The Effect of Tauroursodeoxycholic Acid (TUDCA) Treatment on Pregnancy Outcomes and Vascular Function in a Rat Model of Advanced Maternal Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Blood Pressure Measurements
2.3. Mesenteric and Uterine Artery Vascular Function by Wire Myography
2.4. Expression of Oxidative and ER Stress Markers Using Western Blot Analysis
2.5. Statistical Analyses
3. Results
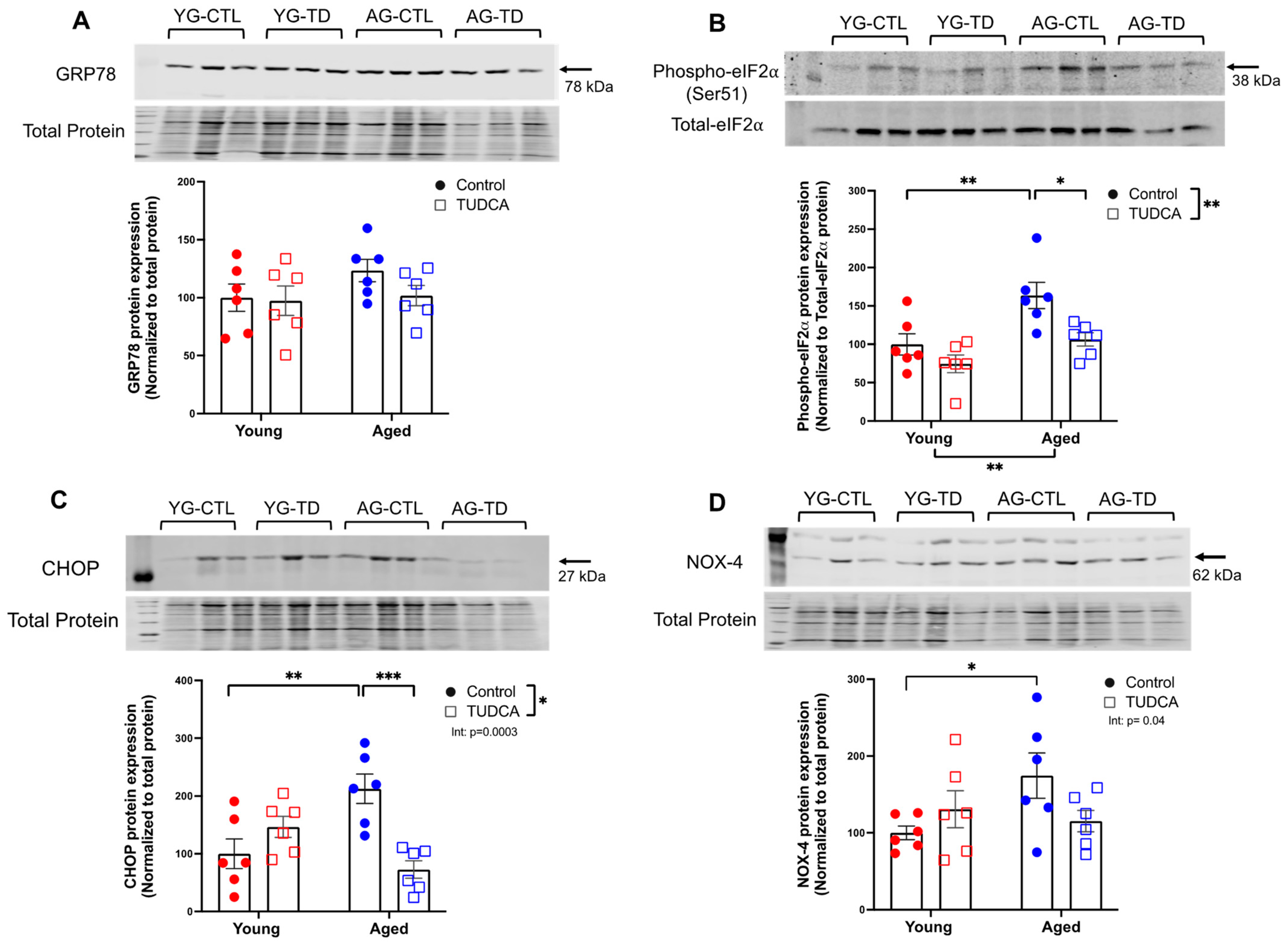
3.1. Increased Expression of ER Stress Markers and NOX-4 in Aged Non-Pregnant and Pregnant Rats
3.2. Reduced ER Stress Protein Expression in TUDCA-Treated Aged Dams
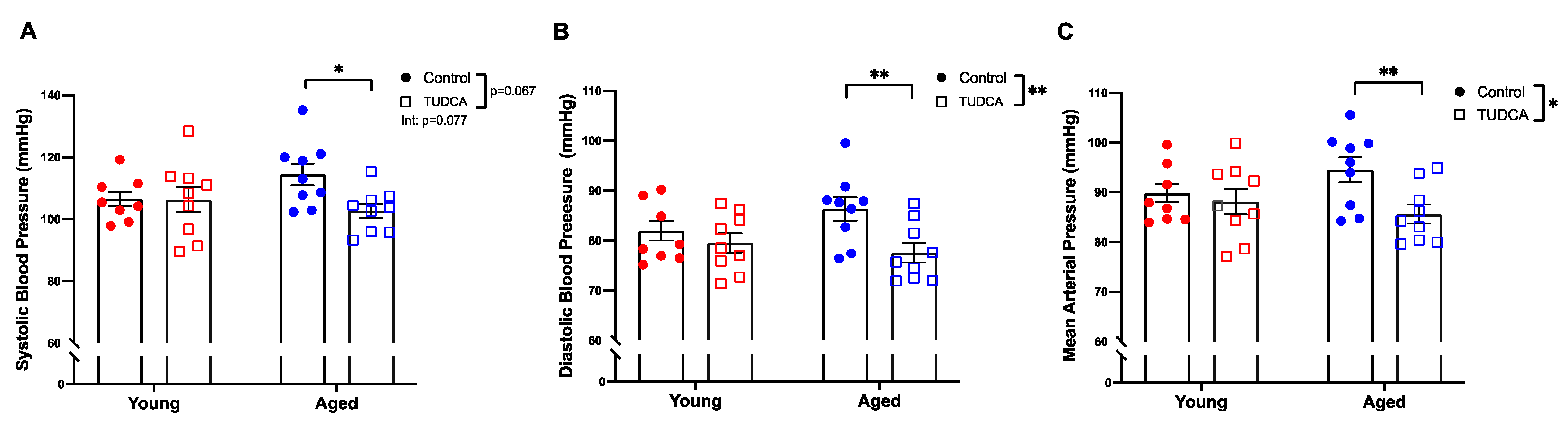
3.3. Reduced Blood Pressure in Aged TUDCA-Treated Dams
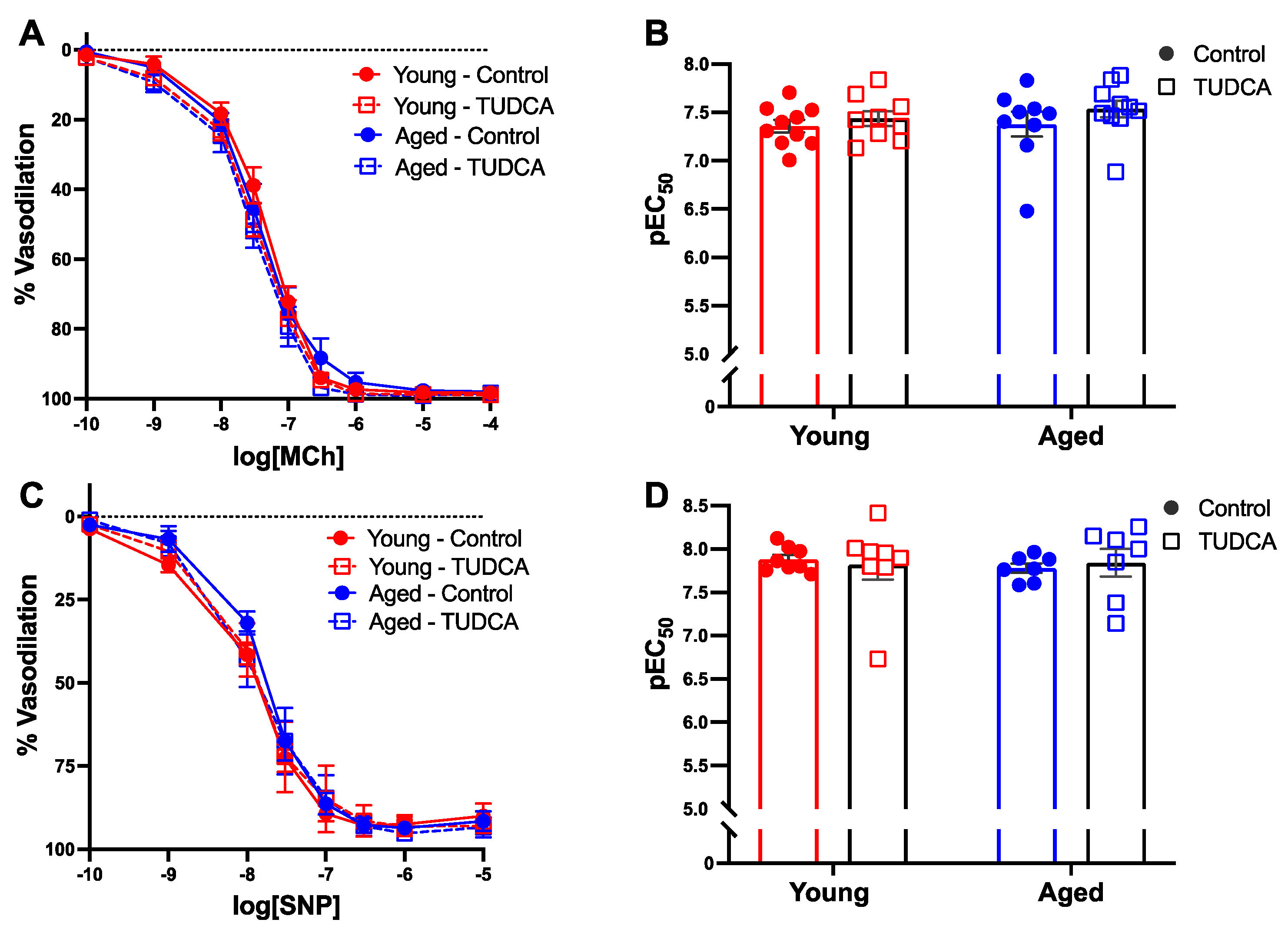
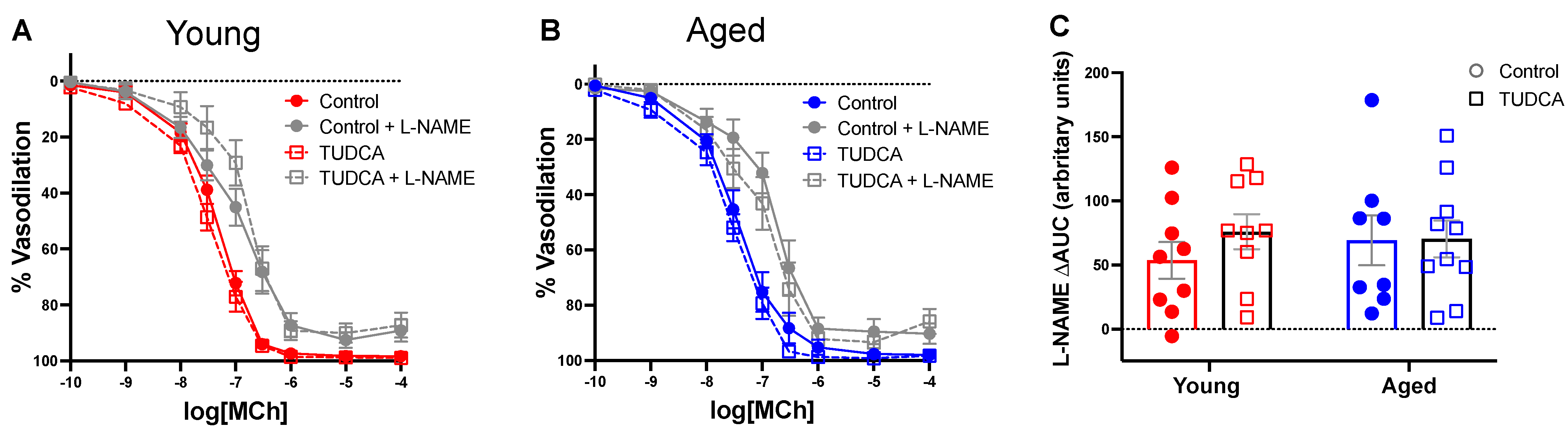
3.4. Mesenteric Artery Vascular Function Was Not Impacted by Age or TUDCA Treatment
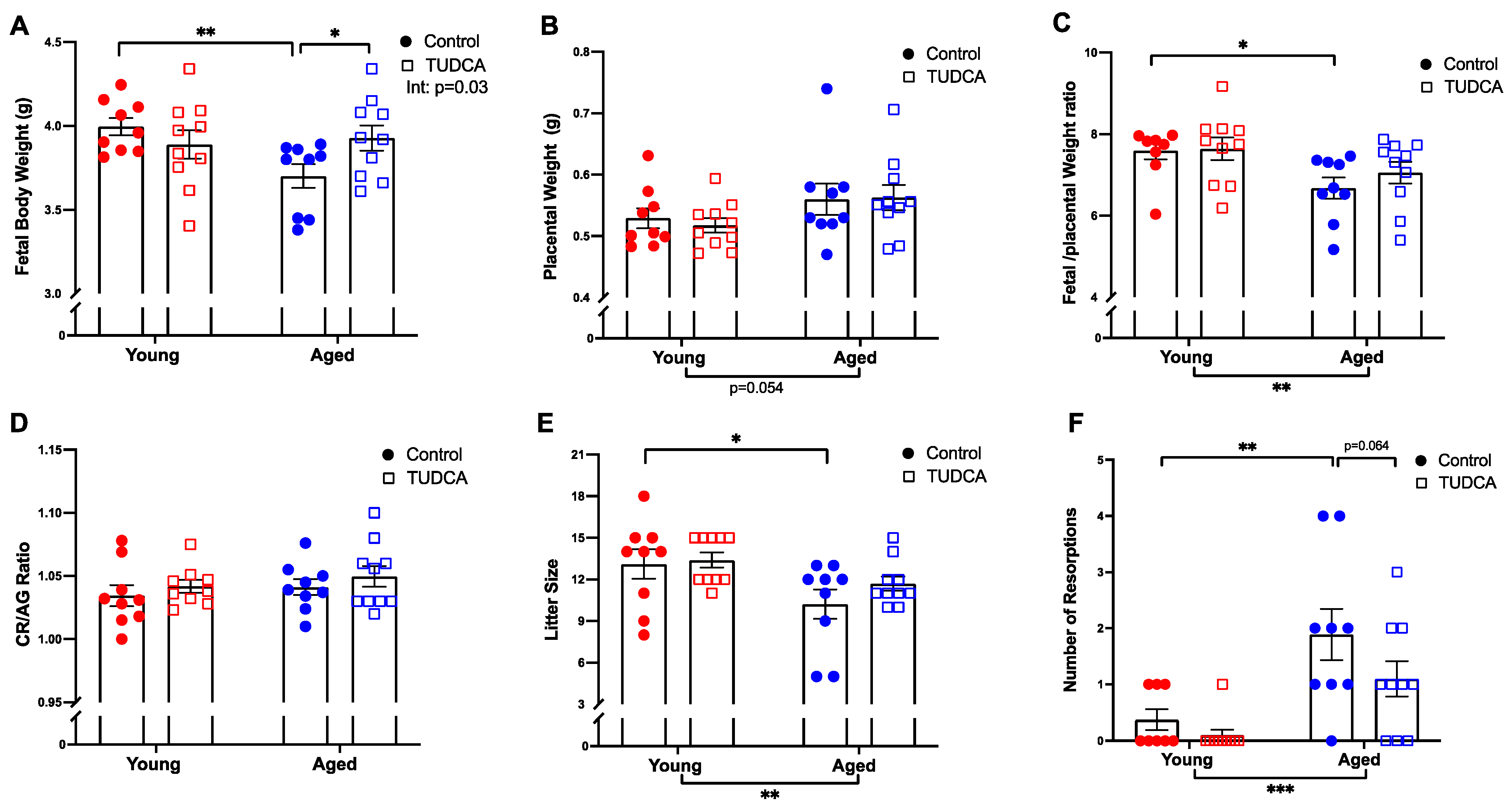
3.5. Increased Fetal Body Weight in Aged TUDCA-Treated Dams
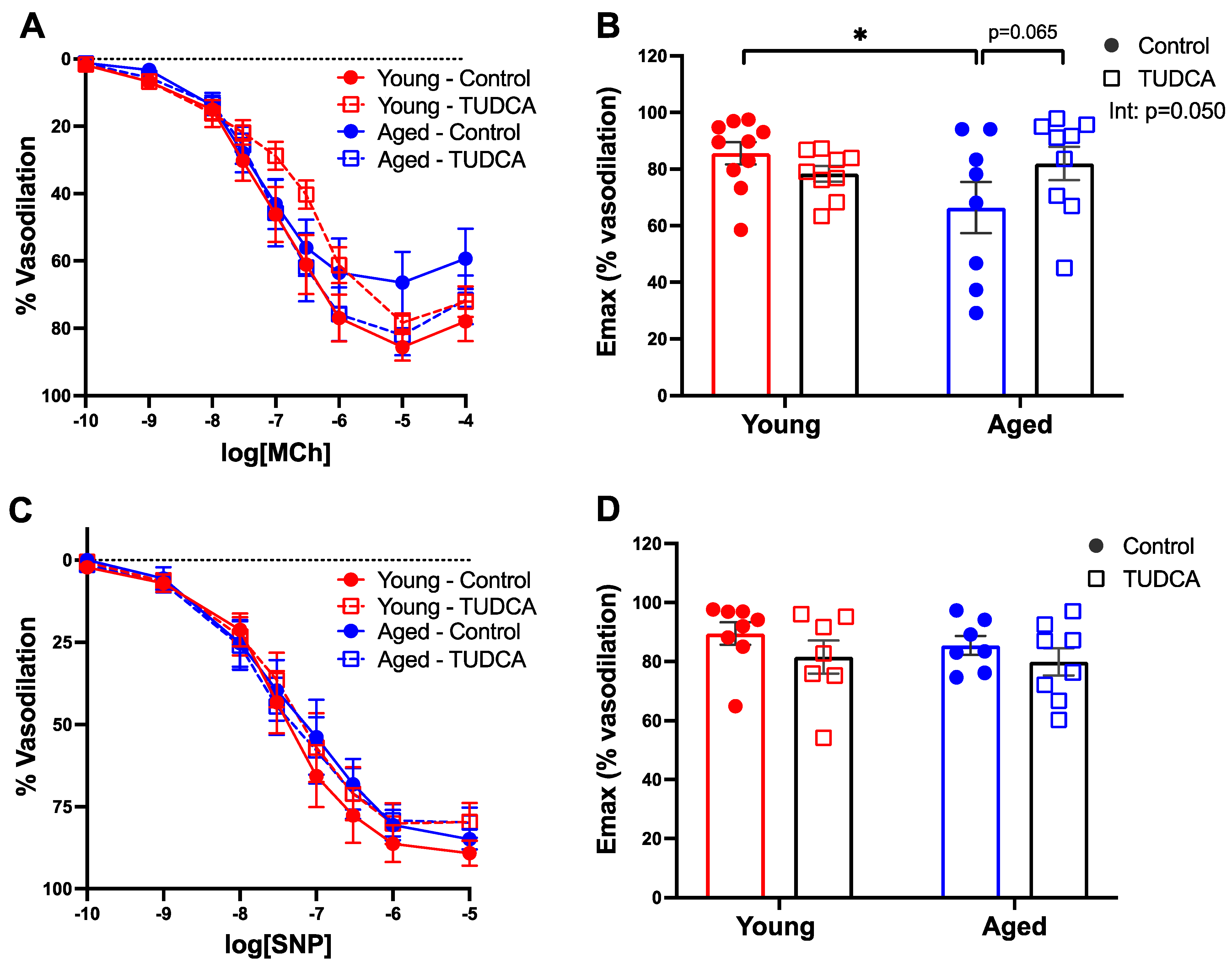
3.6. TUDCA Treatment Tended to Improve Uterine Artery Function in Aged Dams
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garner, R.; Bushnik, T. The children of older first-time mothers in Canada: Their health and development. Genus 2008, 64, 63–81. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Sutton, P.D.; Ventura, S.J.; Menacker, F.; Kirmeyer, S.; Mathews, T.J. Births: Final Data for 2006; National Vital Statistics Reports 2009; CDC: Atlanta, GA, USA, 2009; Volume 57, pp. 1–102.
