Microencapsulation of Chilean Papaya Waste Extract and Its Impact on Physicochemical and Bioactive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection and Preparation of Raw Material
2.3. Preparation of Chilean Papaya Waste Extracts
2.4. Preparation of Microcapsules
2.5. Microencapsulation Yield
2.6. Physicochemical Characterization
2.7. Thermal Analysis
2.8. Total Phenolic (TPC) and Total Flavonoid Contents (TFC)
2.9. Microencapsulation Efficiency (MEE)
2.10. Identification and Quantification of Phenolic Compounds
2.11. Measurement of Antioxidant Capacity
2.12. Cell Culture and Analysis of Intracellular ROS Generation
2.13. Measurement of Antimicrobial Activity
2.14. Statistical Analysis
3. Results and Discussions
3.1. Microencapsulation Yield
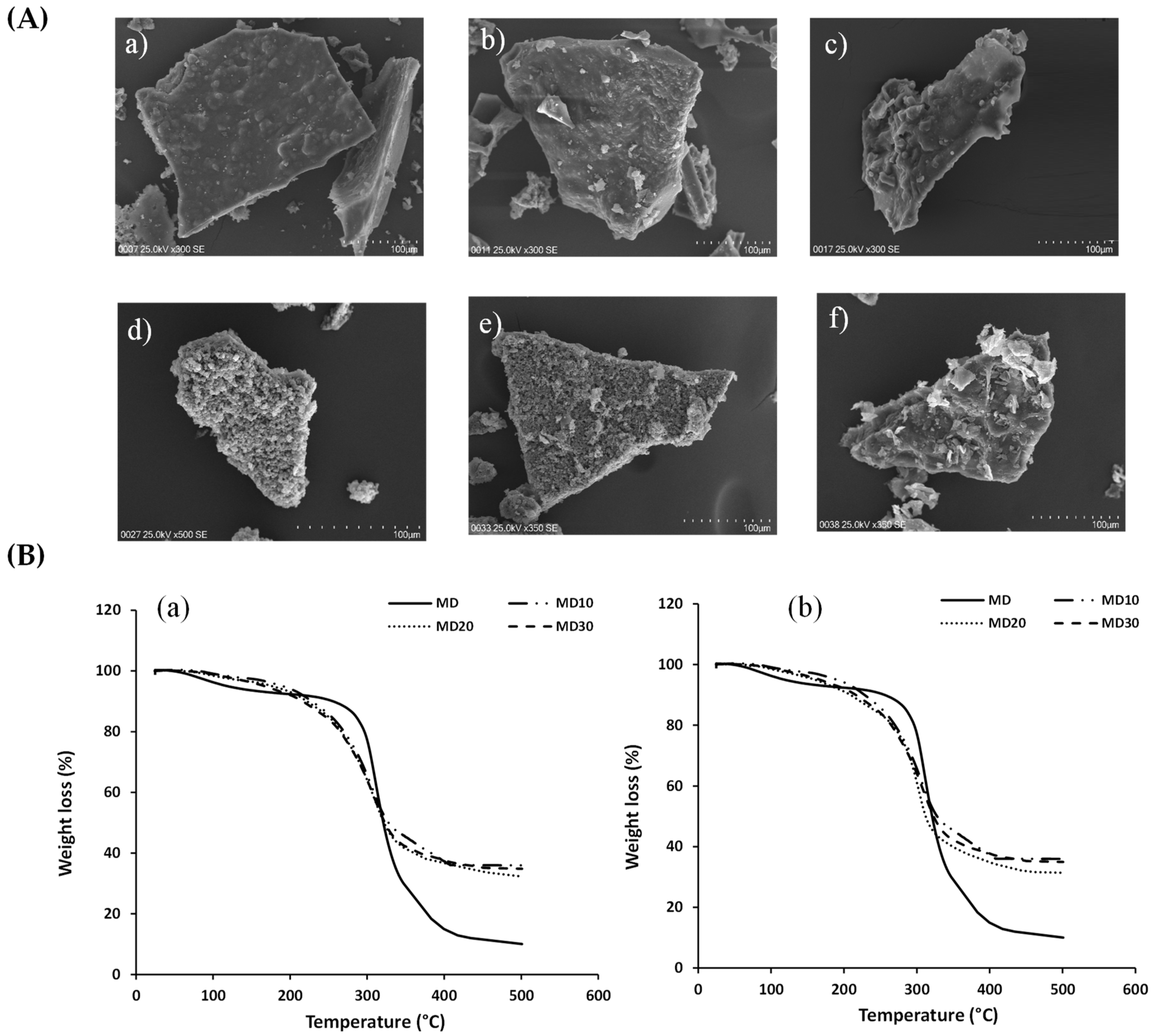
3.2. Physicochemical Characterization and Thermal Analysis
3.3. Phenolic Profile, TPC, TFC, and MEE
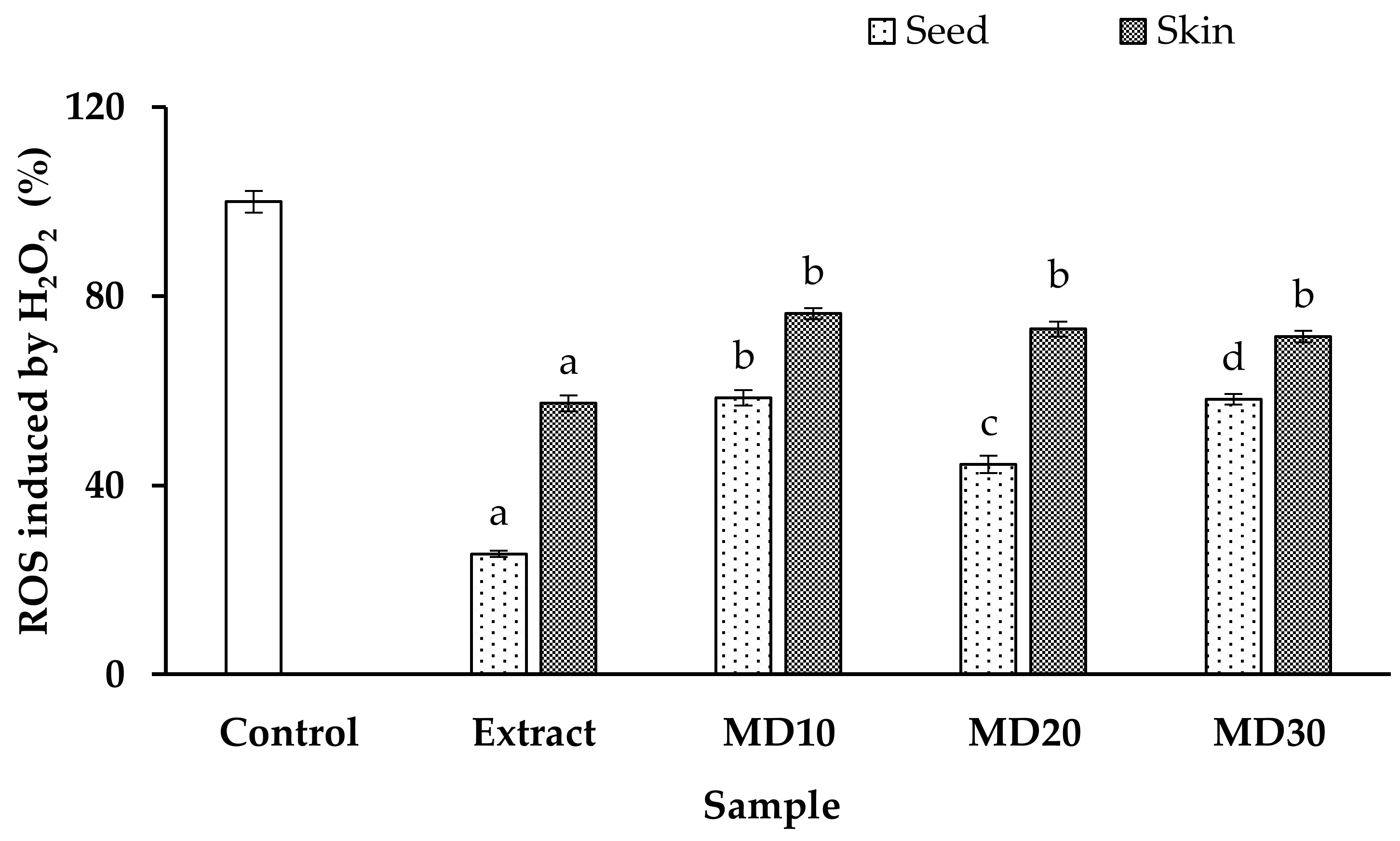
3.4. Antioxidant Capacity and Intracellular ROS Generation
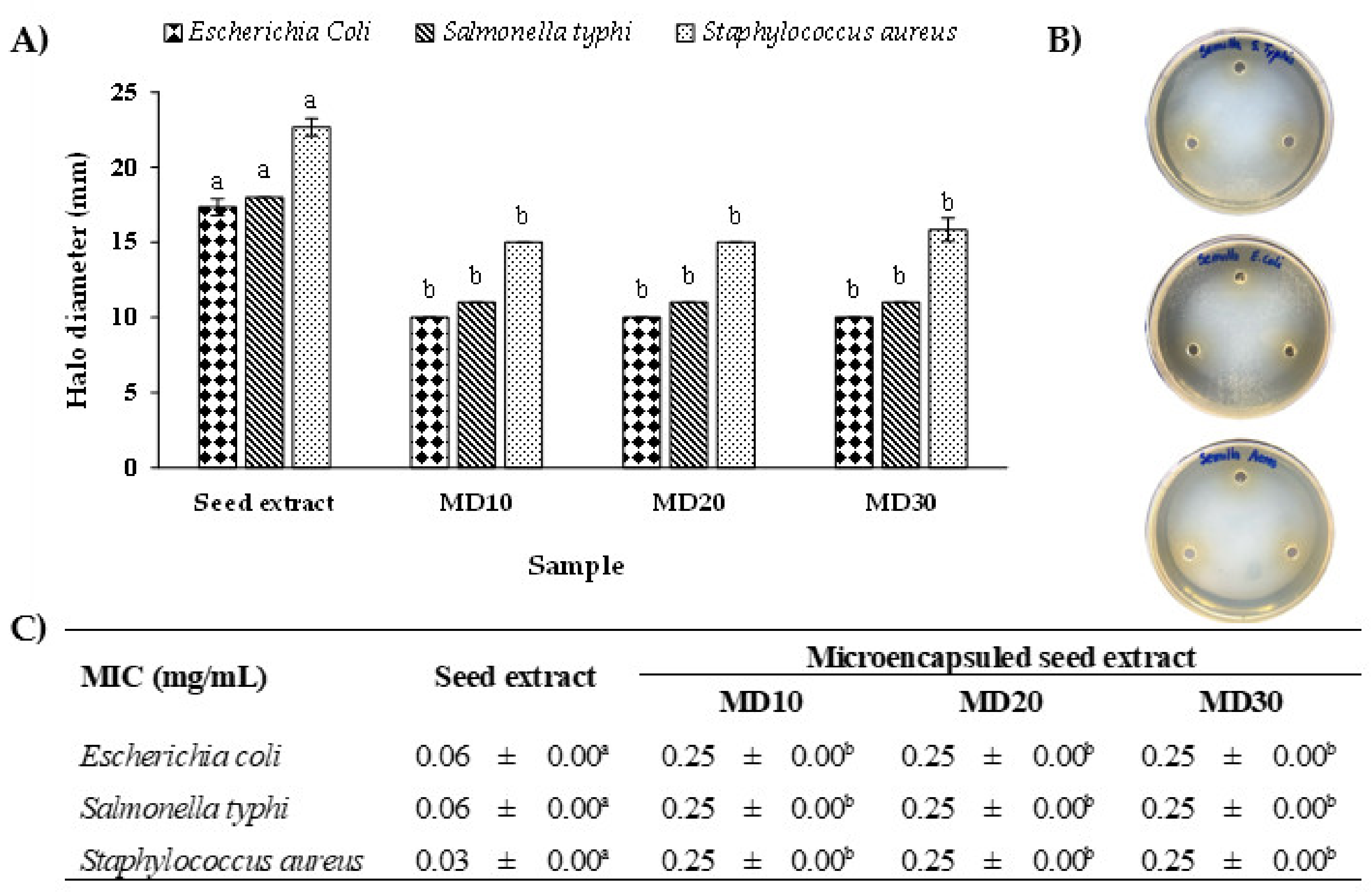
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT—Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Uribe, E.; Delgadillo, A.; Giovagnoli-Vicuña, C.; Quispe-Fuentes, I.; Zura-Bravo, L. Extraction techniques for bioactive compounds and antioxidant capacity determination of Chilean papaya (Vasconcellea pubescens) fruit. J. Chem. 2015, 2015, 347532. [Google Scholar] [CrossRef]
- Pavithra, C.S.; Devi, S.S.; Suneetha, J.W.; Durga Rani, C.V. Nutritional properties of papaya peel. Pharma Innov. J. 2017, 6, 170–173. [Google Scholar]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Iesce, M.R.; Naviglio, D.; Ciaravolo, M.; Vitale, E.; Arena, C. Comparative studies on different citrus cultivars: A revaluation of waste mandarin components. Antioxidants 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Tatasciore, S.; Santarelli, V.; Neri, L.; González Ortega, R.; Faieta, M.; Di Mattia, C.D.; Di Michele, A.; Pittia, P. Freeze-Drying Microencapsulation of Hop Extract: Effect of Carrier Composition on Physical, Techno-Functional, and Stability Properties. Antioxidants 2023, 12, 442. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Parikh, A.; Agarwal, S.; Raut, K. A review on application of maltodextrin in pharmaceutical industry. Int. J. Pharm. Biol. Sci. 2014, 4, 67–74. