Singular Olive Oils from a Recently Discovered Spanish North-Western Cultivar: An Exhaustive 3-Year Study of Their Chemical Composition and In-Vitro Antidiabetic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling
2.3. Olive Oil Extraction
2.4. Olive Oil Characterisation
2.4.1. Quality Parameters
2.4.2. Fatty Acid, Sterol, and Triterpene Dialcohol Composition
2.4.3. Tocopherol Composition
2.4.4. Total Phenols, o-Diphenols, and Antioxidant Capacity
2.5. Evaluation of the Phenolic Profile
2.5.1. Extraction Protocol
2.5.2. LC-MS Analysis
2.6. Assessment of the In-Vitro Antidiabetic Activity
2.7. Statistical Analysis
3. Results
3.1. Effect of Crop Year on Quality-Related Parameters and Sensory Attributes
3.2. Effect of Crop Year on Olive Oil Genuineness-Related Parameters
3.3. Effect of Crop Year on Other Quality-Related Parameters Not Included in Current European Regulations
3.4. Effect of Crop Year on Olive Oil Phenolic Compounds
3.4.1. Total Phenolic Content by Spectrophotometric Methods
3.4.2. Phenolic Content by LC/MS-MS
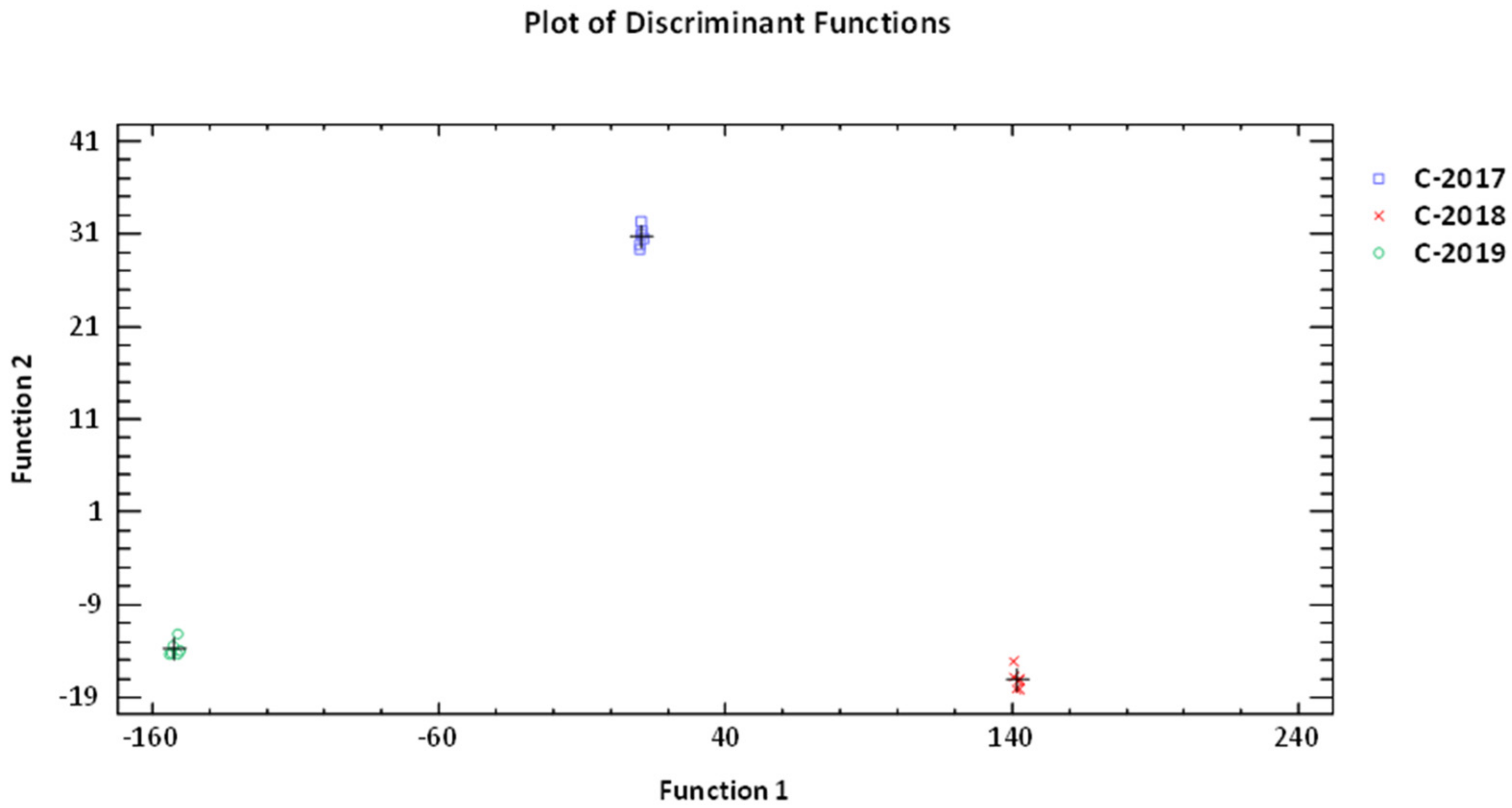
3.4.3. Chemometric Evaluation
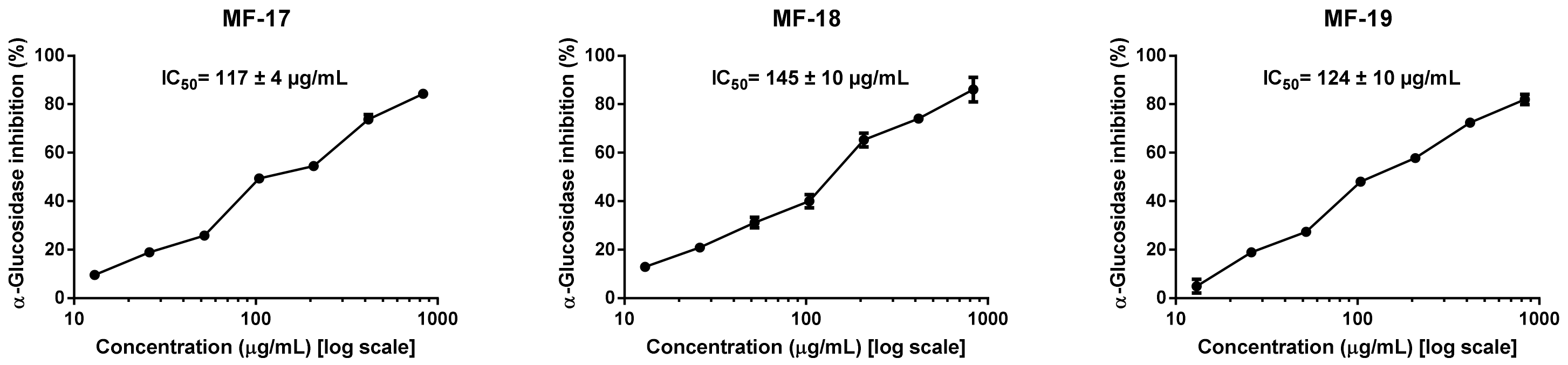
3.5. Effect of Crop Year on Olive Oil Antidiabetic Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gago, P.; Santiago, J.L.; Boso, S.; Martínez, M.C. The forgotten, ancient olive trees of the Spanish northwest: A first molecular and botanical analysis. Span. J. Agric. Res. 2019, 17, e0702. [Google Scholar] [CrossRef]
- Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P. Bases científicas para la creación de una DOP o IGP ‘Aceites de Galicia’. Almazaras 2019, 16. [Google Scholar]
- Zamuz, S.; Purriños, L.; Tomasevic, I.; Domínguez, R.; Brnčić, M.; Barba, J.F.; Lorenzo, J.M. Consumer acceptance and quality parameters of the commercial olive oils manufactured with cultivars grown in Galicia (NW Spain). Foods 2020, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.; Yadav, S.; Garrido-Maestu, A.; Azinheiro, S.; Trujillo, I.; Barros-Velázquez, J.; Prado, M. Evaluation of simple sequence repeats (SSR) and single nucleotide polymorphism (SNP)-based methods in olive varieties from the Northwest of Spain and potential for miniaturization. Food Chem. Mol. Sci. 2021, 3, 100038. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J.; Trujillo, I. Genotypic and phenotypic identification of olive cultivars from north-western Spain and characterization of their extra virgin olive oils in terms of fatty acid composition and minor compounds. Sci. Hortic. 2018, 232, 269–279. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gándara, J. Blending local olive oils with Arbequina or Picual oils produces high quality, distinctive EVOOs. Eur. J. Lipid Sci. Technol. 2015, 117, 1238–1247. [Google Scholar] [CrossRef]