- Canada, S. Births 2009. 2012. Catalogue no. 84F0210X. Available online: http://www.statcan.gc.ca/pub/84f0210x/84f0210x2009000-eng.pdf (accessed on 23 June 2022).
- Khalil, A.; Syngelaki, A.; Maiz, N.; Zinevich, Y.; Nicolaides, K.H. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet. Gynecol. 2013, 42, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Wilson, R.E.; Alio, A.P.; Kirby, R.S. Advanced maternal age and risk of antepartum and intrapartum stillbirth. J. Obstet. Gynaecol. Res. 2008, 34, 843–850. [Google Scholar] [CrossRef]
- Lamminpää, R.; Vehviläinen-Julkunen, K.; Gissler, M.; Heinonen, S. Preeclampsia complicated by advanced maternal age: A registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth 2012, 12, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, P.W.; Freeman, S.B.; Sherman, S.L.; Taft, L.F.; Gu, Y.; Pettay, D.; Flanders, W.D.; Khoury, M.J.; Hassold, T.J. Advanced maternal age and the risk of Down syndrome characterized by the meiotic stage of chromosomal error: A population-based study. Am. J. Hum. Genet. 1996, 58, 628–633. [Google Scholar]
- Kenny, L.; Lavender, T.; McNamee, R.; Neill, S.O.; Mills, T.; Khashan, A.S. Advanced Maternal Age and Adverse Pregnancy Outcome: Evidence from a Large Contemporary Cohort. PLoS ONE 2013, 8, e56583. [Google Scholar] [CrossRef]
- Ludford, I.; Scheil, W.; Tucker, G.; Grivell, R. Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998–2008. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 235–241. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [Green Version]