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Z.; Xia, J.; Kang, Y.; Sun, P.; Xiao, Z.; Niu, Y. Research progress of starch as microencapsulated wall material. Carbohydr. Polym. 2023, 318, 121118. [Google Scholar] [CrossRef] [PubMed]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as wall materials in spray-dried microencapsulation of bioactive compounds: Physicochemical properties and characterization. Crit. Rev. Food Sci. Nutr. 2023, 63, 6983–7015. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Guerrero, R.; Revelo-Cuarán, Y.A.; Osorio-Mora, O.; Arango-Bedoya, O. Extracción asistida con ultrasonido de compuestos fenólicos de dos variedades de papas (Solanum phureja) nativas andinas y evaluación de su actividad antioxidante. Inf. Tecnol. 2020, 31, 43–50. [Google Scholar] [CrossRef]
- Giovagnoli-Vicuña, C.; Briones-Labarca, V.; Romero, M.S.; Giordano, A.; Pizarro, S. Effect of Extraction Methods and In Vitro Bio-Accessibility of Microencapsulated Lemon Extract. Molecules 2022, 27, 4166. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Method of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Lorenzoni Nunes, G.; Bremer Boaventura, B.C.; Silva Pinto, S.; Verruck, S.; Seigi Murakami, F.; Schwinden Prudêncio, E.; Dias de Mello Castanho Amboni, R. Microencapsulation of freeze concentrated Ilex paraguariensis extract by spray drying. J. Food Eng. 2015, 151, 60–68. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of Betabel Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Velásquez, P.; Riquelme, K.; Leyton, F.; Giordano, A.; Gómez, M.; Montenegro, G. Antibacterial potential assessment of Nalca (Gunnera tinctoria Mol.) ethanolic extracts. Nat. Prod. Res. 2020, 35, 5425–5428. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Van Den Berg, R.; Haenen, G.R.M.M.; Van Den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma ( FRAP ) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Garrido, C.; Cho, Y.; Cortés-Rios, J.; Vasquez, D.; Pessoa-Mahana, C.D.; Araya-Maturana, R.; Pessoa-Mahana, H.; Faundez, M. Nitrofuran drugs beyond redox cycling: Evidence of Nitroreduction-independent cytotoxicity mechanism. Toxicol. Appl. Pharmacol. 2020, 401, 115104. [Google Scholar] [CrossRef]