- Salimonti, A.; Simeone, V.; Cesari, G.; Lamaj, F.; Cattivelli, L.; Perri, E.; Desiderio, F.; Fanizzi, F.P.; Del Coco, L.; Zelasco, S. A first molecular investigation of monumental olive trees in Apulia region. Sci. Hortic. 2013, 162, 204–212. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Simal-Gandara, J. Characterization of virgin olive oils produced with autochthonous Galician varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State-of-the-art on functional virgin olive oils enriched with bioactive compounds and their properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar] [CrossRef] [Green Version]
- Etxeberría, U.; de la Garza, A.L.; Campion, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo-González, M.; Grosso, C.; Valentão, P.; Andrade, P.B. α-Glucosidase and α-amylase inhibitors from Myrcia spp.: A stronger alternative to acarbose? J. Pharm. Biomed. Anal. 2016, 118, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B.; Simal-Gándara, J. The involvement of phenolic-rich extracts from Galician autochthonous extra-virgin olive oils against the α-glucosidase and α-amylase inhibition. Food Res. Int. 2019, 116, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2006, 32, 15–31. [Google Scholar] [CrossRef]
- Hadrich, F.; Bouallagui, Z.; Junkyu, H.; Isoda, H.; Sayadi, S. The α-glucosidase and α- amylase enzyme inhibitory of hydroxytyrosol and oleuropein. J. Oleo Sci. 2015, 64, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loizzo, M.R.; Di Lecce, G.; Boselli, E.; Menichini, F.; Frega, N.G. Inhibitory activity of phenolic compounds from extra virgin olive oils on the enzymes involved in diabetes, obesity and hypertension. J. Food Biochem. 2011, 35, 381–399. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and silico structure-activity relationship study. J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Sun, W.I.; Xing, Y.; Xiu, Z.I.; Zhuang, C.I.; Dong, Y.S. Dietary flavonoids and acarbose synergistically inhibit α-glucosidase and lower postprandial blood glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Reboredo-Rodríguez, P.; González-Barreiro, C.; Simal-Gándara, J.; Valentão, P.; Carrasco-Pancorbo, A.; Andrade, P.B.; Cancho-Grande, B. Evaluation of the neuroprotective and antidiabetic potential of phenol-rich extracts from virgin olive oils by in vitro assays. Food Res. Int. 2018, 106, 558–567. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Olmo-García, L.; Figueiredo-González, M.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Effect of olive ripening degree on the antidiabetic potential of biophenols-rich extracts of Brava Gallega virgin olive oils. Food Res. Int. 2020, 137, 109427. [Google Scholar] [CrossRef]
- Lukić, M.; Lukić, I.; Krapac, M.; Sladonja, B.; Piližota, V. Sterols and triterpene diols in olive oil as indicators of variety and degree of ripening. Food Chem. 2013, 136, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Miho, H.; Díez, C.M.; Mena-Bravo, A.; Sánchez de Medina, V.; Moral, J.; Melliou, E.; Magiatis, P.; Rallo, L.; Barranco, D.; Priego-Capote, F. Cultivar influence on variability in olive oil phenolic profiles determined through and extensive germplasm survey. Food Chem. 2018, 266, 192–199. