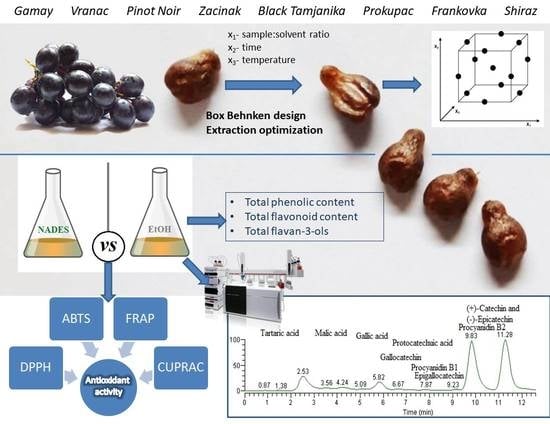
Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Solvent Preparation
2.3. Plant Material and Sample Preparation
2.4. Experimental Design—Determination of Optimal Extraction Conditions
2.5. Extraction Procedure
2.6. HPLC–MS/MS Analyses of Phenolic Compounds in GSEs
2.7. Total Phenol, Flavonoid and Flavan-3-ol Determination
2.8. Antioxidant Activity
2.8.1. Diphenylpicrylhydrazyl (DPPH) Radical Scavenging Microassay
2.8.2. FRAP (Ferric Ion Reducing Antioxidant Power) Microassay
2.8.3. ABTS/TEAC (Trolox Equivalent Antioxidant Capacity) Radical Scavenging Microassay
2.8.4. CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Microassay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Experimental Design—Determination of Optimal Extraction Conditions
3.2. Validation of HPLC–MS/MS Method for Phenolic Compounds Assay
3.3. HPLC–MS/MS Determination of Phenolic Compounds in GSEs
3.4. Content of Total Phenolics, Flavonoids and Flavan-3-ols
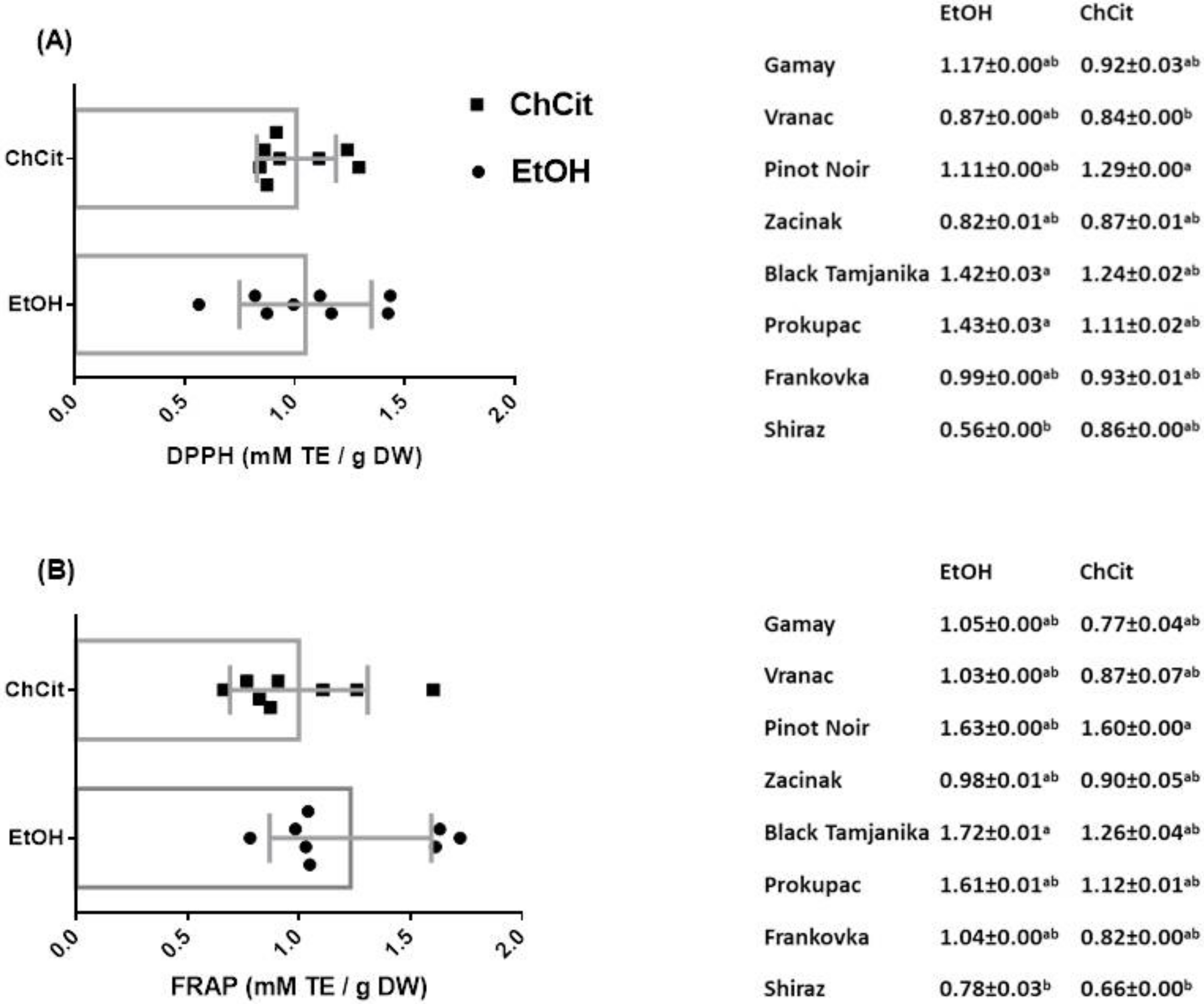
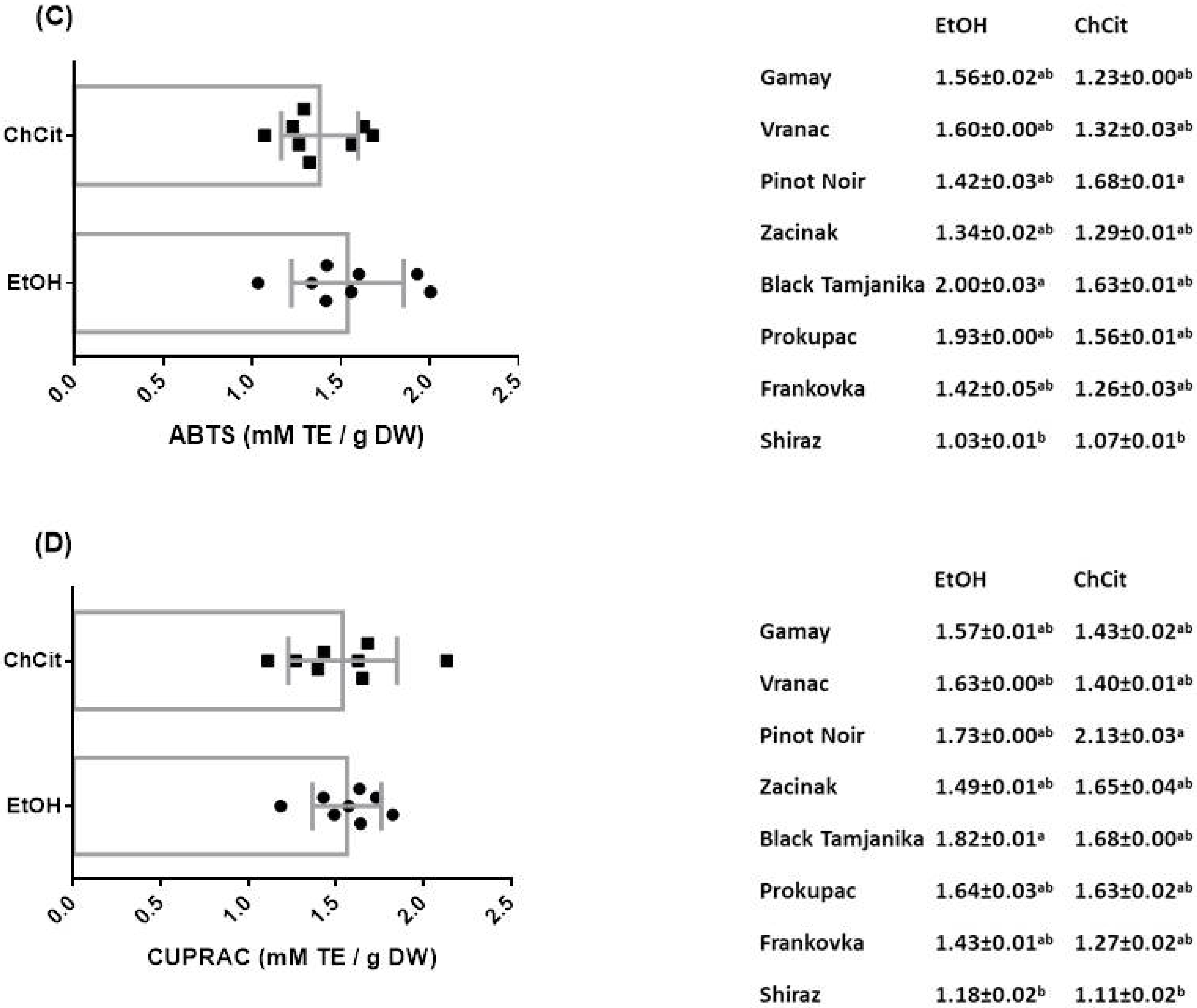
3.5. Antioxidant Activity of GSEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.S.M.; Trindade, H. Comparison of five agro-industrial waste-based composts as growing media for lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Morales, M.P.; Mendívil, M.A.; Juárez, M.C.; Muñoz, L. Using of waste pomace from winery industry to improve thermal insulation of fired clay bricks. Eco-friendly way of building construction. Constr. Build. Mater. 2014, 71, 181–187. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): Study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Godjevac, D.; Tesevic, V.; Velickovic, M.; Vujisic, L.; Vajs, V.; Milosavljevic, S. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J. Serb. Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Mandic, A.I.; Đilas, S.M.; Ćetković, G.S.