimental Design (BBD)
2.4. AVE Characterization
2.4.1. Extraction Yield
2.4.2. Total Phenolic Content (TPC)
2.4.3. Antioxidant Activity
2.4.3.1. DPPH Radical Scavenging Assay
2.4.3.2. FRAP Assay
2.4.4. Aloin Content Determination
2.5. Characterization of AVE Obtained under Optimum Conditions
2.5.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5.2. Thermogravimetric Analysis (TGA)
2.5.3. AVE Phenolic Profile
2.5.3.1. HPLC-MS Qualitative Analysis
2.5.3.2. HPLC-DAD Quantitative Analysis
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results and Discussion
3.1. AVS Characterization
3.2. MAE Optimization
3.2.1. Model Fitting and Analysis
3.2.2. Effect of Extraction Variables on the Extraction Yield
3.2.3. Effect of Extraction Variables on Total Phenolic and Aloin Contents
3.2.4. Effect of Extraction Variables on Antioxidant Activity
3.2.5. Optimal Extraction Conditions
3.2.6. Verification Test under Optimum Extraction Conditions
3.3. Characterization of AVE Obtained at Optimal Extraction Conditions
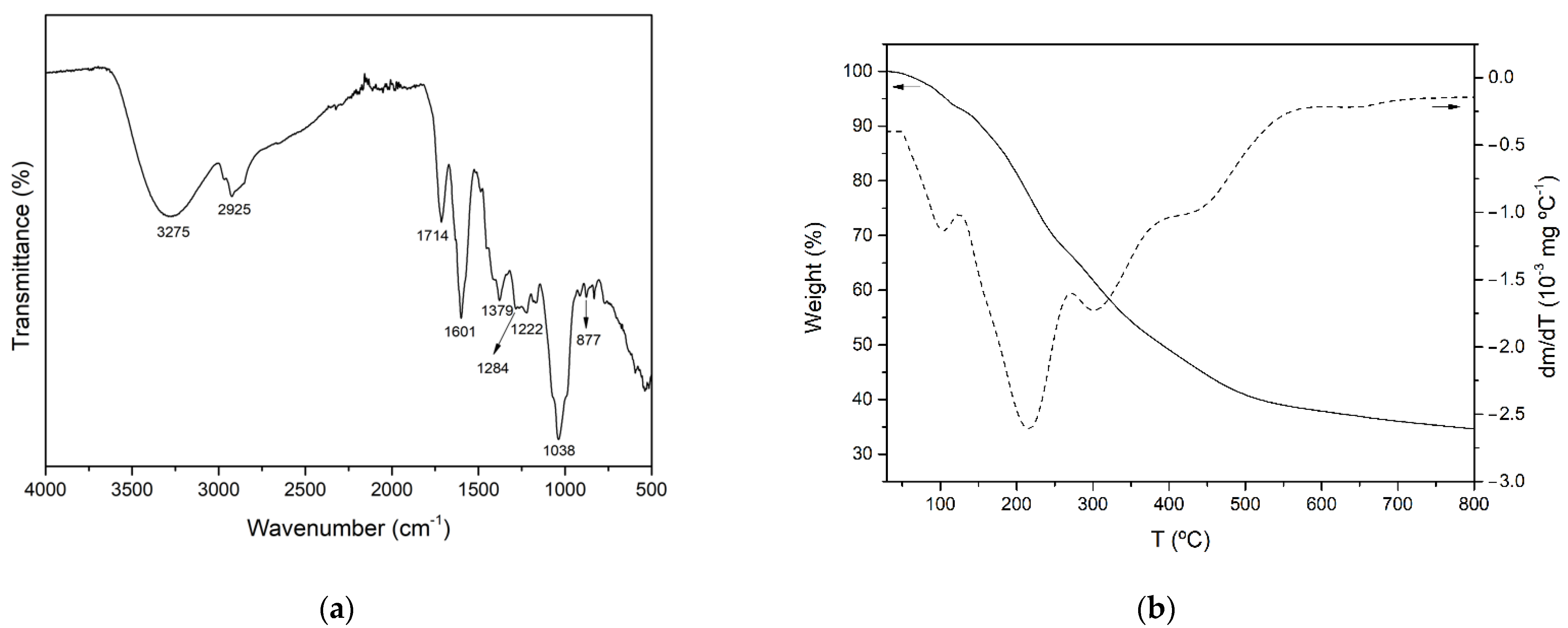
3.3.1. FTIR Analysis
3.3.2. Thermogravimetric Analysis (TGA)
3.3.3. Determination of Phenolic Profile by HPLC
| Peak 1 | tR 2 | (m/z) | Elemental Composition | Tentative Identification | Ref. |
|---|---|---|---|---|---|
| (min) | [M-H]- | ||||
| a | 10.9 | 455 | - | Unknown | [13] |
| b | 13 | 337 | C16H17O8- | cis or trans 5-p-Coumaroylquinic acid | [13] |
| 609 | C27H29O16- | luteolin-6,8-C-diglucoside | [13] | ||
| c | 13.8 | 447 | C22H23O10- | 8-O-methyl-7-hydroxyaloin | [47,84] |
| 447 | C22H23O10- | luteolin-6-C-glucoside | [13] | ||
| d | 15.2 | 433 | C21H21O10- | 7-hydroxyaloin B | [47] |
| 433 | C21H21O10- | 10-hydroxyaloin B | [13,48,76,85] | ||
| 433 | C21H21O10- | 5-hydroxyaloin B | [13] | ||
| e | 15.9 | 433 | C21H21O10- | 7-hydroxyaloin A | [47] |
| 433 | C21H21O10- | 10-hydroxyaloin A | [13,48,76,85] | ||
| 433 | C21H21O10- | 5-hydroxyaloin A | [13] | ||
| f | 17.7 | 505 | C24H25O12- | Dihydroisocoumarin glucoside | [84] |
| g | 20.2 | 459 | C23H23O10- | 6′-malonylnataloin B | [47,48] |
| h | 20.8 | 459 | C23H23O10- | 6′-malonylnataloin A | [47,48] |
| i | 24.2 | 585 | - | Unknown | [13] |
| j | 24.7 | 343 | C18H15O7- | 5,3′-Dihydroxy-6,7,4′-trimethoxyflavone | [48] |
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-López, M.L.; Romaní, A.; Cerqueira, M.A.; Rodríguez-García, R.; Jasso de Rodríguez, D.; Vicente, A.A. Compositional Features and Bioactive Properties of Whole Fraction from Aloe vera Processing. Ind. Crops Prod. 2016, 91, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Pietro Molinari, G.; Colla, G. Phytochemical Constituents and In Vitro Radical Scavenging Activity of Different Aloe Species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of Microwave-Assisted Extraction of Cocoa Bean Shell Waste and Evaluation of Its Antioxidant, Physicochemical and Functional Properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Djaković, S.; Bosiljkov, T.; Halambek, J.; Zorić, Z.; Dragović-Uzelac, V.; Petrović, M.; Rimac Brnčić, S. Valorisation of Tomato Peel Waste as a Sustainable Source for Pectin, Polyphenols and Fatty Acids Recovery Using Sequential Extraction. Waste Biomass Valori. 2020, 11, 4593–4611. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigos, M.C.; Balart, R.; Torres-Giner, S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Methods 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Baruah, A.; Bordoloi, M.; Deka Baruah, H.P. Aloe vera: A Multipurpose Industrial Crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, E.; Darias-Martín, J.; Díaz-Romero, C. Aloe vera as a Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 305–326. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, G.; Arutselvy, B.; Jacob, S. Delignification of Aloe vera Rind by Mild Acid Associated Microwave Pretreatment to Persuade Enhanced Enzymatic Saccharification. Waste Biomass Valori. 2020, 11, 5965–5975. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.-J.; Wu, X.-F.; Zhong, J.-S.; Wan, J.-Z. Effects of Temperature, PH and Light on the Stability of Aloin A and Characterisation of Its Major Degradation Products. Int. J. Food Sci. Technol. 2014, 49, 1773–1779. [Google Scholar] [CrossRef]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research Advances for the Extraction, Analysis and Uses of Anthraquinones: A Review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Pellizzoni, M.; Ruzickova, G.; Kalhotka, L.; Lucini, L. Antimicrobial Activity of Different Aloe barbadensis Mill. and Aloe arborescens Mill. Leaf Fractions. J. Med. Plant Res. 2012, 6, 1975–1981. [Google Scholar] [CrossRef]
- Lucini, L.; Pellizzoni, M.; Molinari, G. Pietro Anthraquinones and β-Polysaccharides Content and Distribution in Aloe Plants Grown under Different Light Intensities. Biochem. Syst. Ecol. 2013, 51, 264–268. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A Review of Toxicity and Adverse Clinical Effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Beland, F.A. An Evaluation of the Biological and Toxicological Properties of Aloe barbadensis (Miller), Aloe vera. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2006, 24, 103–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonta, F.; Bogoni, P.; Masotti, P.; Micali, G. High-Performance Liquid Chromatographic Profiles of Aloe Constituents and Determination of Aloin in Beverages, with Reference to the EEC Regulation for Flavouring Substances. J. Chromatogr. A 1995, 718, 99–106. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-Assisted Extractions of Active Ingredients from Plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical Analysis of Research Trends and Issues in Microwave Assisted Extraction of Phenolics: Have We Really Done Enough. TrAC-Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martínez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: New York, NY, USA, 2013; pp. 15–52. [Google Scholar]
- Kala, H.K.; Mehta, R.; Tandey, R.; Sen, K.K.; Mandal, V. Ten Years of Research on Phenolics (2005–2015): A Status Report. Pacific Sci. Rev. A Nat. Sci. Eng. 2016, 18, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Lu, Z.; Chen, G.; Guan, S.; Liu, X.; Jiang, B.; Ye, M.; Guo, D.A. Phytochemical Analysis of Traditional Chinese Medicine Using Liquid Chromatography Coupled with Mass Spectrometry. J. Chromatogr. A 2009, 1216, 2045–2062. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cao, S.-Y.; Lin, S.-J.; Zhang, J.-R.; Gan, R.-Y.; Li, H.-B. Polyphenolic Profile and Antioxidant Capacity of Extracts from Gordonia Axillaris Fruits. Antioxidants 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef] [Green Version]