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A.; Del Caro, A.; Piga, A.; Vacca, V.; Caboni, M.F.; Gallina Toschi, T. Preliminary characterisation of virgin olive oils obtained from different cultivars in Sardinia. Eur. Food Res. Technol. 2006, 222, 354–361. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic compounds profile of Cornicabra virgin olive oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Salvador, M.D. Chemical and sensory characteristics of extra virgin olive oils produced in central Iberian Peninsula under the protected designation of origin quality scheme. Eur. J. Lipid Sci. Technol. 2019, 121, 1800134. [Google Scholar] [CrossRef]
- Ouni, Y.; Taamalli, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC-ESI-TOF MS method. Food Chem. 2011, 127, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Uceda, M.; Frías, L. Harvest dates. Evolution of the fruit oil content, oil composition and oil quality. In Proceedings of the II Seminario Oleícola Internacional, International Olive Council, Cordoba, Spain, 6–17 October 1975; pp. 125–130. [Google Scholar]
- Meteo Galicia. Informes Meteorolóxicos. Consellería de Medio Ambiente, Territorio e Vivienda. Xunta de Galicia. Available online: https://www.meteogalicia.gal/observacion/estacionshistorico/historico.action?idEst=19023 (accessed on 18 October 2021).
- 31991R2568; Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union: Brussels, Belgium, 1991.
- IOC/T.20/Doc. No 15/Rev. 10; Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Council: Madrid, Spain, 2005.
- 32013R1348; Commission Implementing Regulation (EU) No 1348/2013 of 16 December 2013 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union: Brussels, Belgium, 2013.
- 32015R1833; Commission Implementing Regulation (EU) 2015/1833 of 12 October 2015 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union: Brussels, Belgium, 2015.
- 32016R1227; Commission Implementing Regulation (EU) 2016/1227 of 27 July 2016 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union: Brussels, Belgium, 2016.
- 32019R1604; Commission Implementing Regulation (EU) 2019/1604 of 27 September 2019 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. European Union: Brussels, Belgium, 2019.
- IOC/T.20/Doc. No 22; Method for the Organoleptic Assessment of Extra Virgin Olive Oil Applying to Use a Designation of Origin. International Olive Council: Madrid, Spain, 2005.
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Comparative study of virgin olive oil behavior under Rancimat accelerated oxidation conditions and long-term room temperature storage. J. Agric. Food Chem. 2007, 55, 8231–8236. [Google Scholar] [CrossRef]
- IUPAC. Determination of Tocopherols and Tocotrienols in Vegetable Fats by HPLC. Method No. 2432. In Standard Methods of Analyses of Oils, Fats and Derivatives; Blackwell: Oxford, UK, 1992. [Google Scholar]
- IOC/T.20/Doc. No 29/Rev.1; Determination of Biophenols in Olive Oils by HPLC. International Olive Council: Madrid, Spain, 2009.