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Polyphenolic Composition and Antioxidant Activities of Grape Seed Extract. Int. J. Food Prop. 2008, 11, 713–726. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract: Phenolic extraction from grape seed. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography–mass spectrometry analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Washington, T.L.; Yagiz, Y.; Gu, L. Ultrasound-assisted extraction of phenolic acids, flavonols, and flavan-3-ols from muscadine grape skins and seeds using natural deep eutectic solvents and predictive modelling by artificial neural networking. Ultrason. Sonochem. 2021, 79, 105773. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rosé Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Jiang, J.; Duan, W.; Fan, P.; Li, S.; Wang, L. Flavan-3-ols in Vitis seeds: Their extraction and analysis by HPLC-ESI-MS/MS. Food Res. Int. 2021, 139, 109911. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Elessawy, F.M.; Bazghaleh, N.; Vandenberg, A.; Purves, R.W. Polyphenol profile comparisons of seed coats of five pulse crops using a semi-quantitative liquid chromatography-mass spectrometric method. Phytochem. Anal. 2020, 31, 458–471. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Handbook of Analytical Validation; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-0-8247-0689-0. [Google Scholar]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Stuart, D.A.; Ou, B.; Fan, E.; Ji, H.; Kou, Y. Determination of Total Procyanidins in Selected Chocolate and Confectionery Products Using DMAC. J. AOAC Int. 2010, 93, 89–96. [Google Scholar] [CrossRef]
- Norma, P.M.; Virginia, N.-M.; Ral, R.-H.; Jos, C.E.; Cristbal, N.A. A microassay for quantification of 2,2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Delgado-Andrade, C.; Haro, A.; Rufián-Henares, J.A. A physiologic approach to test the global antioxidant response of foods. The GAR method. Food Chem. 2011, 129, 1926–1932. