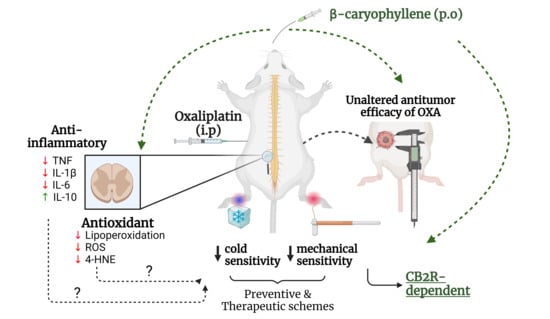
β-Caryophyllene Inhibits Oxaliplatin-Induced Peripheral Neuropathy in Mice: Role of Cannabinoid Type 2 Receptors, Oxidative Stress and Neuroinflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Ethics Statement
2.3. Animal Housing, Randomization, Blinding, and Humane Endpoints
2.4. Tumor Implants
2.5. OXA-Induced Peripheral Neuropathy and Pharmacological Treatments
2.6. Von Frey Test
2.7. Cold Plate Test
2.8. Hargreaves Test
2.9. Tissue Harvesting and Sample Processing
2.10. TBARS Assay
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. DCF Assay
2.13. Statistical Analysis
3. Results
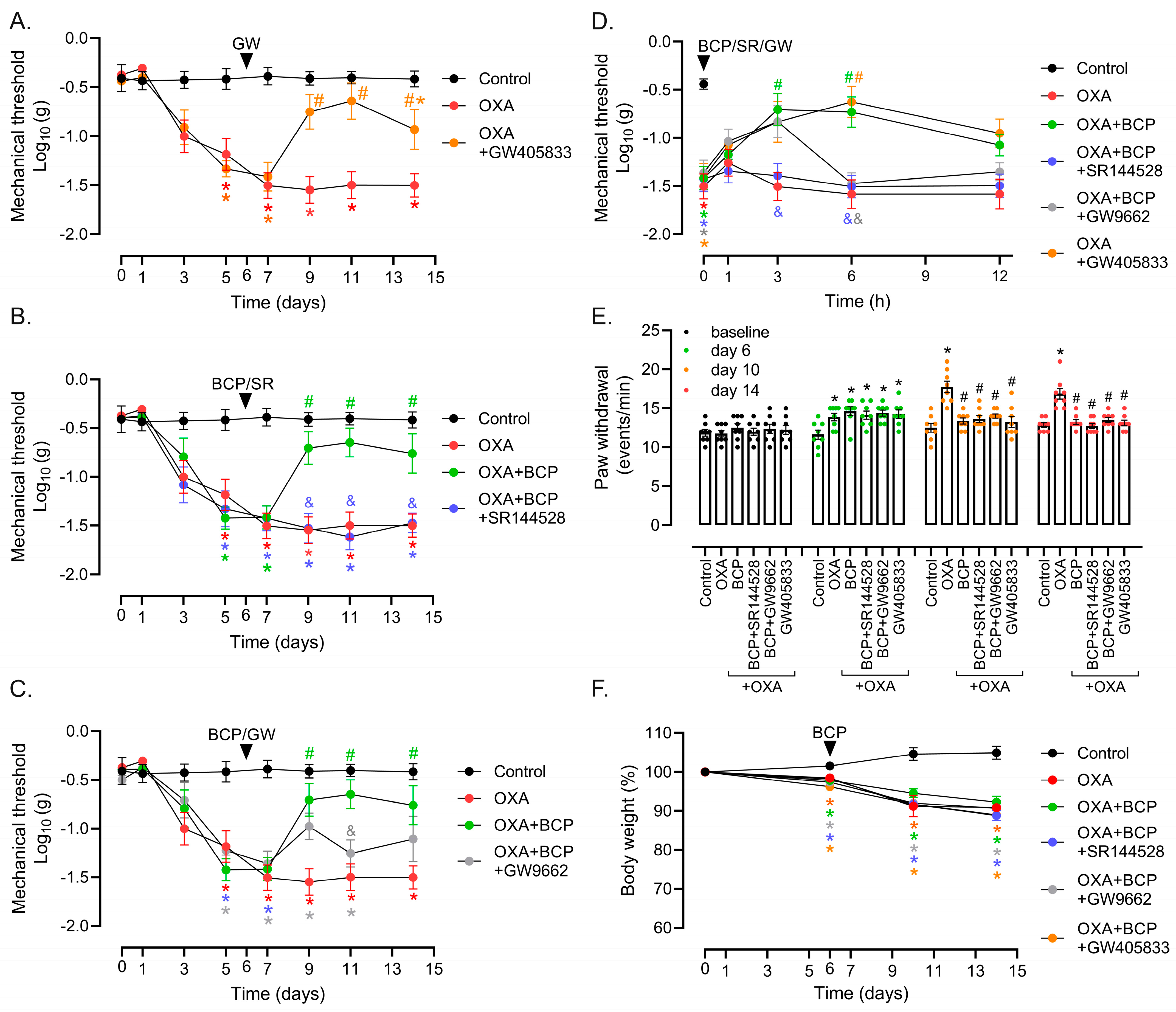
3.1. Preventive and Therapeutic Intervention with BCP Suppresses Mechanical and Cold Allodynia in OXA-Treated Mice
3.2. CB2 Receptor Mediates BCP Anti-Nociceptive Effects in OXA-Induced Peripheral Neuropathy
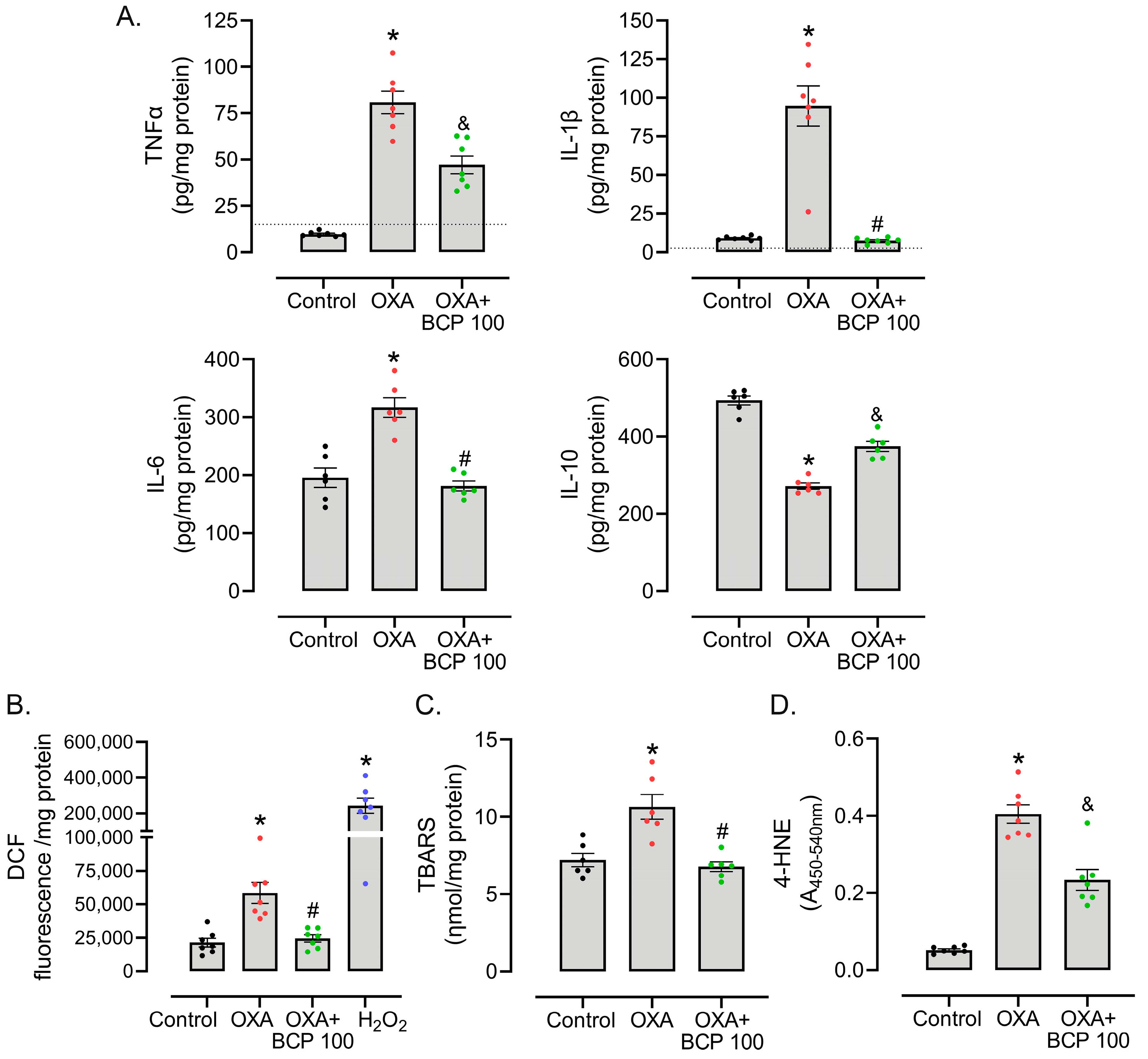
3.3. BCP Decreases ROS Production, Oxidative Damage, and Inflammatory Cytokine Levels in the Spinal Cords of OXA-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Frigeni, B.; Lanzani, F.; Mattavelli, L.; Susani, E.; Alberti, P.; Cortinovis, D.; Bidoli, P. Chemotherapy-Induced Peripheral Neurotoxicity Assessment: A Critical Revision of the Currently Available Tools. Eur. J. Cancer Oxf. Engl. 1990 2010, 46, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, E.G.; Shortland, P.J.; Mahns, D.A. The Incidence of Acute Oxaliplatin-Induced Neuropathy and Its Impact on Treatment in the First Cycle: A Systematic Review. BMC Cancer 2018, 18, 410. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.K.; Smith, E.M.L.; Beutler, A.S.; Ta, L.E.; Lafky, J.M.; Wagner-Johnston, N.D.; Ruddy, K.J.; Dakhil, S.; et al. Clinical Course of Oxaliplatin-Induced Neuropathy: Results from the Randomized Phase III Trial N08CB (Alliance). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Pachman, D.R.; Qin, R.; Seisler, D.; Smith, E.M.L.; Kaggal, S.; Novotny, P.; Ruddy, K.J.; Lafky, J.M.; Ta, L.E.; Beutler, A.S.; et al. Comparison of Oxaliplatin and Paclitaxel-Induced Neuropathy (Alliance A151505). Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Gauchan, P.; Andoh, T.; Kato, A.; Kuraishi, Y. Involvement of Increased Expression of Transient Receptor Potential Melastatin 8 in Oxaliplatin-Induced Cold Allodynia in Mice. Neurosci. Lett. 2009, 458, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Gualdani, R.; Dragoni, E.; Di Cesare Mannelli, L.; Sostegni, S.; Norcini, M.; Gabrielli, G.; la Marca, G.; Richichi, B.; Francesconi, O.; et al. A TRPA1 Antagonist Reverts Oxaliplatin-Induced Neuropathic Pain. Sci. Rep. 2013, 3, 2005. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Prot. Eff. Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef]