- Gorinstein, S.; Martín-Belloso, O.; Katrich, E.; Lojek, A.; Cíz, M.; Gligelmo-Miguel, N.; Haruenkit, R.; Park, Y.; Jung, S.; Trakhtenberg, S. Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. J. Nutr. Biochem. 2003, 14, 154–159. [Google Scholar] [CrossRef]
- Bajoub, A.; Fernández-Gutierrez, A.; Carrasco-Pancorbo, A. Evaluating the potential of phenolic profiles as discriminant features among extra virgin olives oils form Moroccan controlled designations of origin. Food Res. Int. 2016, 84, 41–51. [Google Scholar] [CrossRef]
- MAPAMA. Resolución de la Dirección General de Producciones y Mercados Agrarios de 23 de Octubre de 2017, por la que se Reconocen Oficialmente una Serie de Variedades de Especies Frutales, Propuestas por la Dirección General de Ganadería, Agricultura e Industrias Alimentarias de la Xunta de Galicia. Dirección de Validación. 2017. Available online: https://sede.administracion.gob.es/pagSedeFront/servicios/consultaCSV.htm (accessed on 30 May 2022)(Código Seguro de Verificación: CSV: GEN-68e0-ec35-2596-15ab-d855-e52f-2900-2339).
- Fundación Juana de Vega. Identificadas Veinte Variedades de Olivo Autóctonas de Galicia, Únicas en el Mundo. Available online: https://juanadevega.org/es/noticias/identificadas-veinte-variedades-de-olivo-autoctonas-de-galicia-unicas-en-el-mundo/ (accessed on 25 March 2022).
- Crizel, R.L.; Hoffmann, J.F.; Zandoná, G.P.; Mendonça Shild Lobo, P.M.S.; Jorge, R.O.; Chaves, F.C. Characterization of extra virgin olive oil from Southern Brazil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900347. [Google Scholar] [CrossRef]
- Kelebek, H.; Kesen, S.; Selli, S. Comparative study of bioactive constituents in Turkish olive oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015, 18, 2231–2245. [Google Scholar] [CrossRef]
- Salvador, M.D.; Aranda, G.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality. A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Zarrouk, W.; Haddada, F.M.; Baccouri, B.; Oueslati, I.; Taamalli, W.; Fernandez, X.; Lizzani-Cuvelier, L.; Daoud, D.; Zarrouk, M. Characterization of virgin olive oil from Southern Tunisia. Eur. J. Lipid Sci. Technol. 2008, 110, 81–88. [Google Scholar] [CrossRef]
- Boulkroune, H.; Lazzez, A.; Guissous, M.; Bellik, Y.; Smaoui, S.; Grati Kamoun, N.; Madani, T. Characterization of sterolic and alcoholic fractions of some Algerian olive oils according to the variety and ripening stage. OCL-Oilseeds Fats Crops Lipids 2017, 24, A502. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.G.; León, L.; Pascual, M.; de la Rosa, R.; Belaj, A.; Sanz, C. Analysis of olive (Olea Europaea L.) genetic resources in relation to the content of vitamin E in virgin olive oil. Antioxidants 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, T.H.; López, L.C.; Pereira, J.A.; Cabrera-Vique, C.; Seiquer, I. Comparative analysis of minor bioactive constituents (CoQ10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. J. Food Compos. Anal. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltrán, G.; Ortega, D.; Fernández, A.; Jiménez, A.; Uceda, M. Characterisation of virgin olive oil of Italian olive cultivars: ‘Frantoio’ and ‘Leccino’, grown in Andalusia. Food Chem. 2005, 89, 387–391. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Olmo-García, L.; Ajal, E.A.; Monasterio, R.P.; Hanine, H.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. In-depth two-year study of phenolic profile variability among olive oils from autochthonous and mediterranean varieties in Morocco, as revealed by a LC-MS chemometric profiling approach. Int. J. Mol. Sci. 2017, 218, 52. [Google Scholar] [CrossRef]