- Radojković, M.; Moreira, M.M.; Soares, C.; Fátima Barroso, M.; Cvetanović, A.; Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C. Microwave-Assisted Extraction of Phenolic Compounds from Morus Nigra Leaves: Optimization and Characterization of the Antioxidant Activity and Phenolic Composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The Extraction of Bioactive Compounds from Food Residues Using Microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe vera (Aloe barbadensis Miller) Plant Tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-Assisted Extraction of Phenolic Acids and Flavonoids and Production of Antioxidant Ingredients from Tomato: A Nutraceutical-Oriented Optimization Study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- López, A.; Suárez de Tangil, M.; Vega-Orellana, O.; Ramírez, A.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (Syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Asnin, L.; Assefa, A.D.; Ko, E.Y.; Sharma, K.; Park, S.W. Extraction of Antioxidants from Aloe vera Leaf Gel: A Response Surface Methodology Study. Food Anal. Methods 2014, 7, 1804–1815. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Hu, Q. Evaluation of Antioxidant Potential of Aloe vera (Aloe barbadensis Miller) Extracts. J. Agric. Food Chem. 2003, 51, 7788–7791. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.N.; Yu, R.; Kuan, C.H.; Finley, J.; Mudge, E.M.; Dentali, S. Determination of Aloin A and Aloin B in Aloe vera Raw Materials and Finished Products by High-Performance Liquid Chromatography: Single-Laboratory Validation. J. AOAC Int. 2014, 97, 1323–1328. [Google Scholar] [CrossRef]
- Lee, S.; Do, S.-G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass Spectrometry-Based Metabolite Profiling and Antioxidant Activity of Aloe vera (Aloe barbadensis Miller) in Different Growth Stages. J. Agric. Food Chem. 2012, 60, 11222–11228. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 6123850. [Google Scholar] [CrossRef] [Green Version]
- Zapata, P.J.; Navarro, D.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M. Characterisation of Gels from Different Aloe Spp. as Antifungal Treatment: Potential Crops for Industrial Applications. Ind. Crops Prod. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Ray, A.; Gupta, S.D.; Ghosh, S. Evaluation of Anti-Oxidative Activity and UV Absorption Potential of the Extracts of Aloe vera L. Gel from Different Growth Periods of Plants. Ind. Crops Prod. 2013, 49, 712–719. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, M.; Yadav, A.; Yadav, J.P. Impact of Spatial and Climatic Conditions on Phytochemical Diversity and in Vitro Antioxidant Activity of Indian Aloe vera (L.) Burm.F. S. Afr. J. Bot. 2017, 111, 50–59. [Google Scholar] [CrossRef]
- Ng, Z.X.; Yong, P.H.; Lim, S.Y. Customized Drying Treatments Increased the Extraction of Phytochemicals and Antioxidant Activity from Economically Viable Medicinal Plants. Ind. Crops Prod. 2020, 155, 112815. [Google Scholar] [CrossRef]