- Agnes, J.P.; Santos, V.W.D.; das Neves, R.N.; Gonçalves, R.M.; Delgobo, M.; Girardi, C.S.; Lückemeyer, D.D.; de Ferreira, M.A.; Macedo-Júnior, S.J.; Lopes, S.C.; et al. Antioxidants Improve Oxaliplatin-Induced Peripheral Neuropathy in Tumor-Bearing Mice Model: Role of Spinal Cord Oxidative Stress and Inflammation. J. Pain 2021, 22, 996–1013. [Google Scholar] [CrossRef]
- Sałat, K. Chemotherapy-Induced Peripheral Neuropathy: Part 1-Current State of Knowledge and Perspectives for Pharmacotherapy. Pharmacol. Rep. PR 2020, 72, 486–507. [Google Scholar] [CrossRef]
- Huynh, P.N.; Christensen, S.B.; McIntosh, J.M. RgIA4 Prevention of Acute Oxaliplatin-Induced Cold Allodynia Requires A9-Containing Nicotinic Acetylcholine Receptors and CD3+ T-Cells. Cells 2022, 11, 3561. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of Oxaliplatin and Cisplatin for Dorsal Root Ganglion Neurons Correlates with Platinum-DNA Binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Stojanovska, V.; Pilowsky, P.; Nurgali, K. Platinum Accumulation in the Brain and Alteration in the Central Regulation of Cardiovascular and Respiratory Functions in Oxaliplatin-Treated Rats. Pflugers Arch. 2021, 473, 107–120. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Windebank, A.J. Cisplatin-Induced Apoptosis of DRG Neurons Involves Bax Redistribution and Cytochrome c Release but Not Fas Receptor Signaling. Neurobiol. Dis. 2002, 9, 220–233. [Google Scholar] [CrossRef]
- Calls, A.; Carozzi, V.; Navarro, X.; Monza, L.; Bruna, J. Pathogenesis of Platinum-Induced Peripheral Neurotoxicity: Insights from Preclinical Studies. Exp. Neurol. 2020, 325, 113141. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative Stress and Nerve Damage: Role in Chemotherapy Induced Peripheral Neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Zanardelli, M.; Micheli, L.; Cinci, L.; Failli, P.; Ghelardini, C.; Di Cesare Mannelli, L. Oxaliplatin Neurotoxicity Involves Peroxisome Alterations. PPARγ Agonism as Preventive Pharmacological Approach. PLoS ONE 2014, 9, e102758. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, H.; Liu, Z.-L.; Li, Q.; Qiu, H.-W.; Zeng, L.-J.; Yang, W.; Zhang, X.-Z.; Li, Z.-Y. Activation of STAT3-Mediated CXCL12 up-Regulation in the Dorsal Root Ganglion Contributes to Oxaliplatin-Induced Chronic Pain. Mol. Pain 2017, 13, 1744806917747425. [Google Scholar] [CrossRef]