- Balci-Torun, F.; Ozdemir, F. Encapsulation of strawberry flavour and physicochemical characterization of the encapsulated powders. Powder Technol. 2021, 380, 602–612. [Google Scholar] [CrossRef]
- Vera Zambrano, M.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Awolu, O.O.; Fole, E.T.; Oladeji, O.A.; Ayo-Omogie, H.N.; Olagunju, A.I. Microencapsulation of avocado pear seed (Persea Americana mill) bioactive-rich extracts and evaluation of its antioxidants, in vitro starch digestibility and storage stability. Bull. Natl. Res. Cent. 2022, 46, 1–11. [Google Scholar] [CrossRef]
- Mohd Nawi, N.; Muhamad, I.I.; Mohd Marsin, A. The physicochemical properties of microwave-assisted encapsulated anthocyanins from Ipomoea batatas as affected by different wall materials. Food Sci. Nutr. 2015, 3, 91–99. [Google Scholar] [CrossRef]
- George, T.T.; Oyenihi, A.B.; Rautenbach, F.; Obilana, A.O. Characterization of moringa oleifera leaf powder extract encapsulated in maltodextrin and/or gum arabic coatings. Foods 2021, 10, 3044. [Google Scholar] [CrossRef]
- Ribeiro, M.L.F.F.; Roos, Y.H.; Ribeiro, A.P.B.; Nicoletti, V.R. Effects of maltodextrin content in double-layer emulsion for production and storage of spray-dried carotenoid-rich microcapsules. Food Bioprod. Process. 2020, 124, 208–221. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Karagozlu, M.; Ocak, B.; Özdestan-Ocak, Ö. Effect of Tannic Acid Concentration on the Physicochemical, Thermal, and Antioxidant Properties of Gelatin/Gum Arabic–Walled Microcapsules Containing Origanum onites L. Essential Oil. Food Bioprocess Technol. 2021, 14, 1231–1243. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Favaro-Trindade, C.S. Physico-chemical properties, stability, and potential food applications of shrimp lipid extract encapsulated by complex coacervation. Food Bioprocess Technol. 2018, 11, 1596–1604. [Google Scholar] [CrossRef]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.B. Physical and Functional Properties, Digestibility, and Storage Stability of Spray- and Freeze-Dried Microencapsulated Bioactive Products from Moringa stenopetala Leaves Extract. Ind. Crops Prod. 2020, 156, 112891. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of drying air temperature and carrier agent concentration on the physicochemical properties of açai juice powder. Cienc. Tecnol. Aliment. 2009, 29, 444–450. [Google Scholar] [CrossRef]