- Gongora, M.C.; Wenger, N.K. Cardiovascular Complications of Pregnancy. Int. J. Mol. Sci. 2015, 16, 23905–23928. [Google Scholar] [CrossRef]
- Neiger, R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Davidge, S.T. Effect of Advanced Maternal Age on Pregnancy Outcomes and Vascular Function in the Rat. Hypertension 2015, 65, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasha, M.; Wooldridge, A.L.; Kirschenman, R.; Spaans, F.; Davidge, S.T.; Cooke, C.-L.M. Altered Vascular Adaptations to Pregnancy in a Rat Model of Advanced Maternal Age. Front. Physiol. 2021, 12, 718568. [Google Scholar] [CrossRef]
- Lean, S.C.; Heazell, A.E.P.; Dilworth, M.; Mills, T.; Jones, R.L. Placental Dysfunction Underlies Increased Risk of Fetal Growth Restriction and Stillbirth in Advanced Maternal Age Women. Sci. Rep. 2017, 7, 9677. [Google Scholar] [CrossRef] [PubMed]
- Plant, M.; Armstrong, C.; Ruggiero, A.; Sherrill, C.; Uberseder, B.; Jeffries, R.; Nevarez, J.; Jorgensen, M.J.; Kavanagh, K.; Quinn, M.A. Advanced maternal age impacts physiologic adaptations to pregnancy in vervet monkeys. GeroScience 2020, 42, 1649–1661. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Vallejo, S.; Peiro, C.; Sanchez-Ferrer, C.F.; Rodriguez-Manas, L. Mechanisms involved in the aging-induced vascular dysfunction. Front. Physiol. 2012, 3, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battson, M.L.; Lee, D.M.; Gentile, C.L. