Shallow- and Deep-Water Ophiura Species Produce a Panel of Chlorin Compounds with Potent Photodynamic Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction
2.3. General Analytical Procedures
2.4. UHPLC-PDA-ELSD-MS Analysis
2.5. UHPLC-PDA-CAD-HRMS Analysis, Data-Processing, and Feature-Based Molecular Network Generation
2.6. Chromatographic Optimization and Semi-Preparative Isolation
2.7. Description of the Isolated Compounds
2.8. UV–Vis Spectrophotometry and Fluorescence Spectra
2.9. Relative Singlet Oxygen Quantum Yield Measurements and Calculations
2.10. Cell Culture
2.11. Phototoxicity Assay
3. Results
3.1. Isolation of Multiple Chlorins from Ophiura sarsii
3.2. Absorbance Spectra and Singlet Oxygen Production by Chlorins of O. sarsii
3.3. Phototoxicity of O. sarsii Chlorins against Cancer Cells
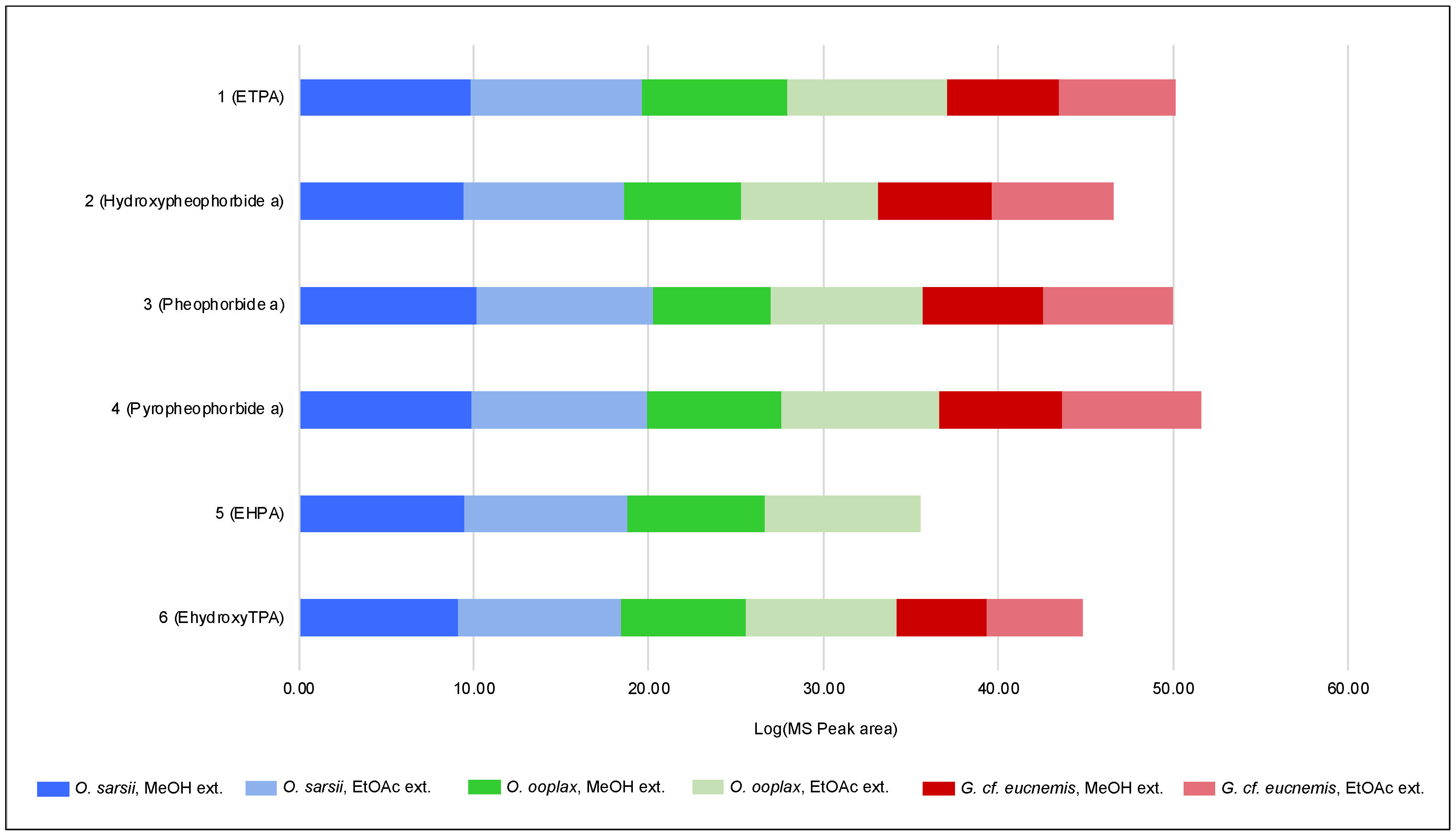
3.4. Identification of Chlorins in a Deep-Sea Pacific Brittle Star O. ooplax and a Deep-Sea Pacific Basket Star Gorgonocephalus cf. eucnemis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Queiros, C.; Garrido, P.M.; Maia Silva, J.; Filipe, P. Photodynamic therapy in dermatology: Beyond current indications. Dermatol. Ther. 2020, 33, e13997. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic disinfection and its role in controlling infectious diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H. (Ed.) Cosmetic Photodynamic Therapy; Karger: Basel, Switzerland, 2016; pp. VIII + 136 p. ISBN 978-3-318-02556-9. [Google Scholar]
- Arnaut, L.G. Chapter 5—Design of porphyrin-based photosensitizers for photodynamic therapy. In Advances in Inorganic Chemistry; Eldik, R.V., Stochel, G., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 63, pp. 187–233. [Google Scholar]
- Pandey, R.K.; Goswami, L.N.; Chen, Y.; Gryshuk, A.; Missert, J.R.; Oseroff, A.; Dougherty, T.J. Nature: A rich source for developing multifunctional agents. Tumor-imaging and photodynamic therapy. Lasers Surg. Med. 2006, 38, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Blagodatski, A.; Xu, J.; Khotimchenko, Y.; Koval, A. Mining Natural Compounds to Target WNT Signaling: Land and Sea Tales. Handb. Exp. Pharmacol. 2021, 269, 215–248. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, Y.S.; Silachev, D.N.; Katanaev, V.L. Marine Natural Products from the Russian Pacific as Sources of Drugs for Neurodegenerative Diseases. Mar. Drugs 2022, 20, 708. [Google Scholar] [CrossRef]
