Bifidobacterium animalis subsp. lactis BPL1™ and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans
Abstract
:1. Introduction
2. Material and Methods
2.1. C. elegans Strains and Maintenance Conditions
2.2. Bacterial Strains and Culture Conditions
2.3. Preparation of LTA from BPL1™
2.4. Fat Reduction Assays
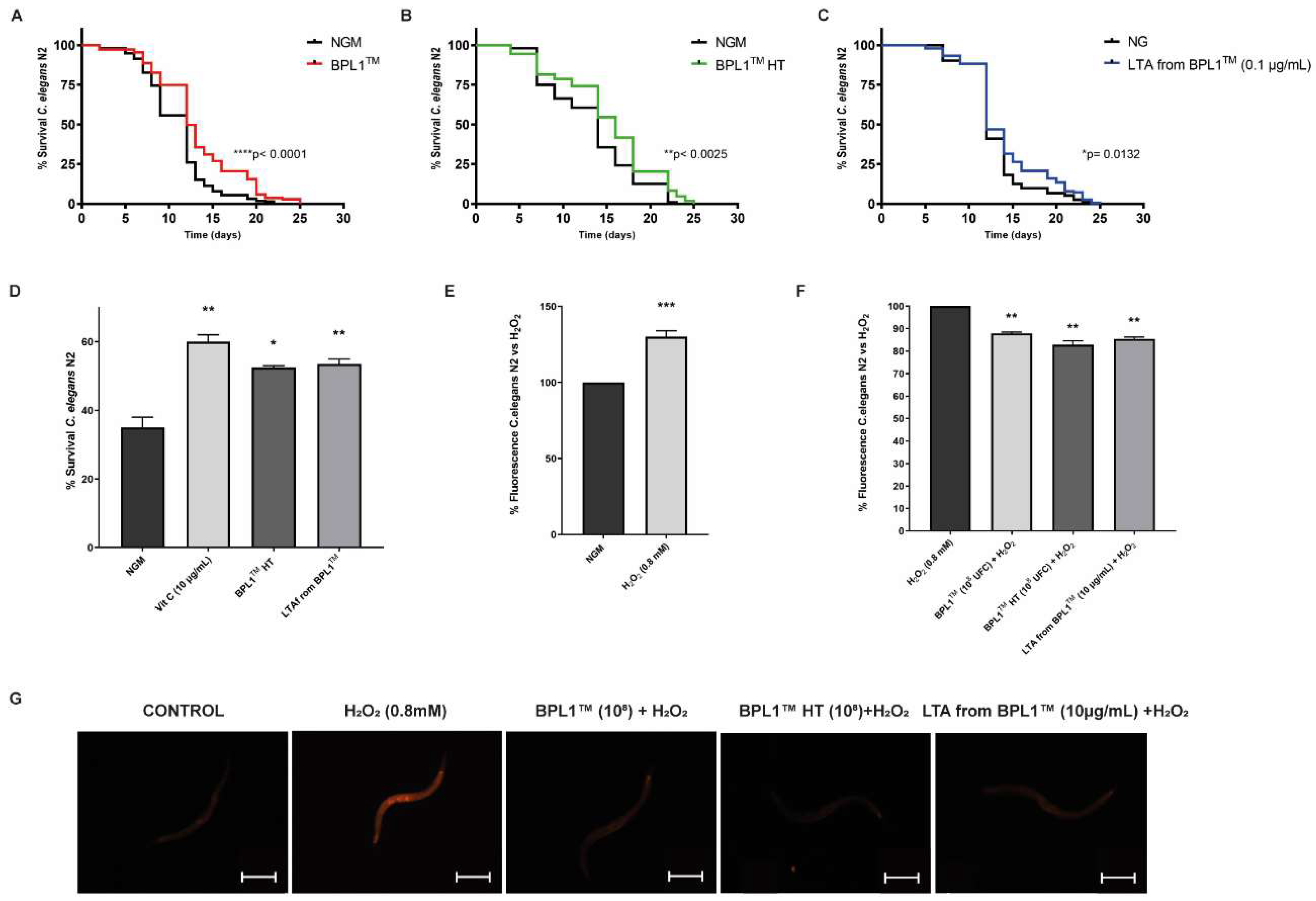
2.5. Lifespan Assays
2.6. Oxidative Stress Assay
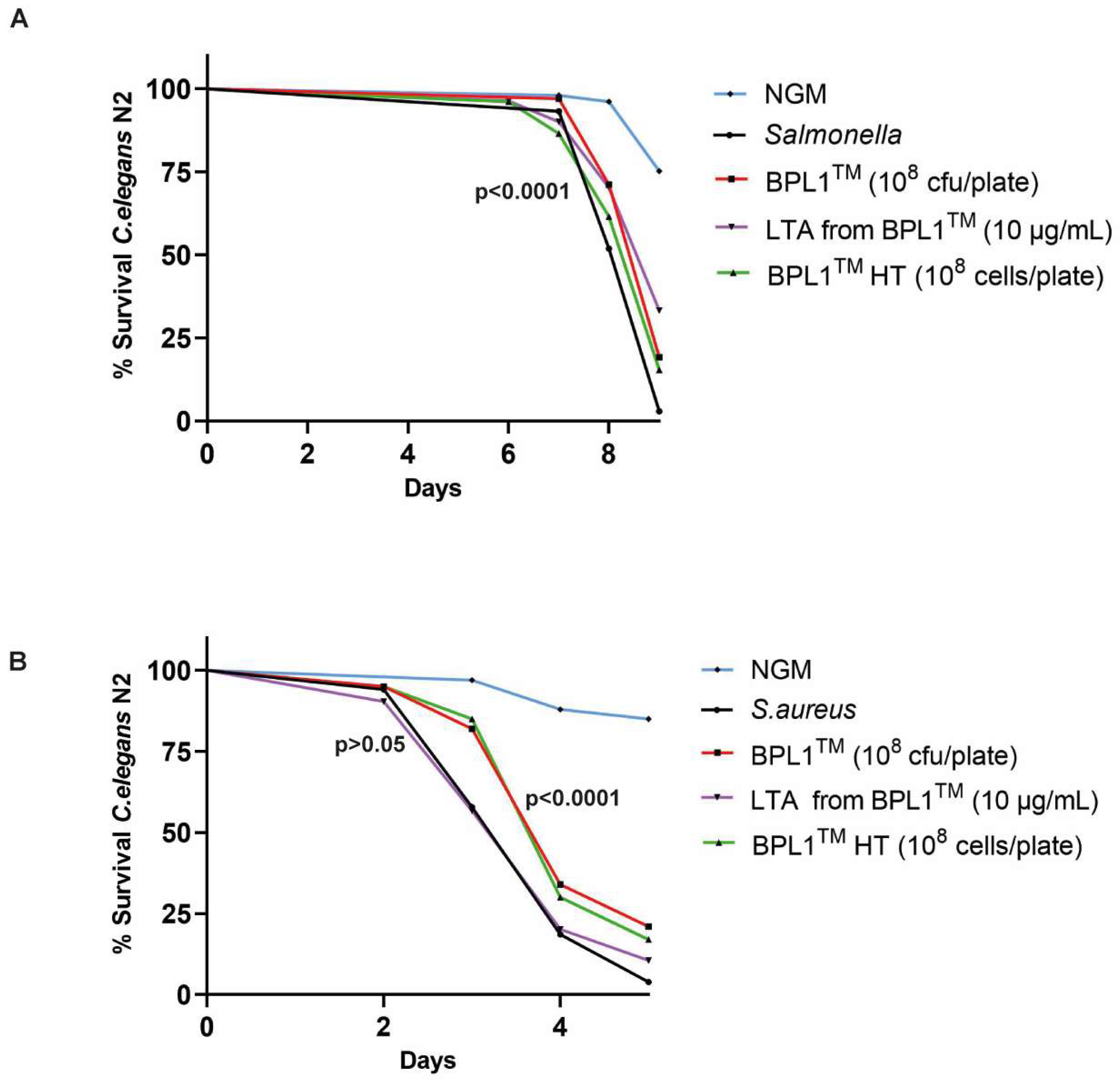
2.7. Infection Assays
2.8. Gut Barrier Integrity
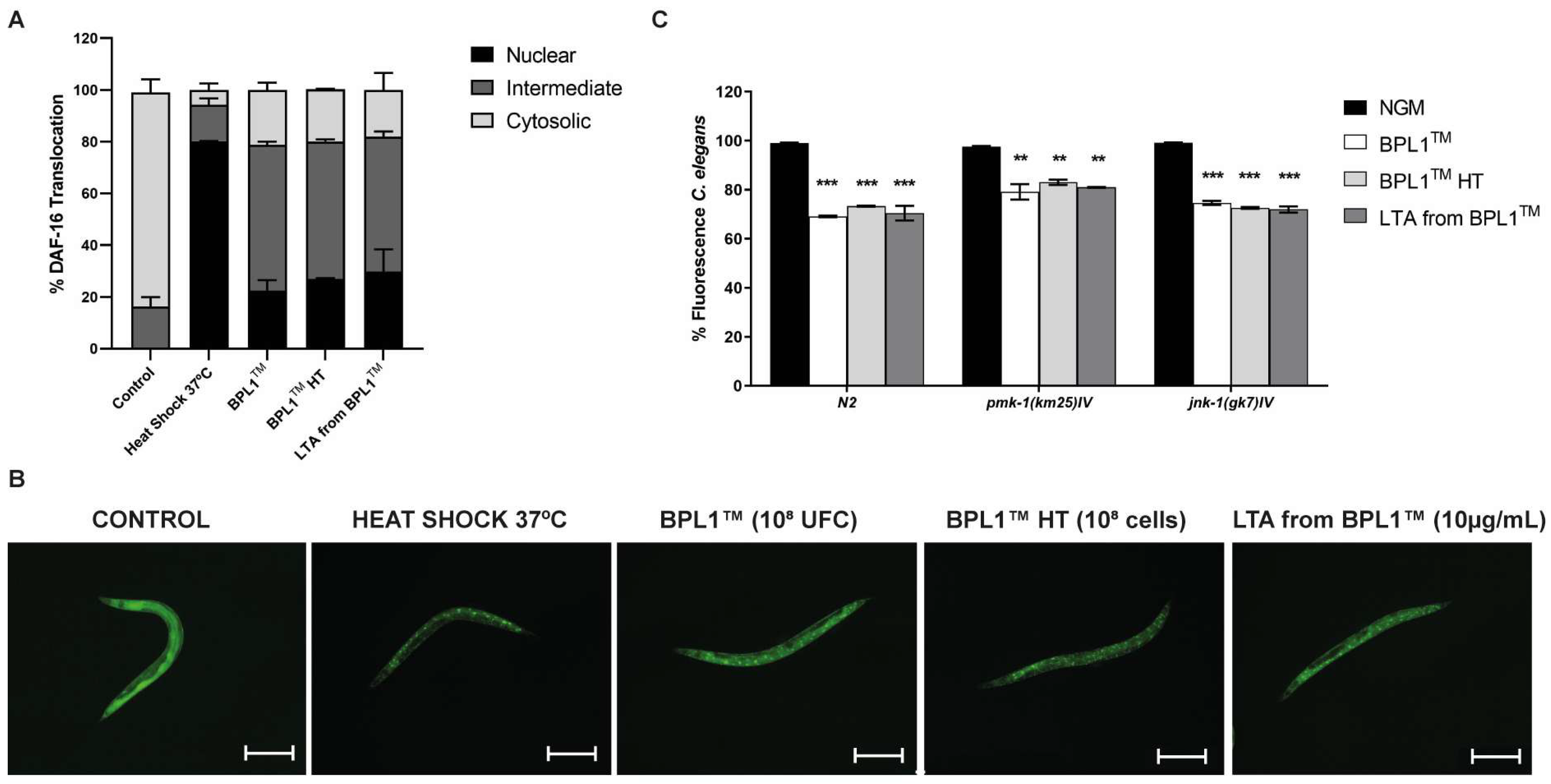
2.9. DAF-16 Nuclear Translocation
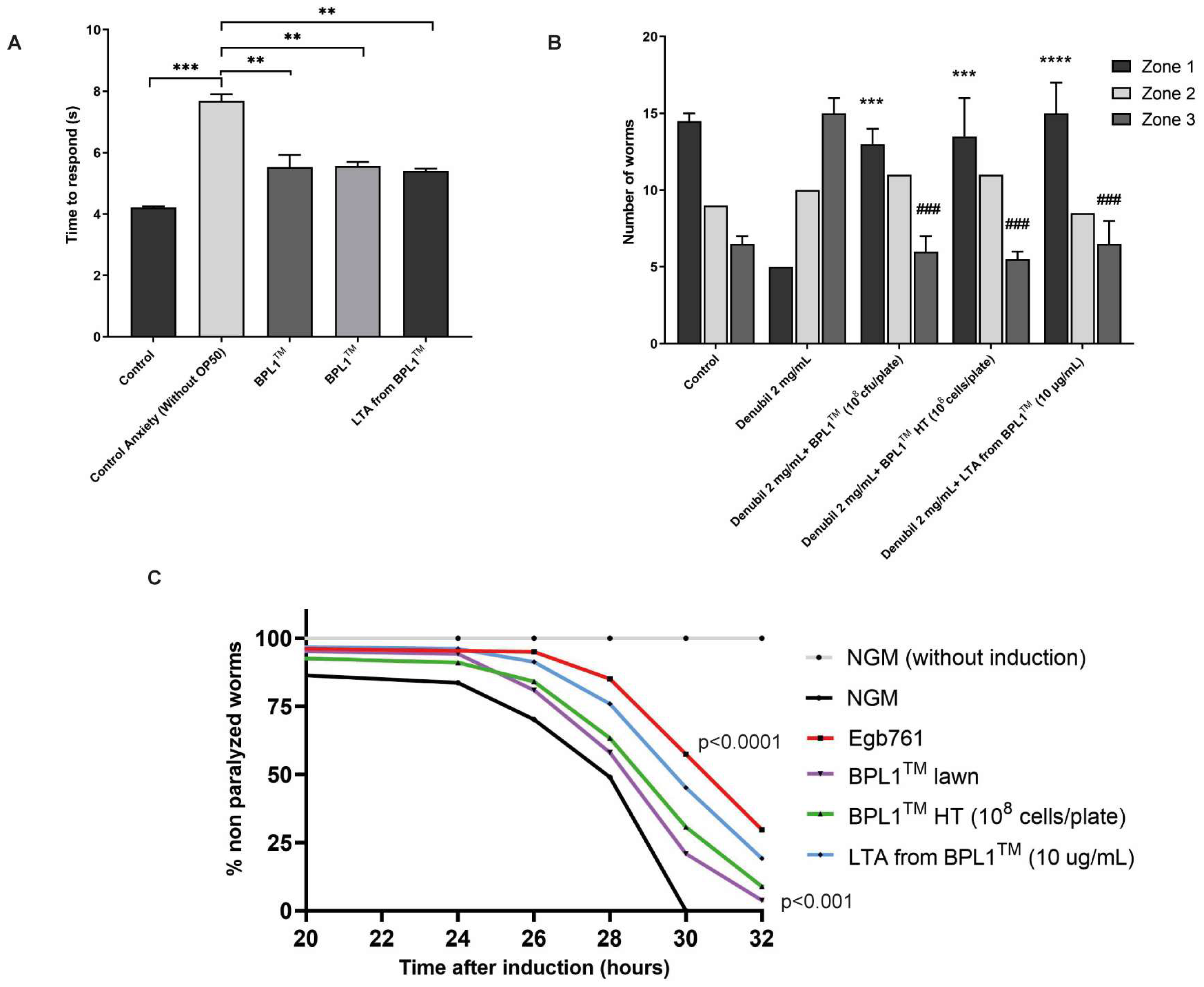
2.10. Behavioral Assays
