A Peptoid-Chelator Selective to Cu2+ That Can Extract Copper from Metallothionein-2 and Lead to the Production of ROS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Purification of the Peptoid Oligomers
2.3. General Method for Water Solubility Test
2.4. EPR Studies
2.5. UV/Visible Spectroscopy
2.6. Synthesis of Metal Complexes for MS Analysis
2.7. Dissociation Constant Calculations
2.8. Protein Sample Preparation
2.9. Circular Dichroism Spectroscopy
2.10. UV/Visible Spectrophotometry for Kinetics and Kinetics of ROS Formation
3. Results and Discussion
3.1. Peptoid Oligomer Design
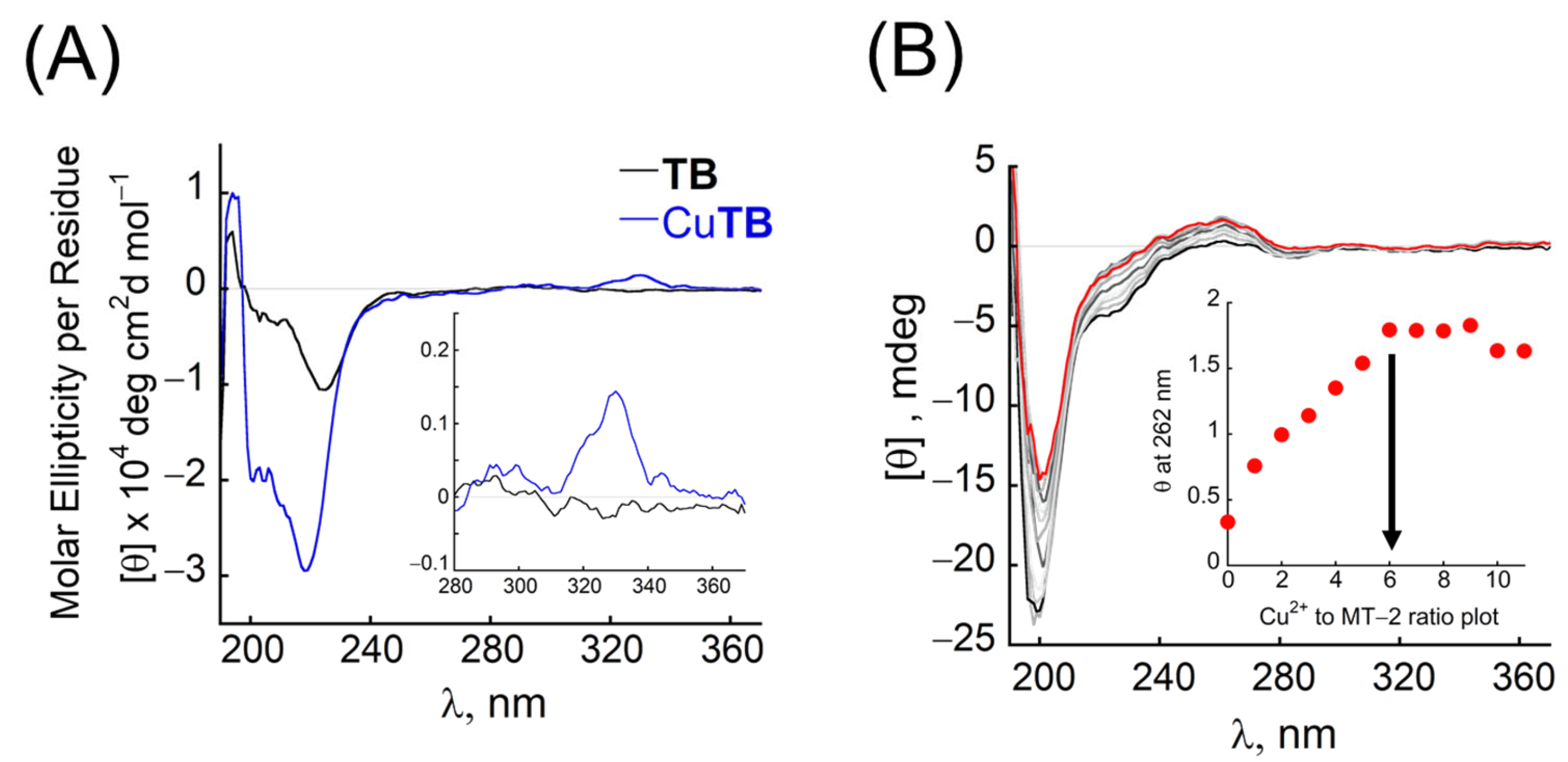
3.2. Characterisation of CuTB Binding
3.3. Extraction of Cu2+ from the Cu-Containing Protein Metallothioneine by TB
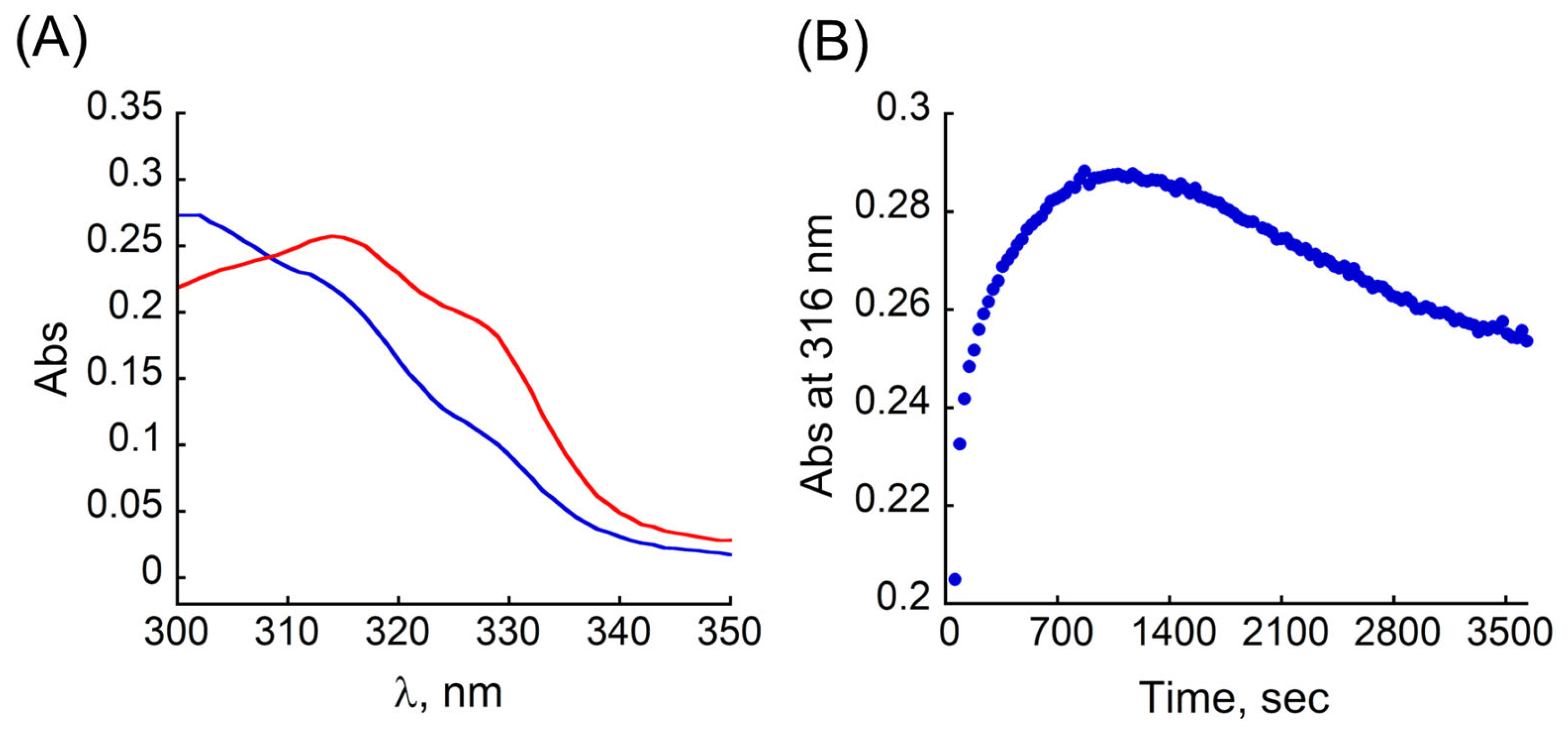
3.4. Re-Distribution of Cu2+ from CuMT2 by TB and ROS Production: A Proof of the Concept
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 18991, 147–163. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.D.; Dameron, C.T. Molecular mechanisms of copper metabolism and the role of the Menkes disease protein. J. Biochem. Mol. Toxicol. 1999, 13, 93–106. [Google Scholar] [CrossRef]
- Delangle, P.; Mintz, E. Chelation therapy in Wilson’s disease: From D-penicillamine to the design of selective bioinspired intracellular Cu(I) chelators. Dalton Trans. 2012, 41, 6359. [Google Scholar] [CrossRef]
- Bush, A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000, 4, 184. [Google Scholar] [CrossRef]
- Montes, S.; Rivera-Mancia, S.; Diaz-Ruiz, A.; Tristan-Lopez, L.; Rios, C. Copper and Copper Proteins in Parkinson’s Disease. Oxid. Med. Cell Longev. 2014, 2014, 147251. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459. [Google Scholar] [CrossRef] [PubMed]
- Theophanides, T.; Anastassopoulou, J. Copper and carcinogenesis. Crit. Rev. Oncol. Hematol. 2002, 42, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Aubert, L.; Nandagopal, N.; Steinhart, Z.; Lavoie, G.; Nourreddine, S.; Berman, J.; Saba-El-Leil, M.K.; Papadopoli, D.; Lin, S.; Hart, T.; et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun. 2020, 11, 3701. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Singh, M.K.; Singh, T.B.; Bhartiya, S.K.; Singh, S.P.; Shukla, V.K. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 2013, 37, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Dundar, T.K.; Aksoy, F.; Mogulkoc, R. Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res. 2017, 175, 57–64. [Google Scholar] [CrossRef]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.T.; Giaccia, A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef]