- Agius, L.; Jaccarini, V.; Ballantine, J.A.; Ferrito, V.; Pelter, A.; Psaila, A.F.; Zammit, V.A. Photodynamic action of bonellin, an integumentary chlorin of Bonellia viridis, Rolando (echiura, bonelliidae). Comp. Biochem. Physiol. B 1979, 63, 109–117. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Gao, Y.H.; Liao, P.Y.; Chen, D.Y.; Sun, N.N.; Nguyen Thi, P.A.; Yan, Y.J.; Wu, X.F.; Chen, Z.L. Comparison between porphin, chlorin and bacteriochlorin derivatives for photodynamic therapy: Synthesis, photophysical properties, and biological activity. Eur. J. Med. Chem. 2018, 160, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, A.; Huber, R.; Marcourt, L.; Chardonnens, E.; Koval, A.; Khotimchenko, Y.S.; Ferreira Queiroz, E.; Wolfender, J.L.; Katanaev, V.L. A Cytotoxic Porphyrin from North Pacific Brittle Star Ophiura sarsii. Mar. Drugs 2021, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, A.; Rodina, E.E.; Silachev, D.; Begun, M.; Babenko, V.A.; Benditkis, A.S.; Kozlov, A.S.; Krasnovsky, A.A.; Khotimchenko, Y.S.; Katanaev, V.L. Chlorin Endogenous to the North Pacific Brittle Star Ophiura sarsii for Photodynamic Therapy Applications in Breast Cancer and Glioblastoma Models. Biomedicines 2022, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.Y. Porphyrins in invertebrates. Ann. New York Acad. Sci. 1975, 244, 662–673. [Google Scholar] [CrossRef]
- Clark, H.L. North Pacific Ophiurans in the Collection of the United States National Museum; United States National Museum: Washington, DC, USA, 1911; Volume 75. [Google Scholar]
- Müller, J.; Troschel, F.H. System der Asteriden.1. Asteriae. 2. Ophiuridae; Vieweg: Braunschweig, Germany, 1842; pp. 1–134. [Google Scholar]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.L.; Allard, P.M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1329. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2010, 27, 431–432. [Google Scholar] [CrossRef]
- Guillarme, D.; Nguyen, D.T.; Rudaz, S.; Veuthey, J.L. Method transfer for fast liquid chromatography in pharmaceutical analysis: Application to short columns packed with small particle. Part II: Gradient experiments. Eur. J. Pharm. Biopharm. 2008, 68, 430–440. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Alfattani, A.; Afzan, A.; Marcourt, L.; Guillarme, D.; Wolfender, J.L. Utility of dry load injection for an efficient natural products isolation at the semi-preparative chromatographic scale. J. Chromatogr. A 2019, 1598, 85–91. [Google Scholar] [CrossRef]
