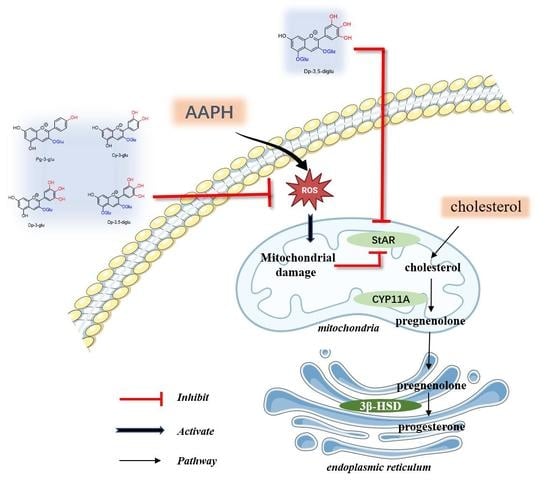
Anthocyanins Prevent AAPH-Induced Steroidogenesis Disorder in Leydig Cells by Counteracting Oxidative Stress and StAR Abnormal Expression in a Structure-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture and Treatments
2.3. Cell Viability Determination
2.4. Measurement of Progesterone
2.5. ROS Determination
2.6. Cell Mitochondrial Membrane Potential Assay
2.7. Protein Extraction and Western Blot
2.8. Antioxidant Properties Assessment
2.9. Molecular Docking
2.10. Data Analysis
3. Results
3.1. AAPH Decreased Progesterone Level in R2C Cells in a Dose-Dependent Manner
3.2. Anthocyanins Improved AAPH-Induced Decrease in Progesterone Synthesis
3.3. Anthocyanin Repaired Oxidative Stress Damage in R2C Cells
3.4. Anthocyanins Reduced Mitochondrial Damage
3.5. Anthocyanins Restored the Expression of StAR for Normal Progesterone Synthesis in Damaged R2C Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, X.; Deng, P.; Zhang, Y.; Liu, R.; Cai, D.; Xu, Q.; Jiang, X.; Sun, J.; Bai, W. The consequence and mechanism of dietary flavonoids on androgen profiles and disorders amelioration. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kruljac, M.; Finnbogadottir, H.; Bobjer, J.; Giraldi, A.; Fugl-Meyer, K.; Giwercman, A. Symptoms of sexual dysfunction among men from infertile couples: Prevalence and association with testosterone deficiency. Andrology 2020, 8, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, S.D.; Patel, P.; Fantus, R.J.; Halpern, J.; Chang, C.; Kargi, A.Y.; Ramasamy, R. Decline in Serum Testosterone Levels Among Adolescent and Young Adult Men in the USA. Eur. Urol. 2021, 7, 886–889. [Google Scholar] [CrossRef]
- Anaissie, J.; Delay, K.J.; Wang, W.; Hatzichristodoulou, G.; Hellstrom, W.J. Testosterone deficiency in adults and corresponding treatment patterns across the globe. Transl. Androl. Urol. 2017, 6, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Barbonetti, A.; D’Andrea, S.; Francavilla, S. Testosterone replacement therapy. Andrology 2020, 8, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhai, L.; Liu, Z.; Wu, S.; Xu, L. Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue. Oxid. Med. Cell. Longev. 2014, 2014, 190945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Lu, J.L.; Sun, J.X.; Jiang, X.W.; Li, X.S.; Li, Y.; Jiao, R.; Tian, L.M.; Bai, W.B. Cyanidin-3-O-glucoside promotes progesterone secretion by improving cells viability and mitochondrial function in cadmium-sulfate-damaged R2C cells. Food Chem. Toxicol. 2019, 128, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Zou, F.; Bai, S.; Jiang, X.; Jiao, R.; Ou, S.; Zhang, H.; Su, Z.; Huang, Y.; et al. Glycidamide inhibits progesterone production through reactive oxygen species-induced apoptosis in R2C Rat Leydig Cells. Food Chem. Toxicol. 2017, 108, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Xu, W.; Zhu, C.J.; Hu, Y.F.; Jiang, X.W.; Ou, S.Y.; Su, Z.J.; Huang, Y.D.; Jiao, R.; Bai, W.B. Cyanidin-3-O-Glucoside protects against 1,3-dichloro-2-propanol-induced reduction of progesterone by up-regulation of steroidogenic enzymes and camp level in Leydig cells. Front. Pharmacol. 2016, 7, 399. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef] [Green Version]
- He, J.A.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Lv, X.; Mu, J.; Wang, W.; Liu, Y.; Lu, X.; Sun, J.; Wang, J.; Ma, Q. Effects and mechanism of natural phenolic acids/fatty acids on copigmentation of purple sweet potato anthocyanins. Curr. Res. Food Sci. 2022, 5, 1243–1250. [Google Scholar] [CrossRef]