- Steinbrueck, A.; Sedgwick, A.C.; Brewster, J.T.; Yan, K.-C.; Shang, Y.; Knoll, D.M.; Vargas-Zuniga, G.I.; He, X.-P.; Tian, H.; Sessler, J.L. Transition metal chelators, pro-chelators, and ionophores as small molecule cancer chemotherapeutic agents. Chem. Soc. Rev. 2020, 49, 3726–3747. [Google Scholar] [CrossRef]
- Guan, D.; Zhao, L.; Shi, X.; Ma, X.; Chen, Z. Copper in cancer: From pathogenesis to therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef]
- Yu, Y.; Wong, J.; Lovejoy, D.B.; Kalinowski, D.S.; Richardson, D.R. Chelators at the Cancer Coalface: Desferrioxamine to Triapine and Beyond. Clin. Cancer Res. 2006, 12, 6876–6883. [Google Scholar] [CrossRef]
- Wadhwa, S.; Mumper, R.J. D-Penicillamine and other low molecular weight thiols: Review of anticancer effects and related mechanisms. Cancer Lett. 2013, 337, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Xiaolong, T.; Zaihua, Y.; Yandong, M.; Wuhua, H.; Zheng, L.; Lixia, Y.; Denghai, M. Copper in cancer: From limiting nutrient to therapeutic target. Front. Oncol. 2023, 2023, 1209156. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Kirshner, J.R.; He, S.; Balasubramanyam, V.; Kepros, J.; Yang, C.-Y.; Zhang, M.; Du, Z.; Barsoum, J.; Bertin, J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 2008, 7, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Reznik, E.; Stokes, M.E.; Krishnamoorthy, L.; Bos, P.H.; Song, Y.; Quartararo, C.E.; Pagano, N.C.; Carpizo, D.R.; de Carvalho, A.C.; et al. Copper-Binding Small Molecule Induces Oxidative Stress and Cell-Cycle Arrest in Glioblastoma-Patient-Derived Cells. Cell Chem. Biol. 2018, 25, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, D.; Song, Z.-L.; Liu, T.; Hou, Y.; Fang, J. Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Med. Res. Rev. 2021, 41, 342–394. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Cheng, Y.; Chudal, L.; Pandey, N.K.; Zhang, J.; Ma, L.; Xi, Q.; Yang, G.; Chen, Y.; et al. Use of copper-cysteamine nanoparticles to simultaneously enable radiotherapy, oxidative therapy and immunotherapy for melanoma treatment. Signal Transduct. Target Ther. 2020, 5, 58. [Google Scholar] [CrossRef]
- Ghasemi, P.; Shafiee, G.; Ziamajidi, N.; Abbasalipourkabir, R. Copper Nanoparticles Induce Apoptosis and Oxidative Stress in SW480 Human Colon Cancer Cell Line. Biol. Trace Elem. Res. 2023, 201, 3746–3754. [Google Scholar] [CrossRef]