- Shi, X.-D.; Nie, S.-P.; Yin, J.-Y.; Que, Z.-Q.; Zhang, L.-J.; Huang, X.-J. Polysaccharide from Leaf Skin of Aloe barbadensis Miller: Part I. Extraction, Fractionation, Physicochemical Properties and Structural Characterization. Food Hydrocoll. 2017, 73, 176–183. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of Microwave-Assisted Extraction of Polyphenols from Myrtus communis L. Leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Radovanović, N.; Ćorović, M.; Šiler-Marinković, S.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. Optimisation of Microwave-Assisted Extraction Parameters for Antioxidants from Waste Achillea millefolium Dust. Ind. Crops Prod. 2015, 77, 333–341. [Google Scholar] [CrossRef]
- Lin, D.; Ma, Q.; Zhang, Y.; Peng, Z. Phenolic Compounds with Antioxidant Activity from Strawberry Leaves: A Study on Microwave-Assisted Extraction Optimization. Prep. Biochem. Biotechnol. 2020, 50, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Djemaa-Landri, K.; Hamri-Zeghichi, S.; Valls, J.; Cluzet, S.; Tristan, R.; Boulahbal, N.; Kadri, N.; Madani, K. Phenolic Content and Antioxidant Activities of Vitis Vinifera L. Leaf Extracts Obtained by Conventional Solvent and Microwave-Assisted Extractions. J. Food Meas. Charact. 2020, 14, 3551–3564. [Google Scholar] [CrossRef]
- Rudić, S.; Dimitrijević-Branković, S.; Dimitrijević, S.; Milić, M. Valorization of Unexploited Artichoke Leaves Dust for Obtaining of Extracts Rich in Natural Antioxidants. Sep. Purif. Technol. 2021, 256, 117714. [Google Scholar] [CrossRef]
- Chang, X.L.; Chen, B.Y.; Feng, Y.M. Water-Soluble Polysaccharides Isolated from Skin Juice, Gel Juice and Flower of Aloe vera Miller. J. Taiwan Inst. Chem. Eng. 2011, 42, 197–203. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Tong, F.; Xin, X.-L.; Aisa, H.A. Optimization of Microwave-Assisted Extraction Using Response Surface Methodology and the Potential Anti-Diabetic Efficacy of Nigella glandulifera Freyn Determined Using the Spectrum–Effect Relationship. Ind. Crops Prod. 2020, 153, 112592. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea Leaves as the Source of Phenolic Compounds, Antioxidants, and Anti-Diabetic Activity Using Microwave-Assisted Extraction Technique. Ind. Crops Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on Phenolic Compounds Stability during Microwave-Assisted Extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milutinović, M.; Radovanović, N.; Rajilić-Stojanović, M.; Šiler-Marinković, S.; Dimitrijević, S.; Dimitrijević-Branković, S. Microwave-Assisted Extraction for the Recovery of Antioxidants from Waste Equisetum Arvense. Ind. Crops Prod. 2014, 61, 388–397. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Concentration-Dependence of Prooxidant and Antioxidant Effects of Aloin and Aloe-Emodin on DNA. Food Chem. 2005, 91, 413–418. [Google Scholar] [CrossRef]
- Sánchez, J.T.; García, A.V.; Martínez-Abad, A.; Vilaplana, F.; Jiménez, A.; Garrigós, M.C. Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe vera Gel. Foods 2020, 9, 1248. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alsaggaf, A.H.A.; Ahmed, N. LC-QTOF-MS Analysis of Phenolics and Saponins Extracted from Aloe vera Leaves via Microwave Technology in Optimal Condition. S. Afr. J. Bot. 2021, 139, 362–373. [Google Scholar] [CrossRef]