2.10.1. Avoidance Behavior
2.10.2. Reduction in Stress
2.11. Paralysis Assays (Alzheimer’s Disease C. elegans Model)
2.12. Statistical Analysis
3. Results
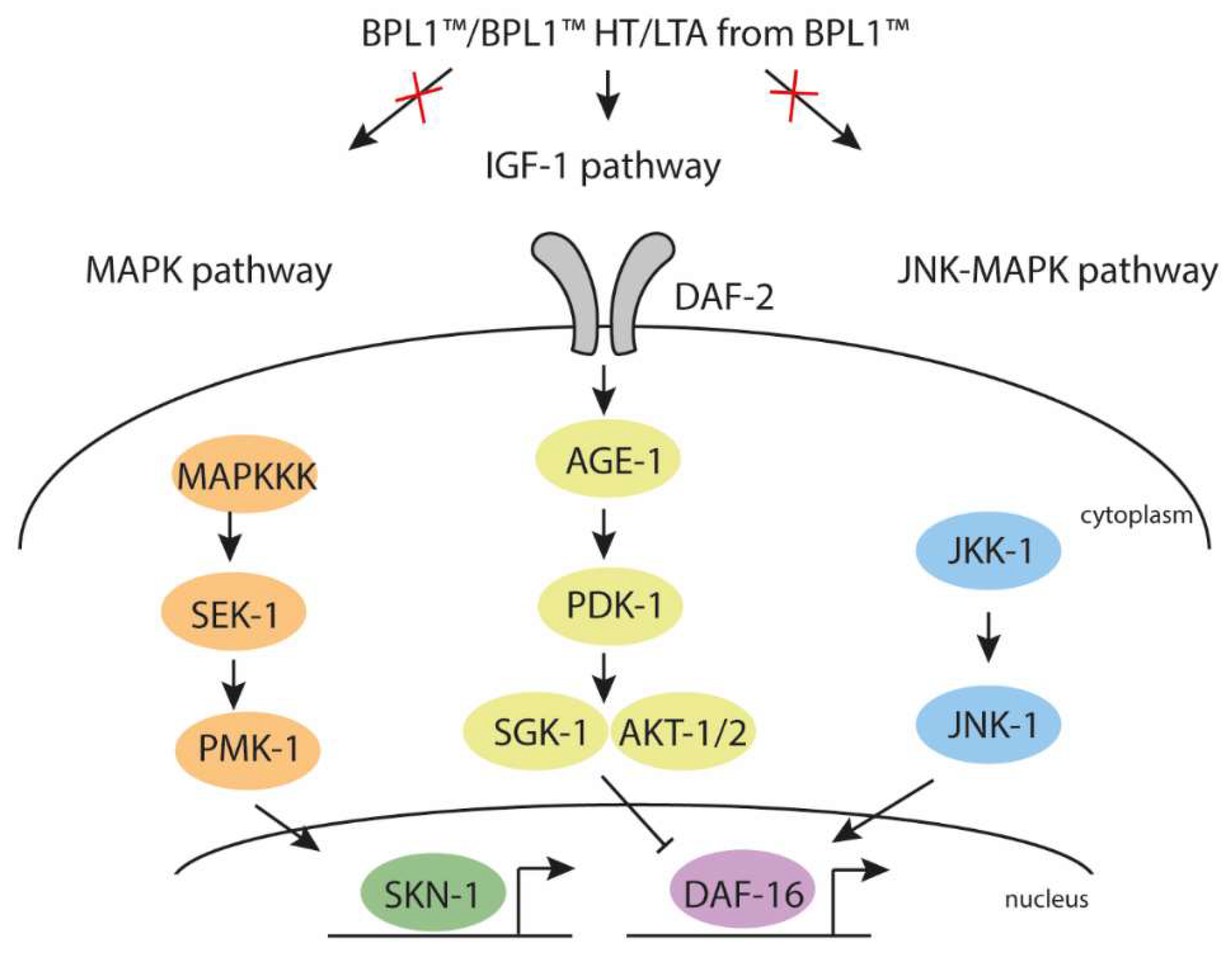
3.1. Fat Reduction Effect of BPL1™, Heat-Treated BPL1™ and LTA from BPL1™ Requires the Insulin-like Signaling Pathway (IGF-1) and Not the MAPK Pathway in C. elegans
3.2. BPL1™, Heat-Treated BPL1™ and LTA from BPL1™ Prolong Lifespan via IGF-1 and Protect against Age-Related Phenotypes in C. elegans
3.3. BPL1™, Heat-Treated BPL1™ and LTA from BPL1™ Exert Protective Effect against Pathogen Infection
3.4. BPL1™, Heat-Treated BPL1™ and Its LTA Improve C. elegans Anxiety and Stress-Related Behaviors and Had Positive Effect on C. elegans Alzheimer’s Model
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. GHE: Life Expectancy and Healthy Life Expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy (accessed on 23 October 2023).