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 12613. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, B.-C.; Zuckermann, R.N. Peptoids: Synthesis, Characterization, and Nanostructures in Comprehensive Biomaterials, 2nd ed.; Ducheyne, P., Healy, K.E., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 53–76. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Turck, C.W.; Cohen, F.E.; Zuckermann, R.N.; Lim, W.A. Exploiting the basis of proline recognition by SH3 and WW domains: Design of N-substituted inhibitors. Science 1998, 282, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Durell, S.R.; Myers, M.C.; Appella, D.H. Probing the Structural Requirements of Peptoids That Inhibit HDM2-p53 Interactions. J. Am. Chem. Soc. 2006, 128, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Udugamasooriya, D.G.; Dineen, S.P.; Brekken, R.A.; Kodadek, T.A. A Peptoid “Antibody Surrogate” That Antagonizes VEGF Receptor 2 Activity. J. Am. Chem. Soc. 2008, 130, 5744–5752. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Chu, T.K.; Dill, K.A.; Zuckermann, R.N. Biomimetic nanostructures: Creating a high-affinity zinc-binding site in a folded nonbiological polymer. J. Am. Chem. Soc. 2008, 130, 8847–8855. [Google Scholar] [CrossRef]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Metallopeptoids. Chem. Commun. 2009, 1, 56–58. [Google Scholar] [CrossRef]
- Della Sala, G.; Nardone, B.; De Riccardis, F.; Izzo, I. Cyclopeptoids: A novel class of phase-transfer catalysts. Org. Biomol. Chem. 2013, 11, 726–731. [Google Scholar] [CrossRef]
- Schettini, R.; Nardone, B.; De Riccardis, F.; Della Sala, G.; Izzo, I. Cyclopeptoids as Phase-Transfer Catalysts for the Enantioselective Synthesis of α-Amino Acids. Eur. J. Org. Chem. 2014, 2014, 7793–7797. [Google Scholar] [CrossRef]
- Schettini, R.; De Riccardis, F.; Della Sala, G.; Izzo, I. Enantioselective Alkylation of Amino Acid Derivatives Promoted by Cyclic Peptoids under Phase-Transfer Conditions. J. Org. Chem. 2016, 81, 2494–2505. [Google Scholar] [CrossRef]
- Ruan, G.; Ghosh, P.; Fridman, N.; Maayan, G. A Di-Copper-Peptoid in a Noninnocent Borate Buffer as a Fast Electrocatalyst for Homogeneous Water Oxidation with Low Overpotential. J. Am. Chem. Soc. 2021, 143, 10614–10623. [Google Scholar] [CrossRef]
- Culf, A.S.; Ouellette, R.J. Solid-Phase Synthesis of N-substituted Glycine Oligomers (α-Peptoids) and Derivatives. Molecules 2010, 15, 5282–5335. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.E.; Ghosh, P.; Maayan, G. Structure and Function of Cu–Peptoid Complexes in Copper Bioinorganic Chemistry; Simaan, A.J., Réglier, M., Eds.; World Scientific: Singapore, 2023; pp. 211–249. [Google Scholar] [CrossRef]