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am. J. Physiol. Circ. Physiol. 2017, 312, H355–H367. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, X.; Seidel, K.; Zhang, M.; Goodman, J.B.; Seta, F.; Hamburg, N.; Han, J. Aging and Hypercholesterolemia Differentially Affect the Unfolded Protein Response in the Vasculature of ApoE−/− Mice. J. Am. Heart Assoc. 2021, 10, e020441. [Google Scholar] [CrossRef]
- DuRose, J.B.; Scheuner, D.; Kaufman, R.J.; Rothblum, L.I.; Niwa, M. Phosphorylation of Eukaryotic Translation Initiation Factor 2α Coordinates rRNA Transcription and Translation Inhibition during Endoplasmic Reticulum Stress. Mol. Cell. Biol. 2009, 29, 4295–4307. [Google Scholar] [CrossRef] [Green Version]
- Baird, T.D.; Wek, R.C. Eukaryotic Initiation Factor 2 Phosphorylation and Translational Control in Metabolism. Adv. Nutr. Int. Rev. J. 2012, 3, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, F.; van Lith, M.; Sweeney, B.; Cain, K.; Bulleid, N.J. Inhibition of IRE1alpha-mediated XBP1 mRNA cleavage by XBP1 reveals a novel regulatory process during the unfolded protein response. Wellcome Open Res. 2017, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Zhang, Q.-H.; Lu, Y.-J.; Ren, K.; Yi, G.-H. Involvement of the IRE1α-XBP1 Pathway and XBP1s-Dependent Transcriptional Reprogramming in Metabolic Diseases. DNA Cell Biol. 2015, 34, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Yung, H.W. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2011, 1, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basar, M.; Bozkurt, I.; Guzeloglu-Kayisli, O.; Sozen, B.; Tekmen, I.; Schatz, F.; Arici, A.; Lockwood, C.J.; Kayisli, U.A. Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro. Fertil. Steril. 2014, 102, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lee, J.E.; Kang, J.W.; Shin, H.Y.; Bin Lee, J.; Jin, D.I. Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development. Int. J. Mol. Sci. 2019, 20, 409. [Google Scholar] [CrossRef] [Green Version]
- Yung, H.W.; Hemberger, M.; Watson, E.D.; Senner, C.E.; Jones, C.P.; Kaufman, R.J.; Charnock-Jones, D.S.; Burton, G.J. Endoplasmic reticulum stress disrupts placental morphogenesis: Implications for human intrauterine growth restriction. J. Pathol. 2012, 228, 554–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Yung, H.-W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30 (Suppl. SA), 43–48. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Meijles, D.N.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.M.; Cahill-Smith, S.; Geng, L.; Du, J.; Brooks, G.; Li, J.-M. Aging-associated metabolic disorder induces Nox2 activation and oxidative damage of endothelial function. Free Radic. Biol. Med. 2017, 108, 940–951. [Google Scholar] [CrossRef]
- Santos, C.X.C.; Nabeebaccus, A.A.; Shah, A.; Camargo, L.D.L.; Filho, S.V.; Lopes, L. Endoplasmic Reticulum Stress and Nox-Mediated Reactive Oxygen Species Signaling in the Peripheral Vasculature: Potential Role in Hypertension. Antioxid. Redox Signal. 2014, 20, 121–134. [Google Scholar] [CrossRef]