- Nadali, N.; Pahlevanlo, A.; Sarabi-Jamab, M.; Balandari, A. Effect of maltodextrin with different dextrose equivalents on the physicochemical properties of spray-dried barberry juice (Berberis vulgaris L.). J. Food Sci. Technol. 2022, 59, 2855–2866. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Locali Pereira, A.R.; Gonçalves Cattelan, M.; Nicoletti, V.R. Microencapsulation of pink pepper essential oil: Properties of spray-dried pectin/SPI double-layer versus SPI single-layer stabilized emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123806. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, M.G.; Amaya-Guerra, C.A.; Quintero-Ramos, A.; de Jesús Ruiz-Anchondo, T.; Gutiérrez-Uribe, J.A.; Baez-González, J.G.; Lardizabal-Gutiérrez, D.; Campos-Venegas, K. Effect of soluble fiber on the physicochemical properties of cactus pear (Opuntia ficus indica) encapsulated using spray drying. Food Sci. Biotechnol. 2014, 23, 755–763. [Google Scholar] [CrossRef]
- Mazuco, R.A.; Cardoso, P.M.M.; Bindaco, É.S.; Scherer, R.; Castilho, R.O.; Faraco, A.A.G.; Ruas, F.G.; Oliveira, J.P.; Guimarães, M.C.C.; de Andrade, T.U.; et al. Maltodextrin and Gum Arabic-Based Microencapsulation Methods for Anthocyanin Preservation in Juçara Palm (Euterpe edulis Martius) Fruit Pulp. Plant Foods Hum. Nutr. 2018, 73, 209–215. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Adriani, L.; Abun, A.; Muchtaridi, M.; Tanuwiria, U.H. The microencapsulation of mangosteen peel extract with maltodextrin from arenga starch: Formulation and characterization. J. Appl. Pharm. Sci. 2019, 9, 33–40. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cortina, A.; Rodríguez-Cortina, J.; Hernández-Carrión, M. Obtention of Sacha Inchi (Plukenetia volubilis Linneo) Seed Oil Fruits: Physicochemical, Morphological, and Controlled Release Characterization. Foods 2022, 11, 3950. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Stucken, K.; Cantuarias, C.; Lamas, F.; García, V.; Pastén, A. Antimicrobial properties of papaya (Vasconcellea pubescens) subjected to low-temperature vacuum dehydration. Innov. Food Sci. Emerg. Technol. 2021, 67, 102563. [Google Scholar] [CrossRef]