- Janes, K.; Wahlman, C.; Little, J.W.; Doyle, T.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Spinal Neuroimmune Activation Is Independent of T-Cell Infiltration and Attenuated by A3 Adenosine Receptor Agonists in a Model of Oxaliplatin-Induced Peripheral Neuropathy. Brain Behav. Immun. 2015, 44, 91–99. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, J.H.; Kim, W.; Yoon, S.H.; Kim, S.K. Anti-Allodynic Effect of Buja in a Rat Model of Oxaliplatin-Induced Peripheral Neuropathy via Spinal Astrocytes and pro-Inflammatory Cytokines Suppression. BMC Complement. Altern. Med. 2017, 17, 48. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Huang, W.; Zhang, Y.; Zhang, K.; Li, Y.; Ouyang, H. Wnt3a/YTHDF1 Regulated Oxaliplatin-Induced Neuropathic Pain via TNF-α/IL-18 Expression in the Spinal Cord. Cell. Mol. Neurobiol. 2023, 43, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Kaabi, J.A.; Ojha, S. A Focused Review on CB2 Receptor-Selective Pharmacological Properties and Therapeutic Potential of β-Caryophyllene, a Dietary Cannabinoid. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar] [CrossRef] [PubMed]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nent, E.; Nozaki, C.; Schmöle, A.-C.; Otte, D.; Zimmer, A. CB2 Receptor Deletion on Myeloid Cells Enhanced Mechanical Allodynia in a Mouse Model of Neuropathic Pain. Sci. Rep. 2019, 9, 7468. [Google Scholar] [CrossRef] [PubMed]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid Signaling in Astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the Cannabinoid CB2 Receptor in Microglial Cells in Response to Inflammatory Stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Parlar, A.; Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Yalçin, A.; Elibol, E.; Özer, M.K.; Üçkardeş, F.; Kara, H. The Exogenous Administration of CB2 Specific Agonist, GW405833, Inhibits Inflammation by Reducing Cytokine Production and Oxidative Stress. Exp. Ther. Med. 2018, 16, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant Natural Sources of the Endocannabinoid (E)-β-Caryophyllene: A Systematic Quantitative Analysis of Published Literature. Int. J. Mol. Sci. 2020, 21, 6540. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Farthing, J.N.; Zvonok, A.M.; Makriyannis, A. Selective Activation of Cannabinoid CB2 Receptors Suppresses Hyperalgesia Evoked by Intradermal Capsaicin. J. Pharmacol. Exp. Ther. 2004, 308, 446–453. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Protects against Diet-Induced Dyslipidemia and Vascular Inflammation in Rats: Involvement of CB2 and PPAR-γ Receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of Peripheral Cannabinoid and Opioid Receptors in β-Caryophyllene-Induced Antinociception. Eur. J. Pain Lond. Engl. 