- Bhandary, B.; Marahatta, A.; Kim, H.-R.; Chae, H.-J. An Involvement of Oxidative Stress in Endoplasmic Reticulum Stress and Its Associated Diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, I. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxidative Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Turpin, C.A.; Sakyi, S.A.; Owiredu, W.K.B.A.; Ephraim, R.K.; Anto, E.O. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 2015, 15, 189. [Google Scholar] [CrossRef] [Green Version]
- Kusaczuk, M. Tauroursodeoxycholate—Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Zeng, M.; Han, Y.; Yan, H.; Tang, H.; Sheng, J.; Hu, H.; Cheng, L.; Xie, Q.; Zhu, Y.; et al. A multicenter, randomized, double-blind trial comparing the efficacy and safety of TUDCA and UDCA in Chinese patients with primary biliary cholangitis. Medicine 2016, 95, e5391. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Diao, Y.F.; Kim, H.R.; Jin, D.I. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE 2012, 7, e40433. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Diao, Y.F.; Kang, J.W.; Lee, J.E.; Kim, D.K.; Jin, D.I. Tauroursodeoxycholic acid improves the implantation and live-birth rates of mouse embryos. Reprod. Biol. 2015, 15, 101–105. [Google Scholar] [CrossRef]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995; The National Academies: Washington, DC, USA, 1995.