- Endo, H.; Hosoya, H.; Koyama, T.; Ichioka, M. Isolation of 10-Hydroxypheophorbide a as a Photosensitizing Pigment from Alcohol-treated Chlorella Cells. Agric. Biol. Chem. 1982, 46, 2183–2193. [Google Scholar] [CrossRef]
- Cheng, H.H.; Wang, H.K.; Ito, J.; Bastow, K.F.; Tachibana, Y.; Nakanishi, Y.; Xu, Z.; Luo, T.Y.; Lee, K.H. Cytotoxic pheophorbide-related compounds from Clerodendrum calamitosum and C. cyrtophyllum. J. Nat. Prod. 2001, 64, 915–919. [Google Scholar] [CrossRef]
- Ohshima, T.; Hirata, M.; Oda, T.; Sasaki, A.; Shiratsuchi, M. Pheophorbide a, a potent endothelin receptor antagonist for both ETA and ETB subtypes. Chem. Pharm. Bull. (Tokyo) 1994, 42, 2174–2176. [Google Scholar] [CrossRef]
- Chen, D.; Lu, S.; Yang, G.; Pan, X.; Fan, S.; Xie, X.; Chen, Q.; Li, F.; Li, Z.; Wu, S.; et al. The seafood Musculus senhousei shows anti-influenza A virus activity by targeting virion envelope lipids. Biochem. Pharm. 2020, 177, 113982. [Google Scholar] [CrossRef]
- Sim, Y.G.; Yoo, J.H.; Oyunbileg, G.; Bayarmaa, B.; Wang, J.J.; Cui, B.C. Preparation of chlorin derivatives as antitumor agents. KR2009027470, 17 March 2009. [Google Scholar]
- Fernandez, J.M.; Bilgin, M.D.; Grossweiner, L.I. Singlet oxygen generation by photodynamic agents. J. Photochem. Photobiol. B 1997, 37, 131–140. [Google Scholar] [CrossRef]
- Eichwurzel, I.; Stiel, H.; Röder, B. Photophysical studies of the pheophorbide a dimer. J. Photochem. Photobiol. B 2000, 54, 194–200. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Chansakaow, S.; Ruangrungsi, N.; Ishikawa, T. Isolation of pyropheophorbide a from the leaves of Atalantia monophylla (ROXB.) CORR. (Rutaceae) as a possible antiviral active principle against herpes simplex virus type 2. Chem. Pharm. Bull. 1996, 44, 1415–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S. Ophiurans of Izu, Japan. J. Fac. Agric. Kyushu Univ. 1942, 7, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.N. Some Ophiuroidea from the Tasman Sea and adjacent waters. New Zealand J. Zool. 1979, 6, 21–51. [Google Scholar] [CrossRef]
- McEnnulty, F.R.; Gowlett-Holmes, K.; Williams, A.; Althaus, F.; Fromont, J.; Poore, G.; O’Hara, T.; Marsh, L.; Kott, P.; Slack-Smith, S.; et al. The deepwater megabenthic invertebrates on the continental margin of Australia (100–1100 m depths): Composition, distribution and novelty. Rec. West Aust. Mus. Suppl. 2011, 80, 1–191. [Google Scholar] [CrossRef]
- Djakonov, A.M. Les Échinodermes Des Mers Arctiques; Academy of sciences of the USSR: Leningrad, Russia, 1933; Volume 8, pp. 1–166. [Google Scholar]