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis galatica Bornm.: A source of multifunctional agents for the management of oxidative damage, Alzheimer’s’s and diabetes mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.LJ.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Cheng, G.; Fa, J.-Q.; Xi, Z.-M.; Zhang, Z.-W. Research on the quality of the wine grapes in corridor area of China. Food Sci. Technol. 2015, 35, 38–44. [Google Scholar] [CrossRef]
- Beslic, Z.; Pantelic, M.; Dabic, D.; Todic, S.; Natic, M.; Tesic, Z. Effect of vineyard floor management on water regime, growth response, yield and fruit quality in Cabernet Sauvignon. Sci. Hortic. 2015, 197, 650–656. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef]
- Bakkalbaşı, E.; Yemiş, O.; Aslanova, D.; Artık, N. Major flavan-3-ol composition and antioxidant activity of seeds from different grape cultivars grown in Turkey. Eur. Food Res. Technol. 2005, 221, 792–797. [Google Scholar] [CrossRef]
- Fuleki, T.; Ricardo da Silva, J.M. Catechin and Procyanidin Composition of Seeds from Grape Cultivars Grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Coletta, A.; Del Coco, L.; Basile, T.; Gambacorta, G.; Fanizzi, F.P. Viticultural practice and winemaking effects on metabolic profile of Negroamaro. Food Chem. 2014, 161, 112–119. [Google Scholar] [CrossRef]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochemical Profiles, Antioxidant and Antibacterial Activities of Grape (Vitis vinifera L.) Seeds and Skin from Organic and Conventional Vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S.J. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.; Row, K. Extraction of catechin compounds from green tea with a new green solvent. Chem. Res. Chin. Univ. 2014, 30, 37–41. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Đorđevski, N.; Stojković, D.; Živković, J.; Pljevljakušić, D.; Ristanović, E.; Nikolić, B.; Ćirić, A. Tamjanika, a Balkan native variety of Vitis vinifera L.: Chemical characterization, antibacterial, and anti-dermatomycosis potential of seed oil. Food Sci. Nutr. 2022, 10, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Radošević, K.; Cvjetko Bubalo, M.; Ganić, K.K.; Redovniković, I.R. COSMOtherm as an Effective Tool for Selection of Deep Eutectic Solvents Based Ready-To-Use Extracts from Graševina Grape Pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]


| Run Order | x1 Sample:Solvent Ratio (w/v) | x2 Time (min) | x3 Temperature (°C) | Y—Experimental TPC (mg GAE/g DW) |
|---|---|---|---|---|
| 1 | 1:10 | 30 | 75 | 86.24 |
| 2 | 2:10 | 30 | 50 | 84.22 |
| 3 | 2:10 | 20 | 25 | 79.85 |
| 4 | 3:10 | 20 | 50 | 78.89 |
| 5 | 2:10 | 40 | 25 | 70.97 |
| 6 | 2:10 | 30 | 50 | 89.70 |
| 7 | 3:10 | 40 | 50 | 76.95 |
| 8 | 2:10 | 30 | 50 | 86.41 |
| 9 | 1:10 | 40 | 50 | 110.75 |
| 10 | 3:10 | 30 | 25 | 53.69 |
| 11 | 1:10 | 20 | 50 | 97.92 |
| 12 | 2:10 | 20 | 75 | 76.08 |
| 13 | 2:10 | 30 | 50 | 89.13 |
| 14 | 3:10 | 30 | 75 | 57.44 |
| 15 | 1:10 | 30 | 25 | 85.34 |
| 16 | 2:10 | 40 | 75 | 78.69 |
| Variety | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gamay | Vranac | Pinot Noir | Zacinak | Black Tamjanika | Prokupac | Frankovka | Shiraz | |
| Phenolic acids | ||||||||
| TA | 1.279 bc | 1.413 abc | 1.719 abc | 1.228 c | 2.844 a | 1.690 abc | 2.501 ab | 1.501 abc |
| MA | 1.088 abc | 0.901 abc | 1.313 abc | 0.362 c | 1.526 ab | 1.853 a | 0.640 bc | 1.327 abc |
| GA | 0.172 c | 0.308 abc | 0.365 abc | 0.580 ab | 0.470 abc | 0.911 a | 0.275 bc | 0.318 abc |
| PA | 0.011 ac | 0.015 ac | 0.008 c | 0.019 ac | 0.008 c | 0.020 ac | <LOQ | 0.027 a |
| Catechins | ||||||||
| GC | 0.004 ab | nd | 0.004 ab | 0.003 b | 0.004 ab | 0.005 a | 0.003 b | nd |
| EGC | 0.012 ab | nd | nd | 0.010 ab | 0.018 ab | 0.022 ab | 0.026 a | 0.005 b |
| C | 1.256 abc | 1.309 abc | 6.978 a | 1.238 c | 2.000 abc | 3.366 ab | 1.709 abc | 1.250 bc |