- Cheang, W.S.; Wong, W.T.; Zhao, L.; Xu, J.; Wang, L.; Lau, C.W.; Chen, Z.Y.; Ma, R.C.; Xu, A.; Wang, N.; et al. PPARδ is required for exercise to attenuate endoplasmic reticulum stress and endothelial dysfunction in diabetic mice. Diabetes 2017, 66, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Lau, Y.S.; Mustafa, M.R.; Choy, K.W.; Chan, S.; Potocnik, S.; Herbert, T.P.; Woodman, O.L. 3′,4′-dihydroxyflavonol ameliorates endoplasmic reticulum stress-induced apoptosis and endothelial dysfunction in mice. Sci. Rep. 2018, 8, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paridaens, A.; Raevens, S.; Devisscher, L.; Bogaerts, E.; Verhelst, X.; Hoorens, A.; Van Vlierberghe, H.; Van Grunsven, L.A.; Geerts, A.; Colle, I. Modulation of the Unfolded Protein Response by Tauroursodeoxycholic Acid Counteracts Apoptotic Cell Death and Fibrosis in a Mouse Model for Secondary Biliary Liver Fibrosis. Int. J. Mol. Sci. 2017, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.-L.M.; Shah, A.; Kirschenman, R.D.; Quon, A.L.; Morton, J.S.; Care, A.S.; Davidge, S.T. Increased susceptibility to cardiovascular disease in offspring born from dams of advanced maternal age. J. Physiol. 2018, 596, 5807–5821. [Google Scholar] [CrossRef]
- Capatina, N.; Hemberger, M.; Burton, G.J.; Watson, E.D.; Yung, H.W. Excessive endoplasmic reticulum stress drives aberrant mouse trophoblast differentiation and placental development leading to pregnancy loss. J. Physiol. 2021, 599, 4153–4181. [Google Scholar] [CrossRef]
- Fabian, E.; Bogner, M.; Elmadfa, I. Age-related modification of antioxidant enzyme activities in relation to cardiovascular risk factors. Eur. J. Clin. Investig. 2011, 42, 42–48. [Google Scholar] [CrossRef]
- Brown, M.K.; Naidoo, N. The endoplasmic reticulum stress response in aging and age-related diseases. Front. Physiol. 2012, 3, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; Kassan, M.; Kadowitz, P.J.; Trebak, M.; Belmadani, S.; Matrougui, K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochim. Biophys. Acta 2014, 1843, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-Y.; Kim, H.-K.; Hoang, T.-H.; Yang, S.; Kim, H.-R.; Chae, H.-J. The correlation of IRE1α oxidation with Nox4 activation in aging-associated vascular dysfunction. Redox Biol. 2020, 37, 101727. [Google Scholar] [CrossRef]
- de Vries, E.; Beuers, U. Ursodeoxycholic acid in pregnancy? J. Hepatol. 2019, 71, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Rudi, J.; Schonig, T.; Stremmel, W. -Therapy with ursodeoxycholic acid in primary biliary cirrhosis in pregnancy-. Z. Gastroenterol. 1996, 34, 188–191. [Google Scholar] [PubMed]
- Pan, X.-L.; Zhao, L.; Li, L.; Li, A.-H.; Ye, J.; Yang, L.; Xu, K.-S.; Hou, X.-H. Efficacy and safety of tauroursodeoxycholic acid in the treatment of liver cirrhosis: A double-blind randomized controlled trial. J. Huazhong Univ. Sci. Technol. 2013, 33, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Phan, A.B.; Gentile, C.L. Tauroursodeoxycholic Acid Reduces Arterial Stiffness and Improves Endothelial Dysfunction in Type 2 Diabetic Mice. J. Vasc. Res. 2017, 54, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Lim, M.; Byeon, S.H.; Lee, Y.-H. Inhibition of endoplasmic reticulum stress improves coronary artery function in the spontaneously hypertensive rats. Sci. Rep. 2016, 6, 31925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, L.K.; Restaino, R.M.; Neuringer, M.; Manrique, C.; Padilla, J. Administration of tauroursodeoxycholic acid prevents endothelial dysfunction caused by an oral glucose load. Clin. Sci. 2016, 130, 1881–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassan, M.; Galán, M.; Partyka, M.; Saifudeen, Z.; Henrion, D.; Trebak, M.; Matrougui, K. Endoplasmic Reticulum Stress Is Involved in Cardiac Damage and Vascular Endothelial Dysfunction in Hypertensive Mice. Arter. Thromb. Vasc. Biol. 2012, 32, 1652–1661. [Google Scholar] [CrossRef] [Green Version]
- Kassan, M.; Galán, M.; Choi, S.K. Endoplasmic Reticulum Stress and Microvascular Endothelial Dysfunction in Diabetes. J. Diabetes Metab. 2011, 2, 108e. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.; Mulvany, M.J. Mesenteric Arcade Arteries Contribute Substantially to Vascular Resistance in Conscious Rats. J. Vasc. Res. 1993, 30, 73–79. [Google Scholar] [CrossRef]