- Barbosa, R.; Villarreal, A.; Rodriguez, C.; De Leon, H.; Gilkerson, R.; Lozano, K. Aloe vera Extract-Based Composite Nanofibers for Wound Dressing Applications. Mater. Sci. Eng. C 2021, 124, 112061. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Herrera, R.; Labidi, J.; Gullón, P. Yerba Mate Waste: A Sustainable Resource of Antioxidant Compounds. Ind. Crops Prod. 2018, 113, 398–405. [Google Scholar] [CrossRef]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and Characterization of Aloe vera Blended Collagen-Chitosan Composite Scaffold for Tissue Engineering Applications. ACS Appl. Mater. Inter. 2013, 5, 7291–7298. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release Kinetics of Rosemary (Rosmarinus officinalis) Polyphenols from Polyvinyl Alcohol (PVA) Electrospun Nanofibers in Several Food Simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun Chitosan-Based Nanofiber Mats Loaded with Garcinia Mangostana Extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.d.F.; Correa, D.S. Biocompatible and Biodegradable Electrospun Nanofibrous Membranes Loaded with Grape Seed Extract for Wound Dressing Application. J. Nanomater. 2019, 2019, 2472964. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Ding, W.; Zhong, J.; Wan, J.; Xie, Z. Simultaneous Qualitative and Quantitative Determination of Phenolic Compounds in Aloe Barbadensis Mill by Liquid Chromatography–Mass Spectrometry-Ion Trap-Time-of-Flight and High Performance Liquid Chromatography-Diode Array Detector. J. Pharm. Biomed. Anal. 2013, 80, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Y.N.; Lee, M.J.; Kim, Y.H.; Lee, W.; Kim, K.H.; Kim, K.T.; Kang, J.S. Identification and Discrimination of Three Common Aloe Species by High Performance Liquid Chromatography–Tandem Mass Spectrometry Coupled with Multivariate Analysis. J. Chromatogr. B 2016, 1031, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Park, J.H.; Kim, N.Y.; Shin, Y.G.; Choi, Y.S.; Gyun, J.; Kim, K.H.; Lee, S.K. Analysis of 13 Phenolic Compounds in Aloe Species by High Performance Liquid Chromatography. Phytochem. Anal. 1998, 9, 186–191. [Google Scholar] [CrossRef]
- Kanama, S.K.; Viljoen, A.M.; Kamatou, G.P.P.P.; Chen, W.; Sandasi, M.; Adhami, H.R.; Van Wyk, B.E. Simultaneous Quantification of Anthrones and Chromones in Aloe Ferox (“Cape Aloes”) Using UHPLC-MS. Phytochem. Lett. 2015, 13, 85–90. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe Ferox Leaf Gel Phytochemical Content, Antioxidant Capacity, and Possible Health Benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef]
- Debnath, T.; Ghosh, M.; Lee, Y.M.; Nath, N.C.D.; Lee, K.-G.; Lim, B.O. Identification of Phenolic Constituents and Antioxidant Activity of Aloe barbadensis Flower Extracts. Food Agric. Immunol. 2018, 29, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, H.; Guo, X.; Abbasi, A.M.; Wang, T.; Li, T.; Fu, X.; Li, J.; Liu, R.H. Comparison of Phytochemical Profiles, Antioxidant and Cellular Antioxidant Activities of Seven Cultivars of Aloe. Int. J. Food Sci. Technol. 2016, 51, 1489–1494. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS Characterization of Bioactive Metabolites from Two Yemeni Aloe Spp. with Antioxidant and Antidiabetic Properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Zhong, J.-S.; Wan, J.-Z.; Ding, W.-J.; Wu, X.-F.; Xie, Z.