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Lu, X.; Wang, X.; Wang, X.; Chen, L.; Guo, H.; Zhang, M.; Li, Y. Bifidobacterium animalis subsp. lactis A6 Alleviates Obesity Associated with Promoting Mitochondrial Biogenesis and Function of Adipose Tissue in Mice. Molecules 2020, 25, 1490. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kurihara, S.; Kibe, R.; Ashida, H.; Benno, Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS ONE 2011, 6, e23652. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr. Microbiol. 2011, 62, 1097–1103. [Google Scholar] [CrossRef]
- Uusitupa, H.-M.; Rasinkangas, P.; Lehtinen, M.J.; Mäkelä, S.M.; Airaksinen, K.; Anglenius, H.; Ouwehand, A.C.; Maukonen, J. Bifidobacterium animalis subsp. lactis 420 for Metabolic Health: Review of the Research. Nutrients 2020, 12, 892. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; González, N.; Chenoll, E.; López-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramón, D.; Genovés, S. Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472. [Google Scholar] [CrossRef]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2022, 15, 805–816. [Google Scholar] [CrossRef]
- Dyczkowska, A.A.; Chabowska-Kita, A. Caenorhabditis elegans as a model organism in obesity research. BioTechnologia 2021, 102, 337–362. [Google Scholar] [CrossRef]
- Caimari, A.; del Bas, J.M.; Boqué, N.; Crescenti, A.; Puiggròs, F.; Chenoll, E.; Martorell, P.; Ramón, D.; Genovés, S.; Arola, L. Heat-killed Bifidobacterium animalis subsp. Lactis CECT 8145 increases lean mass and ameliorates metabolic syndrome in cafeteria-fed obese rats. J. Funct. Foods 2017, 38, 251–263. [Google Scholar] [CrossRef]
- Carreras, N.L.; Martorell, P.; Chenoll, E.; Genovés, S.; Ramón, D.; Aleixandre, A. Anti-obesity properties of the strain Bifidobacterium animalis subsp. lactis CECT 8145 in Zücker fatty rats. Benef. Microbes 2018, 9, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: A randomized controlled trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Amat-Bou, M.; Garcia-Ribera, S.; Climent, E.; Piquer-Garcia, I.; Corripio, R.; Sanchez-Infantes, D.; Villalta, L.; Elias, M.; Jiménez-Chillarón, J.C.; Chenoll, E. Effects of Bifidobacterium animalis Subsp. lactis (BPL1) Supplementation in Children and Adolescents with Prader–Willi Syndrome: A Randomized Crossover Trial. Nutrients 2020, 12, 3123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, Y.; Liu, R.; Zheng, X.; Zhang, M.; Guo, H.; Zhang, H.; Ren, F. The Transcription Factor DAF-16 is Essential for Increased Longevity in C. elegans Exposed to Bifidobacterium longum BB68. Sci. Rep. 2017, 7, 7408. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kiortsis, D.N.; Filippatos, T.D.; Elisaf, M.S. The effects of orlistat on metabolic parameters and other cardiovascular risk factors. Diabetes Metab. 2005, 31, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Forment, J.V.; de Llanos, R.; Montón, F.; Llopis, S.; González, N.; Genovés, S.; Cienfuegos, E.; Monzó, H.; Ramón, D. Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J. Agric. Food Chem. 2011, 59, 2077–2085. [Google Scholar] [CrossRef]
- Ikeda, T.; Yasui, C.; Hoshino, K.; Arikawa, K.; Nishikawa, Y. Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against salmonella enterica serovar enteritidis. Appl. Environ. Microbiol. 2007, 73, 6404–6409. [Google Scholar] [CrossRef]
- Kim, Y.; Mylonakis, E. Caenorhabditis elegans immune conditioning with the probiotic bacterium Lactobacillus acidophilus strain NCFM enhances gram-positive immune responses. Infect. Immun. 2012, 80, 2500–2508. [Google Scholar] [CrossRef]