- Ballus, C.A.; Meinhart, A.D.; De Souza Campos, F.A., Jr.; Godoy, H.T. Total phenolics of virgin olive oils highly correlate with the hydrogen atom transfer mechanism of antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Romero, M.P.; Tovar, M.J.; Ramo, T.; Motilva, M.J. Effect of crop season on the composition of virgin olive oil with protected designation of origin “Les Garrigues”. J. Am. Oil Chem. Soc. 2003, 80, 423–430. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agr. 2016, 96, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.; Hernández, M.; Garrido, I.; Llerena, J.L.; Espinosa, F. Fatty acid and phenolic compound concentrations in eight different monovarietal virgin olive oils from Extremadura and the relationship with oxidative stability. Int. J. Mol. Sci. 2016, 17, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, T.H.; Cabrera-Vique, C.; Seiquer, I. Antioxidant properties of chemical extracts and bioaccessible fractions obtained from six Spanish monovarietal extra virgin olive oils: Assays in Caco-2 cells. Food Funct. 2015, 6, 2375. [Google Scholar] [CrossRef]
- Banco, A.; Trentacoste, E.; Monasterio, R.P. Characterization of virgin olive oils from Spanish olive varieties introduced in Mendoza, Argentina, and their comparison with the autochthonous variety. J. Sci. Food Agric. 2021, 101, 518–524. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Characterization of olive oils from superintensive crops with different ripening degree, irrigation management, and cultivar: (Arbequina, Koroneiki, and Arbosana). Eur. J. Lipid Sci. Technol. 2019, 121, 1800360. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Jimenez, A.; Sanchez-Villasclaras, S.; Uceda, M.; Beltrán, G. Modulation of bitterness and pungency in virgin olive oil from unripe “Picual” fruits. Eur. J. Lipid Sci. Technol. 2015, 117, 1463–1472. [Google Scholar] [CrossRef]
- Borges, T.H.; Serna, A.; López, L.C.; Lara, L.; Nieto, R. Composition and antioxidant properties of Spanish extra virgin olive oil regarding cultivar, harvest year and crop stage. Antioxidants 2019, 8, 217. [Google Scholar] [CrossRef] [Green Version]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Bajoub, A.; Hurtado-Fernández, E.; Ajal, E.A.; Ouazzani, N.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Comprehensive 3-year study of the phenolic profile of Moroccan monovarietal virgin olive oils from the Meknès region. J. Agric. Food Chem. 2015, 63, 4376–4385. [Google Scholar] [CrossRef] [PubMed]
- Hachicha Hbaieb, R.; Kotti, F.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Gargouri, M. Effect of Tunisian olive ripeness on endogenous enzymes and virgin olive oil phenolic composition. J. Food Compos. Anal. 2017, 62, 43–50. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of cultivar and ripening on minor components in Spanish olive fruits and their corresponding virgin olive oils. Food Res. Int. 2008, 41, 433–440. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Garbetta, A.; Ciasca, B.; Linsalata, V.; Minervini, F.; Lattanzio, V.M.T.; Logrieco, A.F.; Cardinali, A. Biophenols from table olive cv Bella di Cerignola: Chemical characterization, bioaccessibility, and intestinal absorption. J. Agric. Food Chem. 2016, 64, 5671–5678. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Olmo-García, L.; Figueiredo-González, M.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Application of the INFOGEST standardized method to assess the digestive stability and bioaccessibility of phenolic compounds from Galician extra-virgin olive oil. J. Agric. Food Chem. 2021, 69, 11592–11605. [Google Scholar] [CrossRef] [PubMed]