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Cao, G.; Li, R.; Liu, J.; Dong, Z.; Xu, L. β-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016, 41, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zuo, T.; Deng, L.; Chen, S.; Yu, W.; Liu, S.; Liu, J.; Wang, X.; Fan, X.; Dong, Z. β-Caryophyllene Suppresses Ferroptosis Induced by Cerebral Ischemia Reperfusion via Activation of the NRF2/HO-1 Signaling Pathway in MCAO/R Rats. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 102, 154112. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; de Bem, A.F. β-Caryophyllene Protects the C6 Glioma Cells against Glutamate-Induced Excitotoxicity through the Nrf2 Pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef]
- Beltramo, M.; Bernardini, N.; Bertorelli, R.; Campanella, M.; Nicolussi, E.; Fredduzzi, S.; Reggiani, A. CB2 Receptor-Mediated Antihyperalgesia: Possible Direct Involvement of Neural Mechanisms. Eur. J. Neurosci. 2006, 23, 1530–1538. [Google Scholar] [CrossRef]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Costa, R.; Calixto, J.B. Antiallodynic Effect of β-Caryophyllene on Paclitaxel-Induced Peripheral Neuropathy in Mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef]
- Valenzano, K.J.; Tafesse, L.; Lee, G.; Harrison, J.E.; Boulet, J.M.; Gottshall, S.L.; Mark, L.; Pearson, M.S.; Miller, W.; Shan, S.; et al. Pharmacological and Pharmacokinetic Characterization of the Cannabinoid Receptor 2 Agonist, GW405833, Utilizing Rodent Models of Acute and Chronic Pain, Anxiety, Ataxia and Catalepsy. Neuropharmacology 2005, 48, 658–672. [Google Scholar] [CrossRef]
- Vera, G.; Cabezos, P.A.; Martín, M.I.; Abalo, R. Characterization of Cannabinoid-Induced Relief of Neuropathic Pain in a Rat Model of Cisplatin-Induced Neuropathy. Pharmacol. Biochem. Behav. 2013, 105, 205–212. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Gu, L.; Yan, J.; Guo, J.; Geng, X.; Shi, H.; Yu, G.; Zhu, C.; Yang, Y.; Zhou, Y.; et al. An Effective and Concise Device for Detecting Cold Allodynia in Mice. Sci. Rep. 2018, 8, 14002. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A New and Sensitive Method for Measuring Thermal Nociception in Cutaneous Hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.; Ribeiro, C.T.; Bortolin, R.C.; Somensi, N.; Fernandes, H.S.; Teixeira, A.A.; Guasselli, M.O.R.; Agani, C.A.J.O.; Souza, N.C.; Grings, M.; et al. Anti-RAGE Antibody Selectively Blocks Acute Systemic Inflammatory Responses to LPS in Serum, Liver, CSF and Striatum. Brain Behav. Immun. 2017, 62, 124–136. [Google Scholar] [CrossRef]