-Y. Multi-Responses Extraction Optimization Combined with High-Performance Liquid Chromatography-Diode Array Detection–Electrospray Ionization-Tandem Mass Spectrometry and Chemometrics Techniques for the Fingerprint Analysis of Aloe barbadensis Miller. J. Pharm. Biomed. Anal. 2015, 107, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of Bioactive Secondary Metabolites and Antioxidant Capacity of Leaf Exudates from Eighteen Aloe Species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The Effect of Different Extraction Techniques on Extraction Yield, Total Phenolic, and Anti-Radical Capacity of Extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]





| Leaf Dimensions 1 | |
| Length (cm) | 64.9 ± 3.7 |
| Width at base (cm) | 12.8 ± 0.8 |
| Thickness (cm) | 2.8 ± 0.3 |
| Waste yield 1,2 | |
| Weight (g) | 780 ± 90 |
| Skin waste (%) | 15.1 ± 2.1 |
| Chemical characterization of AVS 3 | |
| Moisture (g 100 g FW−1) | 84.9 ± 0.8 |
| Ash (g 100 g DW−1) | 15.5 ± 0.1 |
| Protein (g 100 g DW−1) | 6.5 ± 0.2 |
| Lipids (g 100 g DW−1) | 2.4 ± 0.1 |
| Experimental Domain | Response Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Run | Et (%, v/v) | T (°C) | t (min) | V (mL) | Yield (gAVE 100 gAVS−1) | TPC (mgGAE gAVE−1) | DPPH (mgTE gAVE−1) | FRAP (mgTE gAVE−1) | Aloin (mg gAVE−1) |
| 1 | 60 | 80 | 22.5 | 80 | 24.2 | 104.9 ± 1.8 | 59.6 ± 5.1 | 110.7 ± 3.2 | 46.1 ± 0.2 |
| 2 | 40 | 60 | 5.0 | 65 | 24.3 | 86.5 ± 0.9 | 51.4 ± 6.1 | 95.2 ± 3.7 | 39.2 ± 0.2 |
| 3 | 60 | 80 | 22.5 | 50 | 18.8 | 102.8 ± 1.4 | 65.0 ± 6.3 | 120.2 ± 4.2 | 50.3 ± 0.1 |
| 4 | 80 | 60 | 22.5 | 50 | 18.4 | 122.4 ± 1.2 | 68.4 ± 5.3 | 119.3 ± 3.2 | 53.0 ± 0.3 |
| 5 | 40 | 60 | 22.5 | 50 | 22.9 | 91.1 ± 0.4 | 53.5 ± 6.2 | 101.7 ± 1.7 | 42.0 ± 0.1 |
| 6 | 60 | 60 | 40.0 | 50 | 22.5 | 102.4 ± 0.5 | 58.7 ± 4.9 | 109.9 ± 3.8 | 42.7 ± 0.2 |
| 7 | 40 | 80 | 22.5 | 65 | 25.4 | 91.0 ± 0.9 | 61.6 ± 5.2 | 93.0 ± 2.0 | 39.0 ± 0.2 |
| 8 | 60 | 40 | 22.5 | 50 | 21.1 | 101.3 ± 0.5 | 54.9 ± 5.9 | 116.2 ± 3.5 | 48.2 ± 0.3 |
| 9 | 80 | 60 | 22.5 | 80 | 18.2 | 121.3 ± 1.7 | 61.6 ± 4.5 | 132.1 ± 0.7 | 54.2 ± 0.3 |
| 10 | 80 | 60 | 40.0 | 65 | 18.7 | 114.9 ± 1.6 | 59.3 ± 5.0 | 130.7 ± 3.6 | 56.6 ± 0.5 |
| 11 | 60 | 60 | 22.5 | 65 | 24.2 | 105.4 ± 1.1 | 58.3 ± 4.2 | 110.5 ± 5.7 | 46.9 ± 0.4 |
| 12 | 60 | 40 | 40.0 | 65 | 23.2 | 103.6 ± 1.0 | 54.4 ± 6.5 | 108.7 ± 4.2 | 44.5 ± 0.3 |
| 13 | 60 | 60 | 22.5 | 65 | 23.3 | 102.2 ± 1.1 | 57.8 ± 2.1 | 113.8 ± 3.0 | 46.1 ± 0.0 |
| 14 | 60 | 60 | 5.0 | 80 | 23.9 | 98.5 ± 1.1 | 55.2 ± 5.5 | 113.6 ± 3.9 | 45.4 ± 0.2 |
| 15 | 80 | 40 | 22.5 | 65 | 18.8 | 121.4 ± 1.1 | 68.1 ± 5.1 | 134.1 ± 2.8 | 57.5 ± 0.2 |
| 16 | 60 | 60 | 22.5 | 65 | 23.6 | 104.0 ± 1.3 | 55.2 ± 4.9 | 113.3 ± 4.3 | 47.1 ± 0.4 |
| 17 | 80 | 60 | 5.0 | 65 | 20.3 | 117.9 ± 1.2 | 64.3 ± 4.3 | 116.3 ± 5.1 | 52.9 ± 0.1 |
| 18 | 60 | 80 | 40.0 | 65 | 22.7 | 103.6 ± 1.4 | 62.5 ± 4.4 | 118.8 ± 1.9 | 47.1 ± 0.1 |
| 19 | 80 | 80 | 22.5 | 65 | 19.4 | 125.8 ± 1.3 | 73.4 ± 4.6 | 131.5 ± 6.0 | 53.3 ± 0.3 |
| 20 | 40 | 60 | 40.0 | 65 | 25.1 | 89.3 ± 0.4 | 49.9 ± 5.4 | 90.5 ± 2.4 | 36.5 ± 0.1 |
| 21 | 40 | 60 | 22.5 | 80 | 26.3 | 87.4 ± 0.7 | 50.5 ± 5.6 | 99.6 ± 2.5 | 41.0 ± 0.1 |
| 22 | 60 | 60 | 5.0 | 50 | 21.0 | 116.3 ± 2.2 | 62.1 ± 2.9 | 110.4 ± 1.8 | 44.8 ± 0.4 |
| 23 | 60 | 60 | 22.5 | 65 | 24.7 | 106.4 ± 0.9 | 58.8 ± 6.1 | 108.2 ± 1.0 | 43.5 ± 0.2 |