- 32012R0432; Commission Regulation (EU) No 432/2012, of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. European Union: Brussels, Belgium, 2019.
- Olmo-García, L.; Fernández-Fernández, C.; Hidalgo, A.; Vílchez, P.; Fernández-Gutiérrez, A.; Marchal, R.; Carrasco-Pancorbo, A. Evaluating the reliability of specific and global methods to assess the phenolic content of virgin olive oil: Do they drive to equivalent results? J. Chromatogr. A 2019, 1585, 56–69. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.A.; Peres, M.P.; Pereira, J.B. Seeking for sensory differentiated olive oils? The urge to preserve old autochthonous olive cultivars. Food Res. Int. 2020, 128, 1087592. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Sánchez, J.; Segura-Carretero, A.; Menéndez, J.A.; Oliveras-Ferraros, C.; Cerretani, L.; Fernández-Gutiérrez, J.A. Prediction of extra virgin olive oil varieties through their phenolic profile. potential cytotoxic activity against human breast cancer cells. J. Agric. Food Chem. 2010, 58, 9942–9955. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of phenolic and volatile composition of extra virgin of olive paste. LWT Food Sci. Technol. 2018, 87, 523–528. [Google Scholar] [CrossRef]
- Olmo-García, L. Relevant applications in food metabolomics: Characterization of products and by-products derived from the olive tree. Ph.D. Thesis, University of Granada, Granada, Spain, 2018. [Google Scholar]
- Ren, S.; Hinzman, A.A.; Kang, E.L.; Szczesniak, R.D.; Lu, L.J. Computational and statistical analysis of metabolomics data. Metabolomics 2015, 11, 1492–1513. [Google Scholar] [CrossRef]
- Antônio Nunes, C.; Ortiz Alvarenga, V.; de Souza Sant’Ana, A.; Sousa Santos, J.; Granato, D. The use of statistical software in food science and technology: Advantages, limitations and misuses. Food Res. Int. 2015, 75, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219–241. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.S.; Lauridsen, M.B.; Dragsted, L.O.; Nielsen, J.; Staerk, D. Development of a bioassay-coupled HPLC-SPE-ttNMR platform for identification of α-glucosidase inhibitors in apple peel (Malus × domestica Borkh.). Food Chem. 2012, 135, 1692–1699. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]


| MF-17 (RI 3.5) | MF-18 (RI 5.0) | MF-19 (RI 2.3) | Regulated Values for EVOO (EU Reg 2568) | |
|---|---|---|---|---|
| Quality-related indices | ||||
| Free acidity (% oleic acid) | 0.18 ± 0.04 a | 0.15 ± 0.00 a | 0.18 ± 0.01 a | ≤0.80 |
| K232 | 1.91 ± 0.02 b | 1.74 ± 0.03 a | 1.81 ± 0.02 a | ≤2.50 |
| K270 | 0.19 ± 0.01 a | 0.16 ± 0.02 a | 0.16 ± 0.01 a | ≤0.22 |
| AK | <0.01 a | <0.01 a | <0.01 a | ≤0.01 |
| Peroxide value (meq O2/kg oil) | 3.2 ± 0.3 a | 3.2 ± 0.1 a | 3.3 ± 0.3 a | ≤20.0 |
| Rancimat (h) | 43.3 ± 3.6 a | 44.1 ± 1.0 a | 40.7 ± 5.0 a | |
| Sensory analysis | ||||
| Positive attributes | ||||
| Fruity | 4.2 | 4.6 | 4.0 | > 0.0 |
| Bitter | 4.0 | 3.4 | 3.7 | |
| Pungent | 4.4 | 4.1 | 4.5 | |
| Genuineness-related indices | ||||
| Fatty acid composition (% m/m methyl esters) | ||||
| Myristic (C14:0) | 0.012 ± 0.002 a | 0.008 ± 0.000 a | 0.010 ± 0.000 a | ≤0.03 |
| Palmitic (C16:0) | 10.36 ± 0.03 b | 10.44 ± 0.02 b | 9.81 ± 0.12 a | 7.50–20.00 |
| Palmitoleic (C16:1) | 0.620 ± 0.000 a | 0.650 ± 0.000 a | 0.493 ± 0.023 b | 0.30–3.50 |
| Margaric (C17:0) | ˂0.100 ± 0.007 a | ˂0.100 ± 0.000 a | ˂0.100 ± 0.000 a | ≤0.40 |