- Ravikumar, M.; Jain, S.; Miller, R.H.; Capadona, J.R.; Selkirk, S.M. An Organotypic Spinal Cord Slice Culture Model to Quantify Neurodegeneration. J. Neurosci. Methods 2012, 211, 280–288. [Google Scholar] [CrossRef]
- Deng, L.; Guindon, J.; Vemuri, V.K.; Thakur, G.A.; White, F.A.; Makriyannis, A.; Hohmann, A.G. The Maintenance of Cisplatin- and Paclitaxel-Induced Mechanical and Cold Allodynia Is Suppressed by Cannabinoid CB2 Receptor Activation and Independent of CXCR4 Signaling in Models of Chemotherapy-Induced Peripheral Neuropathy. Mol. Pain 2012, 8, 71. [Google Scholar] [CrossRef]
- Deng, L.; Cornett, B.L.; Mackie, K.; Hohmann, A.G. CB1 Knockout Mice Unveil Sustained CB2-Mediated Antiallodynic Effects of the Mixed CB1/CB2 Agonist CP55,940 in a Mouse Model of Paclitaxel-Induced Neuropathic Pain. Mol. Pharmacol. 2015, 88, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Diaz, P.; Bie, B.; Astruc-Diaz, F.; Wu, J.; Yang, H.; Brown, D.L.; Naguib, M. Spinal Gene Expression Profiling and Pathways Analysis of a CB2 Agonist (MDA7)-Targeted Prevention of Paclitaxel-Induced Neuropathy. Neuroscience 2014, 260, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, S.; Madrigal, J.L.; Caso, J.R.; García-Gutiérrez, M.S.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory Role of the Cannabinoid CB2 Receptor in Stress-Induced Neuroinflammation in Mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a Natural Bicyclic Sesquiterpene Attenuates Doxorubicin-Induced Chronic Cardiotoxicity via Activation of Myocardial Cannabinoid Type-2 (CB2) Receptors in Rats. Chem. Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Di Cesare Mannelli, L.; Pacini, A. Oxaliplatin-Induced Blood Brain Barrier Loosening: A New Point of View on Chemotherapy-Induced Neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef]
- Park, S.B.; Lin, C.S.-Y.; Krishnan, A.V.; Goldstein, D.; Friedlander, M.L.; Kiernan, M.C. Oxaliplatin-Induced Neurotoxicity: Changes in Axonal Excitability Precede Development of Neuropathy. Brain J. Neurol. 2009, 132 Pt 10, 2712–2723. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, W. The Role of Satellite Glial Cells, Astrocytes, and Microglia in Oxaliplatin-Induced Neuropathic Pain. Biomedicines 2020, 8, 324. [Google Scholar] [CrossRef]
- Trevisan, G.; Materazzi, S.; Fusi, C.; Altomare, A.; Aldini, G.; Lodovici, M.; Patacchini, R.; Geppetti, P.; Nassini, R. Novel Therapeutic Strategy to Prevent Chemotherapy-Induced Persistent Sensory Neuropathy by TRPA1 Blockade. Cancer Res. 2013, 73, 3120–3131. [Google Scholar] [CrossRef]