- Stöhr, S.; O’Hara, T.D.; Thuy, B. Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS ONE 2012, 7, e31940. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Gomes, N.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine Invertebrate Metabolites with Anticancer Activities: Solutions to the “Supply Problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef]
- Gavrilova, G.S.; Kucheryavenko, A.V. Commercial rearing of the sea cucumber Apostichopus japonicus in Peter the great bay: Methodical peculiarities and results of the work of a mariculture farm in Sukhodol Bight. Russ. J. Mar. Biol. 2010, 36, 539–547. [Google Scholar] [CrossRef]
- O’Nea, W.G.; Jacobi, P.A. Toward a General Synthesis of Chlorins. J. Am. Chem. Soc. 2008, 130, 1102–1108. [Google Scholar] [CrossRef]
- Jacobi, P.A.; Lanz, S.; Ghosh, I.; Leung, S.H.; Löwer, F.; Pippin, D. A New Synthesis of Chlorins. Org. Lett. 2001, 3, 831–834. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimenko, A.; Huber, R.; Marcourt, L.; Tabakaev, D.; Koval, A.; Dautov, S.S.; Dautova, T.N.; Wolfender, J.-L.; Thew, R.; Khotimchenko, Y.; et al. Shallow- and Deep-Water Ophiura Species Produce a Panel of Chlorin Compounds with Potent Photodynamic Anticancer Activities. Antioxidants 2023, 12, 386. https://doi.org/10.3390/antiox12020386
Klimenko A, Huber R, Marcourt L, Tabakaev D, Koval A, Dautov SS, Dautova TN, Wolfender J-L, Thew R, Khotimchenko Y, et al. Shallow- and Deep-Water Ophiura Species Produce a Panel of Chlorin Compounds with Potent Photodynamic Anticancer Activities. Antioxidants. 2023; 12(2):386. https://doi.org/10.3390/antiox12020386
Chicago/Turabian StyleKlimenko, Antonina, Robin Huber, Laurence Marcourt, Dmitry Tabakaev, Alexey Koval, Salim Sh. Dautov, Tatyana N. Dautova, Jean-Luc Wolfender, Rob Thew, Yuri Khotimchenko, and et al. 2023. "Shallow- and Deep-Water Ophiura Species Produce a Panel of Chlorin Compounds with Potent Photodynamic Anticancer Activities" Antioxidants 12, no. 2: 386. https://doi.org/10.3390/antiox12020386
APA StyleKlimenko, A., Huber, R., Marcourt, L., Tabakaev, D., Koval, A., Dautov, S. S., Dautova, T. N., Wolfender, J.-L., Thew, R., Khotimchenko, Y., Queiroz, E. F., & Katanaev, V. L. (2023). Shallow- and Deep-Water Ophiura Species Produce a Panel of Chlorin Compounds with Potent Photodynamic Anticancer Activities. Antioxidants, 12(2), 386. https://doi.org/10.3390/antiox12020386