| Margaroleic (C17:1) | ˂0.100 ± 0.007 a | ˂0.100 ± 0.000 a | ˂0.100 ± 0.000 a | ≤0.60 |
| Stearic (C18:0) | 3.725 ± 0.021 c | 2.820 ± 0.014 b | 3.117 ± 0.120 a | 0.50–5.00 |
| Oleic (C18:1) | 74.75 ± 0.01 a | 76.57 ± 0.02 b | 78.72 ± 0.43 c | 55.00–83.00 |
| Linoleic (C18:2) | 8.900 ± 0.071 c | 7.935 ± 0.007 b | 6.040 ± 0.223 a | 2.50–21.00 |
| Linolenic (C18:3) | 0.565 ± 0.021 a | 0.580 ± 0.000 a | 0.640 ± 0.030 a | ≤1.00 |
| Arachidic (C20:0) | 0.480 ± 0.000 c | 0.380 ± 0.000 a | 0.470 ± 0.000 b | ≤0.60 |
| Eicosenoic (C20:1) | 0.305 ± 0.021 a | 0.315 ± 0.007 a | 0.337 ± 0.006 a | ≤0.50 |
| Behenic (C22:0) | 0.125 ± 0.007 b | 0.110 ± 0.000 a | 0.150 ± 0.000 c | ≤0.20 |
| Lignoceric (C24:0) | ˂0.100 ± 0.007 a | ˂0.100 ± 0.000 a | ˂0.100 ± 0.000 a | ≤0.20 |
| trans-Oleic isomers C18:1 T | 0.017 ± 0.001 a | 0.008 ± 0.000 a | 0.017 ± 0.006 a | ≤0.05 |
| trans-Linoleic + trans-Linolenic | 0.013 ± 0.001 a | 0.010 ± 0.000 a | 0.013 ± 0.006 a | ≤0.05 |
| ∑ SFA | 14.796 ± 0.005 b | 13.853 ± 0.007 a | 13.560 ± 0.217 a | |
| ∑ MUFA | 75.74 ± 0.04 a | 77.63 ± 0.01 b | 79.55 ± 0.41 c | |
| ∑ PUFA | 9.465 ± 0.049 c | 8.515 ± 0.007 b | 6.680 ± 0.195 a | |
| C18:1/C18:2 | 8.399 ± 0.068 a | 9.650 ± 0.011 a | 13.046 ± 0.546 b | |
| ∑ MUFA/∑ PUFA | 8.002 ± 0.046 a | 9.117 ± 0.009 b | 11.916 ± 0.403 c | |
| Sterol relative amounts | ||||
| Cholesterol (%) | 0.245 ± 0.007 a | 0.120 ± 0.000 a | 0.167 ± 0.058 a | ≤0.5 |
| Brassicasterol (%) | nd | nd | <0.100 ± 0.000 | ≤0.1 |
| Campesterol (%) | 2.240 ± 0.028 a | 2.250 ± 0.014 a | 2.433 ± 0.058 b | ≤4.0 |
| Stigmasterol (%) | 0.430 ± 0.014 a | 0.605 ± 0.007 b | 0.633 ± 0.058 b | ≤Campesterol |
| Apparent β-sitosterol (%) | 94.75 ± 0.05 a | 95.33 ± 0.00 a | 95.13 ± 0.25 a | ≥93.0 |
| Δ7-Stigmastenol (%) | 0.340 ± 0.000 b | 0.170 ± 0.000 a | 0.333 ± 0.058 b | ≤0.5 |
| Total sterols (mg/kg) | 1131.0 ± 11.3 a | 1001.4 ± 14.3 a | 1645 ± 407.4 a | ≥1000 |
| Triterpenic alcohols | ||||
| Erythrodiol + Uvaol (%) | 2.430 ± 0.113 ab | 2.185 ± 0.021 a | 2.533 ± 0.058 b | ≤4.5 |
| Tocopherols (mg/kg) | ||||
| α-tocopherol | 184.05 ± 3.89 a | 273.00 ± 1.98 b | 303.30 ± 18.04 b | |
| β-tocopherol | <0.1 ± 0.0 | <0.1 ± 0.0 | <0.1 ± 0.0 | |
| γ-tocopherol | <0.1 ± 0.0 | <0.1 ± 0.0 | <0.1 ± 0.0 | |
| δ-tocopherol | 3.55 ± 0.64 a | 3.15 ± 0.07 a | 7.20 ± 0.10 b | |
| Total tocopherols | 187.60 ± 4.52 a | 276.20 ± 1.98 b | 313.50 ± 18.07 b | |
| Total phenolic content by UV-VIS method | MF-17 (RI 3.5) | MF-18 (RI 5.0) | MF-19 (RI 2.3) | ||||
| Folin-Ciocalteu | mg GA/kg | 675.4 ± 51.0 a | 705.9 ± 20.8 a | 793.1 ± 46.5 b | |||
| orto-Diphenols | mg GA/kg | 196.7 ± 34.8 a | 226.5 ± 14.0 a | 214.5 ± 14.9 a | |||
| Antioxidant capacity | µmol Trolox/kg | 2678.4 ± 269.1 b | 1962.9 ± 73.7 a | 1982.3 ± 144.7 a | |||
| Phenolic profile by LC-MS method | Acronym | [M-H]− | Rt | MF-17 (RI 3.5) | MF-18 (RI 5.0) | MF-19 (RI 2.3) | |
| Secoiridoids | Oleuropein derivatives | ||||||
| Hydroxy oleacein (hydroxy decarboxymethyl oleuropein aglycone) | Hy-DOA | 335 | 13.8 | nd a | 16.2 ± 0.5 c | 2.2 ± 0.4 b | |
| Oleacein (decarboxymethyl oleuropein aglycone) | DOA | 319 | 14.7 | 350.5 ± 41.0 b | 221.3 ± 12.1 a | 542.1 ± 36.5 c | |
| Oleuropein aglycone (isomer I) | OlAgl (Is I) | 377 | 17.5 | 14.9 ± 2.1 b | 17.3 ± 1.2 c | 9.95 ± 0.8 a | |
| Oleuropein aglycone (main peak) | OlAgl (main peak) | 377 | 20.9 | 92.8 ± 7.8 c | 63.5 ± 5.6 b | 36.19 ± 2.8 a | |
| Dehydro oleuropein aglycone | DH-OlAgly | 375 | 20.9 | nd a | 14.5 ± 0.1 c | 0.49 ± 0.04 b | |
| Oleuropein aglycone (isomer II) | OlAgl (Is II) | 377 | 21.7 | 13.0 ± 1.7 a | 21.7 ± 3.1 b | 12.2 ± 2.1 a | |