- Jiang, X.W.; Li, X.S.; Zhu, C.J.; Sun, J.X.; Tian, L.M.; Chen, W.; Bai, W.B. The target cells of anthocyanins in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2018, 59, 921–946. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Li, X.S.; Yao, Z.L.; Yang, D.C.; Jiang, X.W.; Sun, J.X.; Tian, L.M.; Hu, J.; Wu, B.Y.; Bai, W.B. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; Pacheco-Hernandez, M.D.; Paez-Hernandez, M.E.; Rodriguez, J.A.; Galan-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Sun, J.X.; Li, X.H.; Luo, H.X.; Ding, L.J.; Jiang, X.W.; Li, X.S.; Jiao, R.; Bai, W.B. Comparative study on the stability and antioxidant activity of six pyranoanthocyanins based on malvidin-3-glucoside. J. Agric. Food Chem. 2020, 68, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, K.R.; Mitchell, R.T. Effect of environmental and pharmaceutical exposures on fetal testis development and function: A systematic review of human experimental data. Hum. Reprod. Update 2019, 25, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Guo, J.T.; Jiang, X.W.; Sun, J.X.; Tian, L.M.; Jiao, R.; Tang, Y.G.; Bai, W.B. Cyanidin-3-O-glucoside protects against cadmium-induced dysfunction of sex hormone secretion via the regulation of hypothalamus-pituitary-gonadal axis in male pubertal mice. Food Chem. Toxicol. 2019, 129, 13–21. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Hawksworth, D.J.; Burnett, A.L. Nonalcoholic fatty liver disease, male sexual dysfunction, and infertility: Common links, common problems. Sex. Med. Rev. 2020, 8, 274–285. [Google Scholar] [CrossRef]
- Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef]
- Beattie, M.C.; Chen, H.; Fan, J.; Papadopoulos, V.; Miller, P.; Zirkin, B.R. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol. Reprod. 2013, 88, 101–107. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Failla, M.L.; Kumar, P.S.; Giusti, M.M. Anthocyanin structure determines susceptibility to microbial degradation and bioavailability to the buccal mucosa. J. Agric. Food Chem. 2014, 62, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–205. [Google Scholar]
- Bose, H.S.; Lingappa, V.R.; Miller, W.L. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 2002, 417, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Impact of location, type, and number of glycosidic substitutions on the color expression of o-dihydroxylated anthocyanidins. Food Chem. 2018, 268, 416–423. [Google Scholar] [CrossRef] [PubMed]






| Complex | Binding Energy (Kcal/mol) | Intermol Energy (Kcal/mol) | Torsional Energy (Kcal/mol) | Hydrogen Bonds between Ligands and the StAR | ||
|---|---|---|---|---|---|---|
| Distance (Å) | Donor Atom | Acceptor Atom | ||||
| Pelargonidin-3-glucoside-StAR | −4.49 | −7.77 | 3.28 | 1.8 | PG3G:H38 | A:ILE244:O |
| 1.9 | A:ASN256:HD22 | PG3G:O21 | ||||
| 2.0 | A:LEU246:HN | PG3G:O34 | ||||
| 2.0 | PG3G:H35 | A:ILE244:O | ||||
| Cyanidin-3-glucoside-StAR | −4.38 | −7.67 | 3.28 | 1.9 | C3G:H38 | A:ASP245:OD1 |
| 1.9 | C3G:H40 | A:ASP245:OD1 | ||||
| 1.9 | A:TRP249:HE1 | C3G:O35 | ||||
| 2.0 | A:LYS252:HN | C3G:O33 | ||||
| Delphinidin-3-O-glucoside-StAR | −4.36 | −8.24 | 3.88 | 1.8 | DP3G:H12 | A:GLY248:O |
| 1.8 | DP3G:H26 | A:ASN256:OD1 | ||||
| 2.1 | DP3G:H22 | A:LYS117:O | ||||
| 2.1 | A:LEU250:HN | DP3G:O11 | ||||
| Cyanidin-3,5-diglucoside-StAR | −3.18 | −8.55 | 5.37 | 1.9 | C35OG:H21 | A:ASN103:O |
| 2.0 | C35OG:H38 | A:ASP126:OD2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, X.; Wu, N.; Zhu, C.; Jiang, X.; Yuan, K.; Li, Y.; Sun, J.; Bai, W. Anthocyanins Prevent AAPH-Induced Steroidogenesis Disorder in Leydig Cells by Counteracting Oxidative Stress and StAR Abnormal Expression in a Structure-Dependent Manner. Antioxidants 2023, 12, 508. https://doi.org/10.3390/antiox12020508
Hu J, Li X, Wu N, Zhu C, Jiang X, Yuan K, Li Y, Sun J, Bai W. Anthocyanins Prevent AAPH-Induced Steroidogenesis Disorder in Leydig Cells by Counteracting Oxidative Stress and StAR Abnormal Expression in a Structure-Dependent Manner. Antioxidants. 2023; 12(2):508. https://doi.org/10.3390/antiox12020508
Chicago/Turabian StyleHu, Jun, Xusheng Li, Naijun Wu, Cuijuan Zhu, Xinwei Jiang, Kailan Yuan, Yue Li, Jianxia Sun, and Weibin Bai. 2023. "Anthocyanins Prevent AAPH-Induced Steroidogenesis Disorder in Leydig Cells by Counteracting Oxidative Stress and StAR Abnormal Expression in a Structure-Dependent Manner" Antioxidants 12, no. 2: 508. https://doi.org/10.3390/antiox12020508