| 24 | 60 | 40 | 22.5 | 80 | 23.4 | 102.7 ± 2.4 | 53.5 ± 6.2 | 114.6 ± 5.4 | 47.6 ± 0.5 |
| 25 | 60 | 60 | 22.5 | 65 | 24.9 | 108.7 ± 2.3 | 59.1 ± 4.7 | 109.6 ± 9.5 | 43.0 ± 0.3 |
| 26 | 60 | 40 | 5.0 | 65 | 22.1 | 109.4 ± 1.2 | 61.2 ± 7.0 | 109.3 ± 0.2 | 45.6 ± 0.3 |
| 27 | 40 | 40 | 22.5 | 65 | 24.8 | 88.8 ± 0.3 | 53.9 ± 6.2 | 97.7 ± 3.8 | 38.4 ± 0.3 |
| 28 | 60 | 60 | 40.0 | 80 | 23.8 | 99.1 ± 0.6 | 54.1 ± 4.2 | 119.7 ± 14.1 | 44.3 ± 0.1 |
| 29 | 60 | 80 | 5.0 | 65 | 22.8 | 96.8 ± 1.2 | 53.4 ± 5.8 | 106.3 ± 3.5 | 44.4 ± 0.4 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Yield | |||||
| A | 102.08 | 1 | 102.08 | 215.82 | 0.0001 * |
| B | 0.00 | 1 | 0.00 | 0.00 | 0.9685 |
| C | 0.21 | 1 | 0.21 | 0.45 | 0.5386 |
| D | 19.00 | 1 | 19.00 | 40.17 | 0.0032 * |
| AA | 13.00 | 1 | 13.00 | 27.49 | 0.0063 * |
| AB | 0.00 | 1 | 0.00 | 0.00 | 1.0000 |
| AC | 1.44 | 1 | 1.44 | 3.04 | 0.1560 |
| AD | 3.24 | 1 | 3.24 | 6.85 | 0.0590 |
| BB | 5.29 | 1 | 5.29 | 11.19 | 0.0287 * |
| BC | 0.36 | 1 | 0.36 | 0.76 | 0.4322 |
| BD | 2.40 | 1 | 2.40 | 5.08 | 0.0873 |
| CC | 1.26 | 1 | 1.26 | 2.67 | 0.1779 |
| CD | 0.64 | 1 | 0.64 | 1.35 | 0.3094 |
| DD | 9.01 | 1 | 9.01 | 19.04 | 0.0120 * |
| Lack-of-fit | 6.02 | 10 | 0.60 | 1.27 | 0.4399 |
| Pure error | 1.89 | 4 | 0.47 | ||
| Total (corr.) | 157.55 | 28 | |||
| R2 | 0.9498 | ||||
| Adj R2 | 0.8995 | ||||
| TPC | |||||
| A | 2995.68 | 1 | 2995.68 | 497.79 | 0.0000 * |
| B | 0.44 | 1 | 0.44 | 0.07 | 0.8001 |
| C | 13.02 | 1 | 13.02 | 2.16 | 0.2153 |
| D | 41.81 | 1 | 41.81 | 6.95 | 0.0578 |
| AA | 1.15 | 1 | 1.15 | 0.19 | 0.6842 |
| AB | 1.21 | 1 | 1.21 | 0.20 | 0.6771 |
| AC | 8.41 | 1 | 8.41 | 1.40 | 0.3026 |
| AD | 1.69 | 1 | 1.69 | 0.28 | 0.6242 |
| BB | 0.55 | 1 | 0.55 | 0.09 | 0.7777 |
| BC | 39.69 | 1 | 39.69 | 6.60 | 0.0621 |
| BD | 0.12 | 1 | 0.12 | 0.02 | 0.8934 |
| CC | 26.36 | 1 | 26.36 | 4.38 | 0.1045 |
| CD | 52.56 | 1 | 52.56 | 8.73 | 0.0417 * |
| DD | 1.81 | 1 | 1.81 | 0.30 | 0.6125 |
| Lack-of-fit | 212.62 | 10 | 21.26 | 3.53 | 0.1176 |
| Pure error | 24.07 | 4 | 6.02 | ||
| Total (corr.) | 3422.55 | 28 | |||
| R2 | 0.9308 | ||||
| Adj R2 | 0.8617 | ||||
| DPPH | |||||
| A | 460.04 | 1 | 460.04 | 189.86 | 0.0002 * |
| B | 72.52 | 1 | 72.52 | 29.93 | 0.0054 * |
| C | 6.31 | 1 | 6.31 | 2.60 | 0.1819 |
| D | 65.80 | 1 | 65.80 | 27.16 | 0.0065 * |
| AA | 20.94 | 1 | 20.94 | 8.64 | 0.0424 * |
| AB | 1.44 | 1 | 1.44 | 0.59 | 0.4838 |
| AC | 3.06 | 1 | 3.06 | 1.26 | 0.3238 |
| AD | 3.61 | 1 | 3.61 | 1.49 | 0.2893 |
| BB | 40.43 | 1 | 40.43 | 16.69 | 0.0150 * |
| BC | 63.20 | 1 | 63.20 | 26.08 | 0.0069 * |
| BD | 4.00 | 1 | 4.00 | 1.65 | 0.2682 |
| CC | 22.88 | 1 | 22.88 | 9.44 | 0.0372 * |
| CD | 1.32 | 1 | 1.32 | 0.55 | 0.5010 |
| DD | 1.99 | 1 | 1.99 | 0.82 | 0.4165 |
| Lack-of-fit | 108.49 | 10 | 10.85 | 4.48 | 0.0808 |
| Pure error | 9.69 | 4 | 2.42 | ||
| Total (corr.) | 902.51 | 28 | |||
| R2 | 0.8691 | ||||
| Adj R2 | 0.7381 | ||||
| FRAP | |||||
| A | 2892.31 | 1 | 2892.31 | 499.79 | 0.0000 * |
| B | 0.00 | 1 | 0.00 | 0.00 | 0.9910 |
| C | 61.65 | 1 | 61.65 | 10.65 | 0.0310 * |
| D | 13.23 | 1 | 13.23 | 2.29 | 0.2051 |
| AA | 0.71 | 1 | 0.71 | 0.12 | 0.7432 |
| AB | 1.10 | 1 | 1.10 | 0.19 | 0.6850 |
| AC | 91.20 | 1 | 91.20 | 15.76 | 0.0165 * |