- Mahdavee Khazaei, K.; Jafari, S.M.; Ghorbani, M.; Hemmati Kakhki, A. Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr. Polym. 2014, 105, 57–62. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography-UV detection-mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Poblete, J.; Quispe-Fuentes, I.; Uribe, E.; Bilbao-Sainz, C.; Pastén, A. Chemical and bioactive characterization of papaya (Vasconcellea pubescens) under different drying technologies: Evaluation of antioxidant and antidiabetic potential. J. Food Meas. Charact. 2019, 13, 1980–1990. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Luo, J.; Wang, F.; Liu, G.; Zhu, H.; Guo, Y. PVDF grafted Gallic acid to enhance the hydrophilicity and antibacterial properties of PVDF composite membrane. Sep. Purif. Technol. 2021, 259, 118127. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, C.; Huang, B.; Fei, P. Preparation of acylated pectin with gallic acid through enzymatic method and their emulsifying properties, antioxidation activities and antibacterial activities. Int. J. Biol. Macromol. 2020, 165, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M.; Machaczka, M. Recent advances in biological activity, new formulations and prodrugs of ferulic acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Fatemeh Askari, S.; Fazelian, S.; Hossein Ayati, M.; Namazi, N. The roles of quercetin in diabetes mellitus and related metabolic disorders; special focus on the modulation of gut microbiota: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 63, 2990–3003. [Google Scholar] [CrossRef] [PubMed]
- Savych, A.; Marchyshyn, S.; Kyryliv, M.; Bekus, I. Cinnamic acid and its derivatives in the herbal mixtures and their antidiabetic activity. Farmacia 2021, 69, 595–601. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.-B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Turan, D.; Abdik, H.; Sahin, F.; Avşar Abdik, E. Evaluation of the neuroprotective potential of caffeic acid phenethyl ester in a cellular model of Parkinson’s disease. Eur. J. Pharmacol. 2020, 883, 173342. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in nfeuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Handayani, M.N.; Khoerunnisa, I.; Cakrawati, D.; Sulastri, A. Microencapsulation of Dragon Fruit (Hylocereus polyrhizus) Peel Extract Using Maltodextrin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 012099. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, H.S. Effect of microencapsulation on antioxidant and antifungal properties of aqueous extract of pomegranate peel. J. Food Sci. Technol. 2020, 57, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Shin, J.; Park, G.; Oh, J.W. Quercetin-3-glucoside extracted from apple pomace induces cell cycle arrest and apoptosis by increasing intracellular ROS levels. Int. J. Mol. Sci. 2021, 22, 10749. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábí, J.; Tothova, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tian, X.H.; Yi, Y.S.; Jiang, W.S.; Zhou, Y.J.; Cheng, W.J. Luteolin-induced protection of H2O2-induced apoptosis in PC12 cells and the associated pathway. Mol. Med. Rep. 2015, 12, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, J.; Wang, H.; Wang, M.; Zhao, J.; Wu, Z. Protective effect of apple phlorizin on hydrogen peroxide-induced cell damage in HepG2 cells. J. Food Biochem. 2019, 43, 13052. [Google Scholar] [CrossRef]
- Wang, W.; Shang, H.; Li, J.; Ma, Y.; Xu, C.; Ma, J.; Hou, J.; Jiang, Z. Four Different Structural Dietary Polyphenols, Especially Dihydromyricetin, Possess Superior Protective Effect on Ethanol-Induced ICE-6 and AML-12 Cytotoxicity: The Role of CYP2E1 and Keap1-Nrf2 Pathways. J. Agric. Food Chem. 2023, 71, 1518–1530. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Pasten, A.; Cantuarias, C.; Stucken, K.; García, V.; Rodríguez, A.; Valenzuela-Barra, G.; Delporte, C. Effect of High- and Low-Temperature Drying Methods on Fatty Acid Profile and Antimicrobial and Anti-Inflammatory Traits of Papaya (Vasconcellea pubescens). ACS Food Sci. Technol. 2023, 3, 77–84. [Google Scholar] [CrossRef]
- Egbuonu, A.; Harry, E.; Orji, I. Comparative Proximate and Antibacterial Properties of Milled Carica papaya (Pawpaw) Peels and Seeds. Br. J. Pharm. Res. 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Content (%) | |
|---|---|---|
| Seed | Skin | |
| Moisture | 63.71 | 90.32 |
| Protein | 10.92 | 1.65 |
| Lipid | 11.16 | 0.20 |
| Fiber total | 8.33 | 1.82 |
| Ash | 2.02 | 1.22 |
| Carbohydrate | 12.19 | 6.61 |
| Parameters | Microencapsulated Seed Extract | Microencapsulated Skin Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Seed Extract | MD10 | MD20 | MD30 | Skin Extract | MD10 | MD20 | MD30 | |