- Wu, C.W.; Kirshenbaum, K.; Sanborn, T.J.; Patch, J.A.; Huang, K.; Dill, K.A.; Zuckermann, R.N.; Barron, A.E. Structural and Spectroscopic Studies of Peptoid Oligomers with α-Chiral Aliphatic Side Chains. J. Am. Chem. Soc. 2003, 125, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.R.; Crapster, J.A.; Guzei, I.A.; Blackwell, H.E. Extraordinarily Robust Polyproline Type I Peptoid Helices Generated via the Incorporation of α-Chiral Aromatic N-1-Naphthylethyl Side Chains. J. Am. Chem. Soc. 2011, 133, 15559–15567. [Google Scholar] [CrossRef] [PubMed]
- Roy, O.; Dumonteil, G.; Faure, S.; Jouffret, L.; Kriznik, A.; Taillefumier, C. Homogeneous and Robust Polyproline Type I Helices from Peptoids with Nonaromatic α-Chiral Side Chains. J. Am. Chem. Soc. 2017, 139, 13533–13540. [Google Scholar] [CrossRef]
- Crapster, J.A.; Guzei, I.A.; Blackwell, H.E. A Peptoid Ribbon Secondary Structure. Angew. Chem. Int. Ed. 2013, 52, 5079–5084. [Google Scholar] [CrossRef]
- Miller, S.M.; Simon, R.J.; Ng, S.; Zuckermann, R.N.; Kerr, J.M.; Moos, W.H. Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 1995, 35, 20–32. [Google Scholar] [CrossRef]
- Schunk, H.C.; Austin, M.J.; Taha, B.Z.; McClellan, M.S.; Suggs, L.J.; Rosales, A.M. Oxidative degradation of sequence-defined peptoid oligomers. Mol. Syst. Des. Eng. 2023, 8, 92. [Google Scholar] [CrossRef]
- Kwon, Y.; Kodadek, T. Quantitative Evaluation of the Relative Cell Permeability of Peptoids and Peptides. J. Am. Chem. Soc. 2007, 129, 1508–1509. [Google Scholar] [CrossRef]
- Bolt, H.L.; Williams, C.E.J.; Brooks, R.V.; Zuckermann, R.N.; Cobb, S.L.; Bromley, E.H.C. Log D versus HPLC derived hydrophobicity: The development of predictive tools to aid in the rational design of bioactive peptoids. Biopolymers 2017, 108, e23014. [Google Scholar] [CrossRef]
- Kirshenbaum, K.; Barron, A.E.; Goldsmith, R.A.; Armand, P.; Bradley, E.K.; Truong, K.T.V.; Dill, K.A.; Cohen, F.E.; Zuckermann, R.N. Sequence-specific polypeptoids: A diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. USA 1998, 95, 4303–4308. [Google Scholar] [CrossRef]
- Ghosh, T.; Ghosh, P.; Maayan, G. A Copper-Peptoid as a Highly Stable, Efficient, and Reusable Homogeneous Water Oxidation Electrocatalyst. ACS Catal. 2018, 8, 10631–10640. [Google Scholar] [CrossRef]
- Ruan, G.; Engelberg, L.; Ghosh, P.; Maayan, G. A unique Co(iii)-peptoid as a fast electrocatalyst for homogeneous water oxidation with low overpotential. Chem. Commun. 2021, 57, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.E.; Sabater, L.; Baskin, M.; Hureau, C.; Maayan, G. A Water-Soluble Peptoid Chelator that Can Remove Cu2+ from Amyloid-β Peptides and Stop the Formation of Reactive Oxygen Species Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2021, 60, 24588. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Maayan, G. A Water-Soluble Peptoid that Can Extract Cu2+ from Metallothionein via Selective Recognition. Chem. A Eur. J. 2021, 27, 1383. [Google Scholar] [CrossRef]