- Kong, C.; Yehye, W.A.; Abd Rahman, N.; Tan, M.W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Voigt, R.M.; Shaikh, M.; Tang, Y.; Cederbaum, A.I.; Turek, F.W.; Keshavarzian, A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 305, G185–G195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, X.; Jia, R.; Yang, R.; Rui, Q.; Wang, D. Lactic Acid Bacteria Protects Caenorhabditis elegans from Toxicity of Graphene Oxide by Maintaining Normal Intestinal Permeability under different Genetic Backgrounds. Sci. Rep. 2015, 5, 17233. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Sakamoto, K. Killed Bifidobacterium longum enhanced stress tolerance and prolonged life span of Caenorhabditis elegans via DAF-16. Br. J. Nutr. 2018, 120, 872–880. [Google Scholar] [CrossRef]
- Chao, M.Y.; Komatsu, H.; Fukuto, H.S.; Dionne, H.M.; Hart, A.C. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. USA 2004, 101, 15512–15517. [Google Scholar] [CrossRef]
- Link, C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Yanase, S.; Ishii, T.; Hartman, P.S.; Matsumoto, K.; Ishii, N. The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mech. Ageing Dev. 2005, 126, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Holzenberger, M.; Kappeler, L.; De Magalhaes Filho, C. IGF-1 signaling and aging. Exp. Gerontol. 2004, 39, 1761–1764. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Back, P.; Braeckman, B.P.; Matthijssens, F. ROS in aging Caenorhabditis elegans: Damage or signaling? Oxid. Med. Cell. Longev. 2012, 2012, 608478. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.L.; Ewbank, J.J. Caenorhabditis elegans: An emerging genetic model for the study of innate immunity. Nat. Rev. Genet. 2003, 4, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.J.; Vanderleyden, J. Anti-inflammatory potential of probiotics: Lipoteichoic acid makes a difference. Trends Microbiol. 2012, 20, 5–10. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, Q.; Min, M.; Zhang, C.; Peng, S.; Shi, Z. Ability of Lactobacillus plantarum lipoteichoic acid to inhibit Vibrio anguillarum-induced inflammation and apoptosis in silvery pomfret (Pampus argenteus) intestinal epithelial cells. Fish Shellfish Immunol. 2016, 54, 573–579. [Google Scholar] [CrossRef]
- Ching, T.T.; Chiang, W.C.; Chen, C.S.; Hsu, A.L. Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell 2011, 10, 506–519. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Kim, A.H.; Sasaki, T.; Chao, M.V. JNK-interacting protein 1 promotes Akt1 activation. J. Biol. Chem. 2003, 278, 29830–29836. [Google Scholar] [CrossRef]
- Garsin, D.A.; Villanueva, J.M.; Begun, J.; Kim, D.H.; Sifri, C.D.; Calderwood, S.B.; Ruvkun, G.; Ausubel, F.M. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 2003, 300, 1921. [Google Scholar] [CrossRef]
- Zheng, J.; Greenway, F.L. Caenorhabditis elegans as a model for obesity research. Int. J. Obes. 2012, 36, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Ikeda, T.; Yasui, C.; Saeki, S.; Nishikawa, Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology 2013, 14, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, G.; Lim, Y.H. Elucidating the Mechanism of Weissella-dependent Lifespan Extension in Caenorhabditis elegans. Sci. Rep. 2015, 5, 17128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.N.; Corkins, M.E.; Li, J.C.; Singh, K.; Parsons, S.; Tucey, T.M.; Sorkaç, A.; Huang, H.; Dimitriadi, M.; Sinclair, D.A.; et al. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 2016, 154, 30–42. [Google Scholar] [CrossRef]
- Chandler-Brown, D.; Choi, H.; Park, S.; Ocampo, B.R.; Chen, S.; Le, A.; Sutphin, G.L.; Shamieh, L.S.; Smith, E.D.; Kaeberlein, M. Sorbitol treatment extends lifespan and induces the osmotic stress response in Caenorhabditis elegans. Front. Genet. 2015, 6, 316. [Google Scholar] [CrossRef]
- Cheng, C.L.; Gao, T.Q.; Wang, Z.; Li, D.D. Role of insulin/insulin-like growth factor 1 signaling pathway in longevity. World J. Gastroenterol. 2005, 11, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Ashrafi, K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis. Model. Mech. 2009, 2, 224–229. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Naclerio, G.A.; Onyedibe, K.I.; Sintim, H.O. Lipoteichoic Acid Biosynthesis Inhibitors as Potent Inhibitors of S. aureus and E. faecalis Growth and Biofilm Formation. Molecules 2020, 25, 2277. [Google Scholar] [CrossRef]