| AD | 55.50 | 1 | 55.50 | 9.59 | 0.0363 * |
| BB | 28.42 | 1 | 28.42 | 4.91 | 0.0910 |
| BC | 42.90 | 1 | 42.90 | 7.41 | 0.0528 |
| BD | 15.60 | 1 | 15.60 | 2.70 | 0.1759 |
| CC | 22.66 | 1 | 22.66 | 3.92 | 0.1189 |
| CD | 10.89 | 1 | 10.89 | 1.88 | 0.2420 |
| DD | 56.67 | 1 | 56.67 | 9.79 | 0.0352 * |
| Lack-of-fit | 214.98 | 10 | 21.50 | 3.71 | 0.1088 |
| Pure error | 23.15 | 4 | 5.79 | ||
| Total (corr.) | 3548.47 | 28 | |||
| R2 | 0.9329 | ||||
| Adj R2 | 0.8658 | ||||
| aloin | |||||
| A | 696.16 | 1 | 696.16 | 186.04 | 0.0002 * |
| B | 0.21 | 1 | 0.21 | 0.06 | 0.8230 |
| C | 0.03 | 1 | 0.03 | 0.01 | 0.9330 |
| D | 0.48 | 1 | 0.48 | 0.13 | 0.7383 |
| AA | 11.79 | 1 | 11.79 | 3.15 | 0.1505 |
| AB | 5.76 | 1 | 5.76 | 1.54 | 0.2825 |
| AC | 10.24 | 1 | 10.24 | 2.74 | 0.1734 |
| AD | 1.21 | 1 | 1.21 | 0.32 | 0.6000 |
| BB | 8.55 | 1 | 8.55 | 2.29 | 0.2051 |
| BC | 3.61 | 1 | 3.61 | 0.96 | 0.3816 |
| BD | 3.24 | 1 | 3.24 | 0.87 | 0.4048 |
| CC | 7.87 | 1 | 7.87 | 2.10 | 0.2205 |
| CD | 0.25 | 1 | 0.25 | 0.07 | 0.8088 |
| DD | 4.67 | 1 | 4.67 | 1.25 | 0.3266 |
| Lack-of-fit | 37.29 | 10 | 3.73 | 1.00 | 0.5501 |
| Pure error | 14.97 | 4 | 3.74 | ||
| Total (corr.) | 809.15 | 28 | |||
| R2 | 0.9354 | ||||
| Adj R2 | 0.8708 |
| Response | Et (%) | T (°C) | t (min) | V (mL) | Predicted Value |
|---|---|---|---|---|---|
| Yield | 40.0 | 67.7 | 26.7 | 80.0 | 26.8 g AVE 100 g AVS−1 |
| TPC | 80.0 | 40.0 | 5.0 | 56.0 | 127.4 mg GAE g AVE−1 |
| DPPH | 80.0 | 80.0 | 40.0 | 52.7 | 73.4 mg TE gAVE−1 |
| FRAP | 80.0 | 54.4 | 39.9 | 80.0 | 140.5 mg TE gAVE−1 |
| aloinMAX | 80.0 | 40.0 | 29.4 | 80.0 | 59.0 mg gAVE−1 |
| aloinMIN | 40.0 | 40.3 | 40.0 | 61.8 | 35.4 mg gAVE−1 |
| Peak 1 | Compound | (m/z) [M-H]- | tR (min) | Calibration Range (mg kg−1) | Linearity (R2) | LOD (mg kg−1) | LOQ (mg kg−1) | RSD 2 (%) | AVE (mg 100 gAVS−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | aloesin | 393 | 9.3 | 0.06–61.80 | 0.9998 | 0.164 | 0.546 | 2.6 | 292.6 ± 0.5 |
| 2 | chlorogenic acid | 353 | 10.5 | 0.05–99.70 | 0.9960 | 0.213 | 0.711 | 1.3 | 80.0 ± 0.2 |
| 3 | orientin | 447 | 14.4 | 0.01–11.20 | 0.9991 | 0.061 | 0.203 | 2.3 | 46.5 ± 0.1 |
| 4 | aloeresin D | 555 | 19.1 | 0.05–9.82 | 0.9971 | 0.383 | 1.275 | 2.6 | 39.7 ± 1.1 |
| 5 | aloin B | 417 | 19.2 | 0.10–100.60 | 0.9998 | 0.087 | 0.292 | 1.1 | 308.1 ± 0.6 |
| 6 | aloin A | 417 | 20.0 | 0.20–202.10 | 0.9999 | 0.278 | 0.926 | 0.9 | 702.0 ± 2.0 |
| 7 | cinnamic acid | 147 | 25.2 | 0.004–3.700 | 0.9992 | 0.029 | 0.095 | 1.4 | 13.6 ± 0.5 |
| 8 | aloe emodin | 269 | 29.4 | 0.001–0.900 | 0.9961 | 0.018 | 0.061 | 1.8 | 3.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solaberrieta, I.; Jiménez, A.; Garrigós, M.C. Valorization of Aloe vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants 2022, 11, 1058. https://doi.org/10.3390/antiox11061058
Solaberrieta I, Jiménez A, Garrigós MC. Valorization of Aloe vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants. 2022; 11(6):1058. https://doi.org/10.3390/antiox11061058
Chicago/Turabian StyleSolaberrieta, Ignacio, Alfonso Jiménez, and María Carmen Garrigós. 2022. "Valorization of Aloe vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition" Antioxidants 11, no. 6: 1058. https://doi.org/10.3390/antiox11061058