- Maayan, G.; Yoo, B.; Kirshenbaum, K. Heterocyclic amines for the construction of peptoid oligomers bearing multi-dentate ligands. Tetrahedron Lett. 2008, 49, 335–338. [Google Scholar] [CrossRef]
- Baskin, M.; Panz, L.; Maayan, G. Versatile ruthenium complexes based on 2,2′-bipyridine modified peptoids. Chem. Commun. 2016, 52, 10350–10353. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Moos, W.H.; Kent, S.B.H. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Darapaneni, C.M.; Kaniraj, P.J.; Maayan, G. Water soluble hydrophobic peptoids via a minor backbone modification. Org. Biomol. Chem. 2018, 16, 1480–1488. [Google Scholar] [CrossRef]
- Xiao, Z.; Wedd, A.G. The challenges of determining metal–protein affinities. Nat. Prod. Rep. 2010, 27, 768–789. [Google Scholar] [CrossRef]
- Zhang, L.; Koay, M.; Maher, M.J.; Xiao, Z.; Wedd, A.G. Intermolecular transfer of copper ions from the CopC protein of Pseudomonas syringae. Crystal structures of fully loaded Cu(I)Cu(II) forms. J. Am. Chem. Soc. 2006, 128, 5834–5850. [Google Scholar] [CrossRef]
- Mehlenbacher, M.R.; Elsiesy, R.; Lakha, R.; Villones, R.L.E.; Orman, M.; Vizcarra, C.L.; Meloni, G.; Wilcox, D.E.; Austin, R.N. Metal binding and interdomain thermodynamics of mammalian metallothionein-3: Enthalpically favoured Cu+ supplants entropically favoured Zn2+ to form Cu4+ clusters under physiological Conditions. Chem. Sci. 2022, 13, 5289. [Google Scholar] [CrossRef]
- Baskin, M.; Maayan, G. A rationally designed metal-binding helical peptoid for selective recognition processes. Chem. Sci. 2016, 7, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Housecroft, C.E. More hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Behar, A.E.; Maayan, G. The First Cu(I)-Peptoid Complex: Enabling Metal Ion Stability and Selectivity via Backbone Helicity. Chem. A Eur. J. 2023, 29, e202301118. [Google Scholar] [CrossRef] [PubMed]
- Baldo Lucchese, B.; Humphreys, K.J.; Lee, D.-H.; Incarvito, C.D.; Sommer, R.D.; Rheingold, A.L.; Karlin, K.D. Mono-, Bi-, and Trinuclear CuII-Cl Containing Products Based on the Tris(2-pyridylmethyl)amine Chelate Derived from Copper(I) Complex Dechlorination Reactions of Chloroform. Inorg. Chem. 2004, 43, 5987–5998. [Google Scholar] [CrossRef] [PubMed]
- Garribba, E.; Micera, G. The Determination of the Geometry of Cu(II) Complexes: An EPR Spectroscopy Experiment. J. Chem. Educ. 2006, 83, 1229. [Google Scholar] [CrossRef]