| Moisture content (g/100 g) | 22.9 ± 1.2 a | 9.3 ± 0.1 b | 7.0 ± 0.2 c | 6.2 ± 1.2 c | 23.8 ± 0.1 a | 9.9 ± 0.0 b | 9.0 ± 0.7 b | 4.9 ± 0.2 c |
| Aw (Dimensionless) | 0.517 ± 0.011 a | 0.368 ± 0.012 b | 0.353 ± 0.005 b | 0.297 ± 0.030 c | 0.503 ± 0.004 a | 0.334 ± 0.005 b | 0.282 ± 0.003 c | 0.282 ± 0.003 c |
| Hygroscopicity (g/100 g) | 34.2 ± 1.8 a | 23.3 ± 1.2 b | 22.5 ± 2.0 bc | 20.5 ± 1.4 c | 31.5 ± 2.4 a | 11.8 ± 1.8 b | 13.9 ± 0.9 b | 13.1 ± 1.5 b |
| Solubility in water (%) | 5.3 ± 0.0 a | 20.7 ± 0.7 b | 13.4 ± 0.5 c | 4.3 ± 0.6 d | 9.6 ± 0.1 a | 20.3 ± 0.6 b | 19.1 ± 0.9 b | 16.1 ± 0.2 c |
| Lightness (L*) | 24.7 ± 1.1 a | 45.3 ± 0.7 b | 52.0 ± 0.1 c | 60.4 ± 0.1 d | 31.1 ± 1.3 a | 78.6 ± 0.7 b | 68.1 ± 1.0 c | 66.9 ± 0.2 c |
| a* color coordinate | 7.9 ± 0.2 a | 10.9 ± 1.2 b | 11.0 ± 0.9 b | 13.6 ± 0.8 c | 7.2 ± 0.2 a | 6.8 ± 0.3 a | 11.5 ± 0.1 b | 13.1 ± 1.1 c |
| b* color coordinate | 11.6 ± 0.1 a | 13.5 ± 0.3 b | 13.2 ± 0.3 b | 18.8 ± 0.0 c | 25.8 ± 0.4 a | 33.6 ± 0.7 b | 41.8 ± 0.4 c | 47.7 ± 0.5 d |
| Size of microcapsule (µm) | - | 243.5 ± 15.9 | 242.3 ± 24.3 | 328.8 ± 22.8 | - | 247.8 ± 23.5 | 538.9 ± 16.2 | 537.3 ± 12.7 |
| Phenolic Profile (µg/mL) | Extract | Microencapsulated Extract | ||
|---|---|---|---|---|
| MD10 | MD20 | MD30 | ||
| Seed Samples | ||||
| Gallic acid | 171.0 ± 0.4 a | 18.6 ± 0.1 b | 7.1 ± 0.1 c | 32.2 ± 0.1 d |
| Ferulic acid | 4.1 ± 0.1 a | 8.0 ± 0.1 b | 5.1 ± 0.1 c | 4.0 ± 0.1 d |
| Chlorogenic acid | 159.2 ± 0.3 a | 558.6 ± 0.9 b | 398.6 ± 0.2 c | 334.1 ± 0.3 d |
| Caffeic acid | 7.8 ± 0.2 a | ND | ND | ND |
| Coumaric acid | 12.3 ± 0.1 a | 8.4 ± 0.1 b | 20.0 ± 0.2 c | 20.1 ± 0.1 c |
| Rutin | 1878.6 ± 0.8 a | 2093.7 ± 0.8 b | 2126.4 ± 0.9 c | 1927.0 ± 0.9 d |
| Quercetin | 67.9 ± 0.2 a | 79.2 ± 0.2 b | 76.2 ± 0.4 c | 69.1 ± 0.2 d |
| Skin Samples | ||||
| Gallic acid | 21.0 ± 0.2 a | 26.8 ± 0.1 b | 13.4 ± 0.1 c | 3.0 ± 0.1 d |
| Ferulic acid | 7.3 ± 0.1 a | 6.6 ± 0.1 b | 4.9 ± 0.1 c | 5.1 ± 0.1 c |
| Chlorogenic acid | 194.5 ± 0.5 a | 321.2 ± 0.2 b | 180.5 ± 0.2 c | 648.5 ± 0.5 d |
| Caffeic acid | 35.5 ± 0.2 a | ND | ND | ND |
| Coumaric acid | 7.0 ± 0.1 a | 15.3 ± 0.1 b | 12.2 ± 0.3 c | 10.0 ± 0.1 d |
| Rutin | 1682.7 ± 0.9 a | 2114.4 ± 0.8 b | 2013.1 ± 0.9 c | 2016.7 ± 0.9 d |
| Quercetin | 63.6 ± 0.3 a | 70.0 ± 0.2 b | 66.4 ± 0.7 c | 92.7 ± 0.6 d |