- Cooke, C.-L.M.; Davidge, S.T. Pregnancy-Induced Alterations of Vascular Function in Mouse Mesenteric and Uterine Arteries. Biol. Reprod. 2003, 68, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Spitler, K.M.; Matsumoto, T.; Webb, R.C. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am. J. Physiol. Circ. Physiol. 2013, 305, H344–H353. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Carmo, J.M.D.; Aberdein, N.; Zhou, X.; Williams, J.M.; Silva, A.; Hall, J. Synergistic Interaction of Hypertension and Diabetes in Promoting Kidney Injury and the Role of Endoplasmic Reticulum Stress. Hypertension 2017, 69, 879–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coan, P.M.; Angiolini, E.; Sandovici, I.; Burton, G.J.; Constancia, M.; Fowden, A.L. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. 2008, 586, 4567–4576. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Lager, S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front. Physiol. 2016, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, J.E.; Johnston, B.M. Nutrition and fetal growth. Reprod. Fertil. Dev. 1995, 7, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.; Glazier, J.; D’Souza, S. Placental transporter activity and expression in relation to fetal growth. Exp. Physiol. 1997, 82, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Peleg, D.; Kennedy, C.M.; Hunter, S.K. Intrauterine growth restriction: Identification and management. Am. Fam. Physician 1998, 58, 453–460. [Google Scholar] [PubMed]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Groves, J.A.; Lee, A.; Yildirir, G.; Zachara, N.E. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 2013, 18, 535–558. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.-H.; Hsieh, T.-T.; Wu, C.-P.; Li, M.-J.; Yeh, Y.-L.; Chen, S.-F. Mammalian target of rapamycin signaling is a mechanistic link between increased endoplasmic reticulum stress and autophagy in the placentas of pregnancies complicated by growth restriction. Placenta 2017, 60, 9–20. [Google Scholar] [CrossRef]
- Hart, B.; Morgan, E.; Alejandro, E.U. Nutrient sensor signaling pathways and cellular stress in fetal growth restriction. J. Mol. Endocrinol. 2019, 62, R155–R165. [Google Scholar] [CrossRef] [Green Version]
- Gosden, R.G. Maternal Age: A Major Factor Affecting the Prospects and Outcome of Pregnancy. Ann. N. Y. Acad. Sci. 1985, 442, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G. Chromosomal anomalies of preimplantation mouse embryos in relation to maternal age. Reproduction 1973, 35, 351–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.-J.; Zhu, Y.-M.; He, W.-H.; Liu, A.-X.; Dong, M.-Y.; Jin, M.; Sheng, J.-Z.; Huang, H.-F. Endoplasmic reticulum stress induced by oxidative stress in decidual cells: A possible mechanism of early pregnancy loss. Mol. Biol. Rep. 2012, 39, 9179–9186. [Google Scholar] [CrossRef]
- Osol, G.; Moore, L.G. Maternal Uterine Vascular Remodeling During Pregnancy. Microcirculation 2014, 21, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Pepe, G.J. Regulation of Uterine Spiral Artery Remodeling: A Review. Reprod. Sci. 2020, 27, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; D’Errico, J.N.; Stapleton, P.A. Uterine Vascular Control Preconception and During Pregnancy. Compr. Physiol. 2021, 11, 1871–1893. [Google Scholar] [PubMed]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Song, R.; Romero, M.; Dasgupta, C.; Min, J.; Hatcher, D.; Xiao, D.; Blood, A.; Wilson, S.M.; Zhang, L. Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca2+ Sparks and Spontaneous Transient Outward Currents in Uterine Arteries via Heightened Endoplasmic Reticulum/Oxidative Stress. Hypertension 2020, 76, 930–942. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasha, M.; Kirschenman, R.; Wooldridge, A.; Spaans, F.; Cooke, C.-L.M.; Davidge, S.T. The Effect of Tauroursodeoxycholic Acid (TUDCA) Treatment on Pregnancy Outcomes and Vascular Function in a Rat Model of Advanced Maternal Age. Antioxidants 2022, 11, 1275. https://doi.org/10.3390/antiox11071275
Pasha M, Kirschenman R, Wooldridge A, Spaans F, Cooke C-LM, Davidge ST. The Effect of Tauroursodeoxycholic Acid (TUDCA) Treatment on Pregnancy Outcomes and Vascular Function in a Rat Model of Advanced Maternal Age. Antioxidants. 2022; 11(7):1275. https://doi.org/10.3390/antiox11071275
Chicago/Turabian StylePasha, Mazhar, Raven Kirschenman, Amy Wooldridge, Floor Spaans, Christy-Lynn M. Cooke, and Sandra T. Davidge. 2022. "The Effect of Tauroursodeoxycholic Acid (TUDCA) Treatment on Pregnancy Outcomes and Vascular Function in a Rat Model of Advanced Maternal Age" Antioxidants 11, no. 7: 1275. https://doi.org/10.3390/antiox11071275