- Das, K.; Datta, A.; Sinha, C.; Huang, J.-H.; Garribba, E.; Hsiao, C.-S.; Hsu, C.-L. End-to-End Thiocyanato-Bridged Helical Chain Polymer and Dichlorido-Bridged Copper(II) Complexes with a Hydrazone Ligand: Synthesis, Characterisation by Electron Paramagnetic Resonance and Variable-Temperature Magnetic Studies, and Inhibitory Effects on Human Colorectal Carcinoma Cells. ChemistryOpen 2012, 1, 80–89. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Bioinorganic Chemistry of Mammalian Metallothioneins. Chem. Rev. 2021, 121, 14594–14648. [Google Scholar] [CrossRef]
- Alies, B.; Sasaki, I.; Proux, O.; Sayen, S.; Guillon, E.; Faller, P.; Hureau, C. Zn impacts Cu coordination to amyloid-β, the Alzheimer’s peptide, but not the ROS production and the associated cell toxicity. Chem. Commun. 2013, 49, 1214–1216. [Google Scholar] [CrossRef]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.; Hibbert, D.; Gülçin, İ.; Demirci Çekiç, S.; Güçlü, K.; Özyürek, M.; Çelik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Esmieu, C.; Guettas, D.; Conte-daban, A.; Sabater, L.; Faller, P.; Hureau, C. Copper-Targeting Approaches in Alzheimer’s Disease: How To Improve the Fallouts Obtained from in Vitro Studies. Inorg. Chem. 2019, 58, 13509–13527. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, J.P.; Tyurin, V.A.; Tyurina, Y.T.; Borisenko, G.G.; Korotaeva, A.; Pitt, B.R.; Lazo, J.S.; Kagan, V.E. Redox Regulation of Copper–Metallothionein. Arch. Biochem. Biophys. 1999, 363, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Faller, P.; Vaša´k, M. Redox Silencing of Copper in Metal-linked Neurodegenerative Disorders: REACTION OF Zn7METALLOTHIONEIN-3 WITH Cu2+ IONS. J. Biol. Chem. 2007, 282, 16068–16078. [Google Scholar] [CrossRef] [PubMed]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted Delivery Methods for Anticancer Drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, W.; Lib, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef] [PubMed]
- Calvaresia, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]
- Harvey, D. Modern Analytical Chemistry; Wiley: New York, NY, USA, 2000; p. 316. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behar, A.E.; Maayan, G. A Peptoid-Chelator Selective to Cu2+ That Can Extract Copper from Metallothionein-2 and Lead to the Production of ROS. Antioxidants 2023, 12, 2031. https://doi.org/10.3390/antiox12122031
Behar AE, Maayan G. A Peptoid-Chelator Selective to Cu2+ That Can Extract Copper from Metallothionein-2 and Lead to the Production of ROS. Antioxidants. 2023; 12(12):2031. https://doi.org/10.3390/antiox12122031
Chicago/Turabian StyleBehar, Anastasia Esther, and Galia Maayan. 2023. "A Peptoid-Chelator Selective to Cu2+ That Can Extract Copper from Metallothionein-2 and Lead to the Production of ROS" Antioxidants 12, no. 12: 2031. https://doi.org/10.3390/antiox12122031