| Ligstroside derivatives | |||||||
| Oleocanthal (decarboxymethyl ligstroside aglycone) | DLA | 303 | 17.0 | 703.8 ± 59.1 b | 120.6 ± 9.7 a | 1060. 9 ± 123.0 c | |
| Ligstroside aglycone (isomer I) | LigAgl (Is I) | 361 | 20.8 | 98.1 ± 11.2 c | 11.4 ± 2.1 a | 21.3 ± 3.0 b | |
| Ligstroside aglycone (main peak) | LigAgl (main peak) | 361 | 23.3 | 226.8 ± 27.5 b | 162.2 ± 31.6 a | 204.7 ± 18.9 b | |
| Ligstroside aglycone (isomer II) | LigAgl (Is II)) | 361 | 23.6 | 47.1 ± 9.7 a | 45.7 ± 8.7 a | 47.4 ± 5.5 a | |
| Sub-total | 1547.04 | 694.45 | 1937.60 | ||||
| Simple phenols | Oxidised Hydroxytyrosol | O-HTy | 151 | 2.4 | nd a | 14.00 ± 0.06 c | 0.029 ± 0.005 b |
| Hydroxytyrosol | HTy | 153 | 6.5 | 17.8 ± 1.6 b | 19.5 ± 1.4 b | 3.0 ± 0.3 a | |
| Tyrosol | Ty | 137 | 8.2 | 10.6 ± 1.8 c | 5.7 ± 0.8 b | 2.5 ± 0.2 a | |
| Hydroxytyrosol acetate | HTy-Ac | 195 | 12.6 | 9.7 ± 0.4 b | 27.4 ± 2.3 c | 4.6 ± 0.3 a | |
| Sub-total | 38.13 | 66.64 | 10.12 | ||||
| Flavonoids | Luteolin | Lut | 285 | 16.0 | 0.8 ± 0.2 a | 5.0 ± 0.4 c | 1.65 ± 0.08 b |
| Apigenin | Api | 269 | 18.7 | 0.31 ± 0.05 a | 0.73 ± 0.06 b | 0.348 ± 0.004 a | |
| Diosmetin | Dios | 299 | 19.3 | 0.20 ± 0.03 b | 0.77 ± 0.04 c | 0.03 ± 0.02 a | |
| Sub-total | 1.33 | 6.48 | 2.03 | ||||
| Phenolic acids | Vanillic acid | Van | 167 | 9.2 | nd a | 0.09 ± 0.02 c | 0.07 ± 0.01 b |
| p-Coumaric acid | p-Cou | 163 | 11.2 | nd a | 0.33 ± 0.05 b | 0.058 ± 0.004 a | |
| Ferulic acid | Fer | 193 | 11.7 | 0.036 ± 0.001 c | 0.015 ± 0.002 b | 0.0099 ± 0.0001 a | |
| Sub-total | 0.04 | 0.43 | 0.13 | ||||
| Lignans | Pinoresinol | Pin | 357 | 16.7 | 0.255 ± 0.048 b | 0.472 ± 0.044 c | 0.161 ± 0.006 a |
| Sub-total | 0.25 | 0.47 | 0.16 | ||||
| Non-phenolic but structurally related compounds | Quinic acid | Quin | 191 | 2.0 | 0.357 ± 0.024 b | 0.169 ± 0.019 a | 0.394 ± 0.037 b |
| Elenolic acid derivatives | |||||||
| Desoxy elenolic acid | Desoxy-EA | 225 | 11.7 | 43.8 ± 4.6 a | 117.1 ± 5.2 b | 52.7 ± 5.0 a | |
| Elenolic acid | EA | 241 | 13.6 | 206.2 ± 19.4 b | 122.6 ± 24.6 a | 431.3 ± 36.6 c | |
| Sub-total | 250.34 | 239.90 | 484.47 | ||||
| TOTAL (mg each compound/kg) | 1837.13 | 1008.39 | 2434.52 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo-González, M.; Olmo-García, L.; Reboredo-Rodríguez, P.; Serrano-García, I.; Leuyacc-del Carpio, G.; Cancho-Grande, B.; Carrasco-Pancorbo, A.; González-Barreiro, C. Singular Olive Oils from a Recently Discovered Spanish North-Western Cultivar: An Exhaustive 3-Year Study of Their Chemical Composition and In-Vitro Antidiabetic Potential. Antioxidants 2022, 11, 1233. https://doi.org/10.3390/antiox11071233
Figueiredo-González M, Olmo-García L, Reboredo-Rodríguez P, Serrano-García I, Leuyacc-del Carpio G, Cancho-Grande B, Carrasco-Pancorbo A, González-Barreiro C. Singular Olive Oils from a Recently Discovered Spanish North-Western Cultivar: An Exhaustive 3-Year Study of Their Chemical Composition and In-Vitro Antidiabetic Potential. Antioxidants. 2022; 11(7):1233. https://doi.org/10.3390/antiox11071233
Chicago/Turabian StyleFigueiredo-González, María, Lucía Olmo-García, Patricia Reboredo-Rodríguez, Irene Serrano-García, Glenda Leuyacc-del Carpio, Beatriz Cancho-Grande, Alegría Carrasco-Pancorbo, and Carmen González-Barreiro. 2022. "Singular Olive Oils from a Recently Discovered Spanish North-Western Cultivar: An Exhaustive 3-Year Study of Their Chemical Composition and In-Vitro Antidiabetic Potential" Antioxidants 11, no. 7: 1233. https://doi.org/10.3390/antiox11071233