| EC | 1.393 abc | 0.623 c | 4.782 a | 1.406 abc | 1.595 abc | 3.107 ab | 2.950 abc | 1.080 bc |
| Procyanidins | ||||||||
| PB1 | 1.444 abc | 1.134 bc | 2.267 ab | 1.083 c | 2.162 abc | 2.269 a | 1.337 abc | 1.617 abc |
| PB2 | 0.054 ab | 0.012 b | 0.112 a | 0.018 ab | 0.026 ab | 0.065 ab | 0.065 ab | 0.013 b |
| Variety | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gamay | Vranac | Pinot Noir | Zacinak | Black Tamjanika | Prokupac | Frankovka | Shiraz | |
| Phenolic acids | ||||||||
| TA | 1.121 a | 0.349 abc | 0.129 c | 0.383 abc | 0.201 abc | 0.724 ab | 0.161 bc | 0.510 abc |
| MA | 1.028 ab | 0.806 ab | 0.175 b | 0.822 ab | <LOQ | 1.050 a | 0.635 ab | 0.888 ab |
| GA | 0.140 c | 0.240 abc | 0.294 abc | 0.516 a | 0.326 abc | 0.509 ab | 0.183 bc | 0.241 abc |
| PA | 0.007 bc | 0.017 ab | 0.008 abc | 0.010 abc | 0.014 abc | 0.009 abc | 0.006 c | 0.026 a |
| Catechins | ||||||||
| GC | nd | nd | nd | nd | 0.004 a | 0.004 a | 0.003 b | nd |
| EGC | 0.001 a | 0.001 a | 0.001 a | 0.002 a | 0.005 a | 0.002 a | 0.007 a | 0.001 a |
| C | 0.672 abc | 0.712 abc | 3.126 a | 0.911 abc | 1.018 abc | 1.220 ab | 0.619 bc | 0.469 c |
| EC | 0.494 abc | 0.184 bc | 1.950 a | 0.608 abc | 0.494 abc | 0.714 abc | 0.769 ab | 0.121 c |
| Procyanidins | ||||||||
| PB1 | 1.350 abc | 1.247 c | 3.833 a | 1.611 abc | 1.743 abc | 2.026 ab | 1.307 bc | 1.311 abc |
| PB2 | 0.027 bc | 0.031 abc | 0.080 a | 0.037 abc | 0.049 abc | 0.038 abc | 0.062 ab | 0.024 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants 2022, 11, 1595. https://doi.org/10.3390/antiox11081595
Dabetic N, Todorovic V, Malenovic A, Sobajic S, Markovic B. Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants. 2022; 11(8):1595. https://doi.org/10.3390/antiox11081595
Chicago/Turabian StyleDabetic, Nevena, Vanja Todorovic, Andjelija Malenovic, Sladjana Sobajic, and Bojan Markovic. 2022. "Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents" Antioxidants 11, no. 8: 1595. https://doi.org/10.3390/antiox11081595