- Quartilho, A.; Mata, H.P.; Ibrahim, M.M.; Vanderah, T.W.; Porreca, F.; Makriyannis, A.; Malan, T.P. Inhibition of Inflammatory Hyperalgesia by Activation of Peripheral CB2 Cannabinoid Receptors. Anesthesiology 2003, 99, 955–960. [Google Scholar] [CrossRef]
- Sagar, D.R.; Kelly, S.; Millns, P.J.; O’Shaughnessey, C.T.; Kendall, D.A.; Chapman, V. Inhibitory Effects of CB1 and CB2 Receptor Agonists on Responses of DRG Neurons and Dorsal Horn Neurons in Neuropathic Rats. Eur. J. Neurosci. 2005, 22, 371–379. [Google Scholar] [CrossRef]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The Effects of β-Caryophyllene Oxide and Trans-Nerolidol on the Efficacy of Doxorubicin in Breast Cancer Cells and Breast Tumor-Bearing Mice. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Ganju, R.K. Synthetic Cannabinoid Receptor Agonists Inhibit Tumor Growth and Metastasis of Breast Cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Fiorenzani, P.; Pessina, F.; Pinassi, J.; Aglianò, M.; Miragliotta, V.; Aloisi, A.M. The CB2 Agonist β-Caryophyllene in Male and Female Rats Exposed to a Model of Persistent Inflammatory Pain. Front. Neurosci. 2020, 14, 850. [Google Scholar] [CrossRef]
- Eeswara, A.; Pacheco-Spiewak, A.; Jergova, S.; Sagen, J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS ONE 2023, 18, e0282920. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnes, J.P.; dos Santos, B.; das Neves, R.N.; Luciano, V.M.M.; Benvenutti, L.; Goldoni, F.C.; Schran, R.G.; Santin, J.R.; Quintão, N.L.M.; Zanotto-Filho, A. β-Caryophyllene Inhibits Oxaliplatin-Induced Peripheral Neuropathy in Mice: Role of Cannabinoid Type 2 Receptors, Oxidative Stress and Neuroinflammation. Antioxidants 2023, 12, 1893. https://doi.org/10.3390/antiox12101893
Agnes JP, dos Santos B, das Neves RN, Luciano VMM, Benvenutti L, Goldoni FC, Schran RG, Santin JR, Quintão NLM, Zanotto-Filho A. β-Caryophyllene Inhibits Oxaliplatin-Induced Peripheral Neuropathy in Mice: Role of Cannabinoid Type 2 Receptors, Oxidative Stress and Neuroinflammation. Antioxidants. 2023; 12(10):1893. https://doi.org/10.3390/antiox12101893
Chicago/Turabian StyleAgnes, Jonathan Paulo, Barbara dos Santos, Raquel Nascimento das Neves, Vitória Maria Marques Luciano, Larissa Benvenutti, Fernanda Capitanio Goldoni, Roberta Giusti Schran, José Roberto Santin, Nara Lins Meira Quintão, and Alfeu Zanotto-Filho. 2023. "β-Caryophyllene Inhibits Oxaliplatin-Induced Peripheral Neuropathy in Mice: Role of Cannabinoid Type 2 Receptors, Oxidative Stress and Neuroinflammation" Antioxidants 12, no. 10: 1893. https://doi.org/10.3390/antiox12101893