- Gareau, M.G. Cognitive Function and the Microbiome. Int. Rev. Neurobiol. 2016, 131, 227–246. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Hare, E.E.; Loer, C.M. Function and evolution of the serotonin-synthetic bas-1 gene and other aromatic amino acid decarboxylase genes in Caenorhabditis. BMC Evol. Biol. 2004, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, S.; O’Connor, V.; Holden-Dye, L.; Walker, R.J. The regulation of feeding and metabolism in response to food deprivation in Caenorhabditis elegans. Invert. Neurosci. 2010, 10, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S. Crossing the Worm-Brain Barrier by Using Caenorhabditis elegans to Explore Fundamentals of Human Psychiatric Illness. Mol. Neuropsychiatry 2018, 3, 170–179. [Google Scholar] [CrossRef]
- De Fruyt, N.; Yu, A.J.; Rankin, C.H.; Beets, I.; Chew, Y.L. The role of neuropeptides in learning: Insights from C. elegans. Int. J. Biochem. Cell Biol. 2020, 125, 105801. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef]
| Strain | Treatment | Worms (n) | Mid Lifespan (Days) | Final Lifespan (Days) | Log Rank χ2 | ρ-Value | Significance |
|---|---|---|---|---|---|---|---|
| N2 (wild-type) | E. coli OP50 | 200 | 12 | 22 | |||
| BPL1 | 200 | 13 | 25 | 36.6 | <0.0001 | **** | |
| daf-16(mgDf50) | E. coli OP50 | 200 | 13 | 22 | |||
| BPL1 | 200 | 13 | 23 | 0.04417 | 0.83 | NS | |
| daf-2(e1370) | E. coli OP50 | 200 | 26 | 53 | |||
| BPL1 | 200 | 26 | 54 | 0.666 | 0.4145 | NS | |
| pmk-1(km25) | E. coli OP50 | 200 | 13 | 22 | |||
| BPL1 | 200 | 15 | 27 | 55.26 | <0.0001 | **** | |
| jnk-1(gk7) | E. coli OP50 | 200 | 10 | 20 | |||
| BPL1 | 200 | 13 | 22 | 82.25 | <0.0001 | **** | |
| N2 (wild-type) | E. coli OP50 | 100 | 15 | 23 | |||
| BPL1 HT | 100 | 16 | 26 | 9.152 | 0.0025 | ** | |
| daf-16(mgDf50) | E. coli OP50 | 100 | 12 | 23 | |||
| BPL1 HT | 100 | 12 | 23 | 1.183 | 0.2768 | NS | |
| daf-2(e1370) | E. coli OP50 | 100 | 18 | 53 | |||
| BPL1 HT | 100 | 19 | 55 | 0.1213 | 0.7276 | NS | |
| pmk-1(km25) | E. coli OP50 | 100 | 15 | 23 | |||
| BPL1 HT | 100 | 18 | 24 | 9.439 | 0.0021 | ** | |
| jnk-1(gk7) | E. coli OP50 | 100 | 13 | 22 | |||
| BPL1 HT | 100 | 17 | 23 | 10.94 | 0.0009 | *** | |
| N2 (wild-type) | E. coli OP50 | 200 | 12 | 23 | |||
| LTA from BPL1 | 200 | 12 | 24 | 6.14 | 0.0132 | * | |
| daf-16(mgDf50) | E. coli OP50 | 200 | 13 | 23 | |||
| LTA from BPL1 | 200 | 13 | 23 | 0.02458 | 0.76 | NS | |
| daf-2(e1370) | E. coli OP50 | 200 | 39 | 58 | |||
| LTA from BPL1 | 200 | 36 | 60 | 1.533 | 0.2157 | NS | |
| pmk-1(km25) | E. coli OP50 | 200 | 13 | 26 | |||
| LTA from BPL1 | 200 | 14 | 27 | 8.368 | 0.0038 | ** | |
| jnk-1(gk7) | E. coli OP50 | 200 | 12 | 26 | |||
| LTA from BPL1 | 200 | 15 | 27 | 9.251 | 0.0024 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaguer, F.; Barrena, M.; Enrique, M.; Maicas, M.; Álvarez, B.; Tortajada, M.; Chenoll, E.; Ramón, D.; Martorell, P. Bifidobacterium animalis subsp. lactis BPL1™ and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans. Antioxidants 2023, 12, 2107. https://doi.org/10.3390/antiox12122107
Balaguer F, Barrena M, Enrique M, Maicas M, Álvarez B, Tortajada M, Chenoll E, Ramón D, Martorell P. Bifidobacterium animalis subsp. lactis BPL1™ and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans. Antioxidants. 2023; 12(12):2107. https://doi.org/10.3390/antiox12122107
Chicago/Turabian StyleBalaguer, Ferran, Marta Barrena, María Enrique, Miren Maicas, Beatriz Álvarez, Marta Tortajada, Empar Chenoll, Daniel Ramón, and Patricia Martorell. 2023. "Bifidobacterium animalis subsp. lactis BPL1™ and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans" Antioxidants 12, no. 12: 2107. https://doi.org/10.3390/antiox12122107