| Assay | Microencapsulated Seed Extract | Microencapsulated Skin Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Seed Extract | MD10 | MD20 | MD30 | Skin Extract | MD10 | MD20 | MD30 | |
| TPC (mg/g DW) | 44.49 ± 1.68 a | 41.11 ± 3.32 a | 44.20 ± 0.50 a | 32.85 ± 3.00 b | 29.24 ± 1.99 a | 16.06 ± 0.82 b | 11.69 ± 1.13 c | 10.28 ± 1.89 c |
| Surface TPC (mg/g DW) | - | 4.91 ± 0.48 a | 3.46 ± 0.26 b | 3.66 ± 0.22 b | - | 2.76 ± 0.10 a | 3.12 ± 0.17 b | 3.52 ± 0.21 c |
| TFC (mg/g DW) | 1.02 ± 0.01 a | 0.89 ± 0.06 b | 0.90 ± 0.04 b | 0.44 ± 0.04 c | 6.41 ± 0.03 a | 1.05 ± 0.08 b | 1.32 ± 0.07 c | 0.75 ± 0.07 d |
| Surface TFC (mg/g DW) | - | 0.08 ± 0.01 a | 0.02 ± 0.00 b | 0.01 ± 0.00 c | - | 0.05 ± 0.00 a | 0.09 ± 0.00 b | 0.05 ± 0.00 c |
| MEE of TPC (%) | - | 86.61 ± 2.83 a | 92.24 ± 0.53 b | 88.84 ± 0.39 a | - | 82.80 ± 0.74 a | 71.06 ± 0.26 b | 68.91 ± 2.71 b |
| MEE of TFC (%) | - | 90.85 ± 0.12 a | 98.00 ± 0.08 b | 97.02 ± 0.29 b | - | 95.53 ± 0.12 a | 93.36 ± 0.21 b | 92.70 ± 0.15 b |
| ADPPH (µmol/g DW) | 12.06 ± 0.11 a | 8.23 ± 0.08 b | 12.12 ± 0.15 a | 10.05 ± 0.2 c | 11.45 ± 0.10 a | 10.45 ± 0.14 b | 7.67 ± 0.23 c | 9.60 ± 0.46 d |
| AABTS (µmol/g DW) | 258.38 ± 1.33 a | 148.31 ± 1.64 b | 203.50 ± 4.06 c | 176.82 ± 7.93 d | 270.46 ± 1.35 a | 221.89 ± 7.91 b | 110.81 ± 5.60 c | 163.03 ± 5.29 d |
| AFRAP (µmol/g DW) | 271.89 ± 4.97 a | 208.71 ± 6.21 b | 236.34 ± 4.11 c | 212.01 ± 7.23 b | 206.45 ± 8.50 a | 163.70 ± 8.46 b | 138.24 ± 6.79 c | 163.63 ± 6.90 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, Y.; Giovagnoli-Vicuña, C.; Faúndez, M.; Giordano, A. Microencapsulation of Chilean Papaya Waste Extract and Its Impact on Physicochemical and Bioactive Properties. Antioxidants 2023, 12, 1900. https://doi.org/10.3390/antiox12101900
Fuentes Y, Giovagnoli-Vicuña C, Faúndez M, Giordano A. Microencapsulation of Chilean Papaya Waste Extract and Its Impact on Physicochemical and Bioactive Properties. Antioxidants. 2023; 12(10):1900. https://doi.org/10.3390/antiox12101900
Chicago/Turabian StyleFuentes, Yihajara, Claudia Giovagnoli-Vicuña, Mario Faúndez, and Ady Giordano. 2023. "Microencapsulation of Chilean Papaya Waste Extract and Its Impact on Physicochemical and Bioactive Properties" Antioxidants 12, no. 10: 1900. https://doi.org/10.3390/antiox12101900





