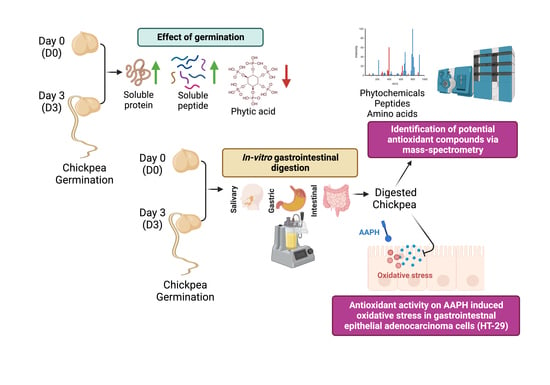
Germination and Simulated Gastrointestinal Digestion of Chickpea (Cicer arietinum L.) in Exhibiting In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Germinated Chickpeas
2.3. Protein Extraction and Total Soluble Protein Content
2.4. Total Peptide Content of the Germinated Chickpeas
2.5. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Phytic Acid Determination
2.7. Simulated Gastrointestinal Digestion
2.8. Cell Culture
2.9. In Vitro Antioxidant Activity
2.10. Identification of Free Amino Acids
2.11. Small Compound Identification through Untargeted HILIC LC-MS/MS
2.12. Statistical Analysis
3. Results
3.1. Whole Germinated Chickpea Nutritional Analysis
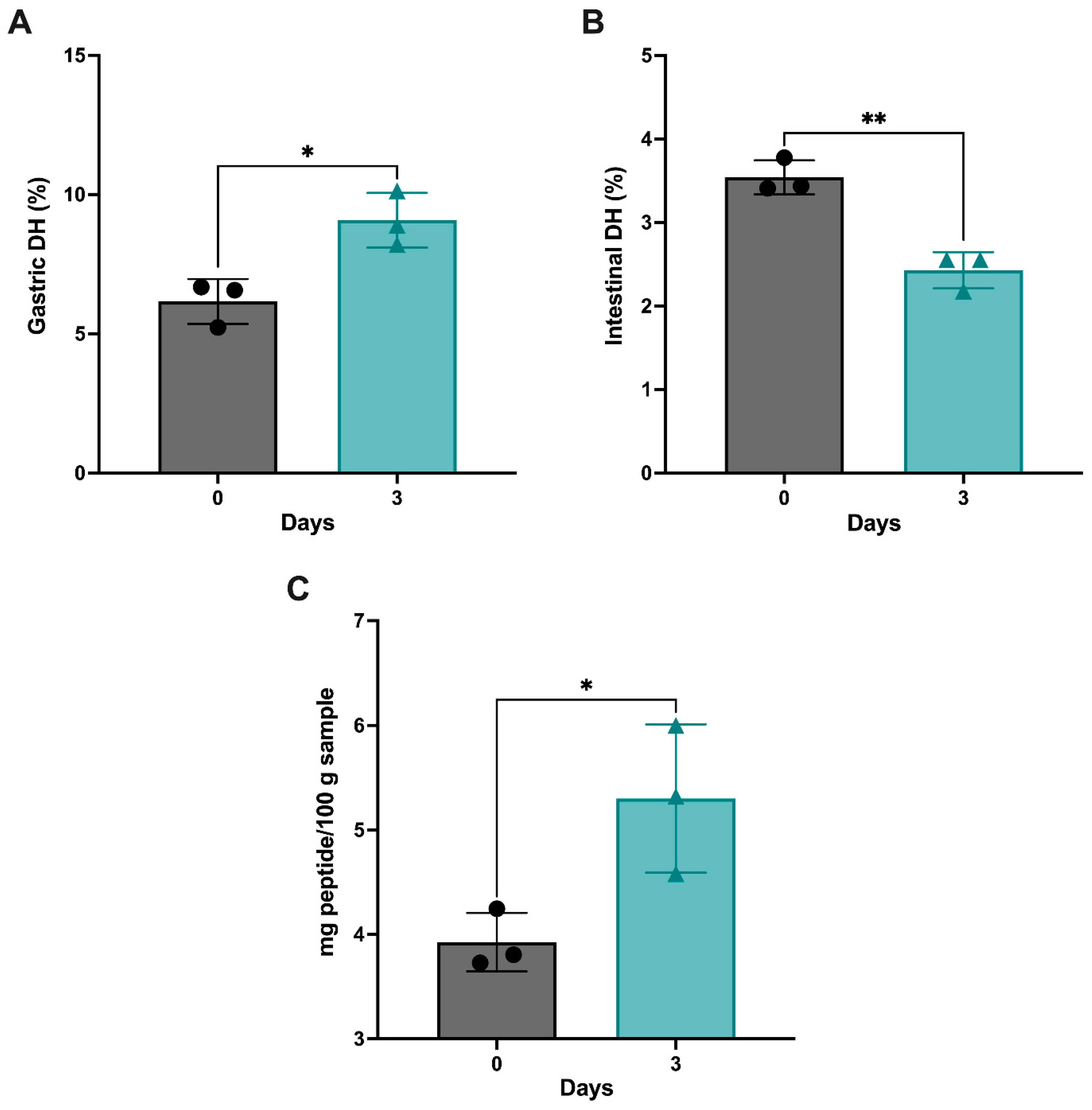
3.2. Proteolytic Activity of the Germinated and GI-Digested Chickpeas
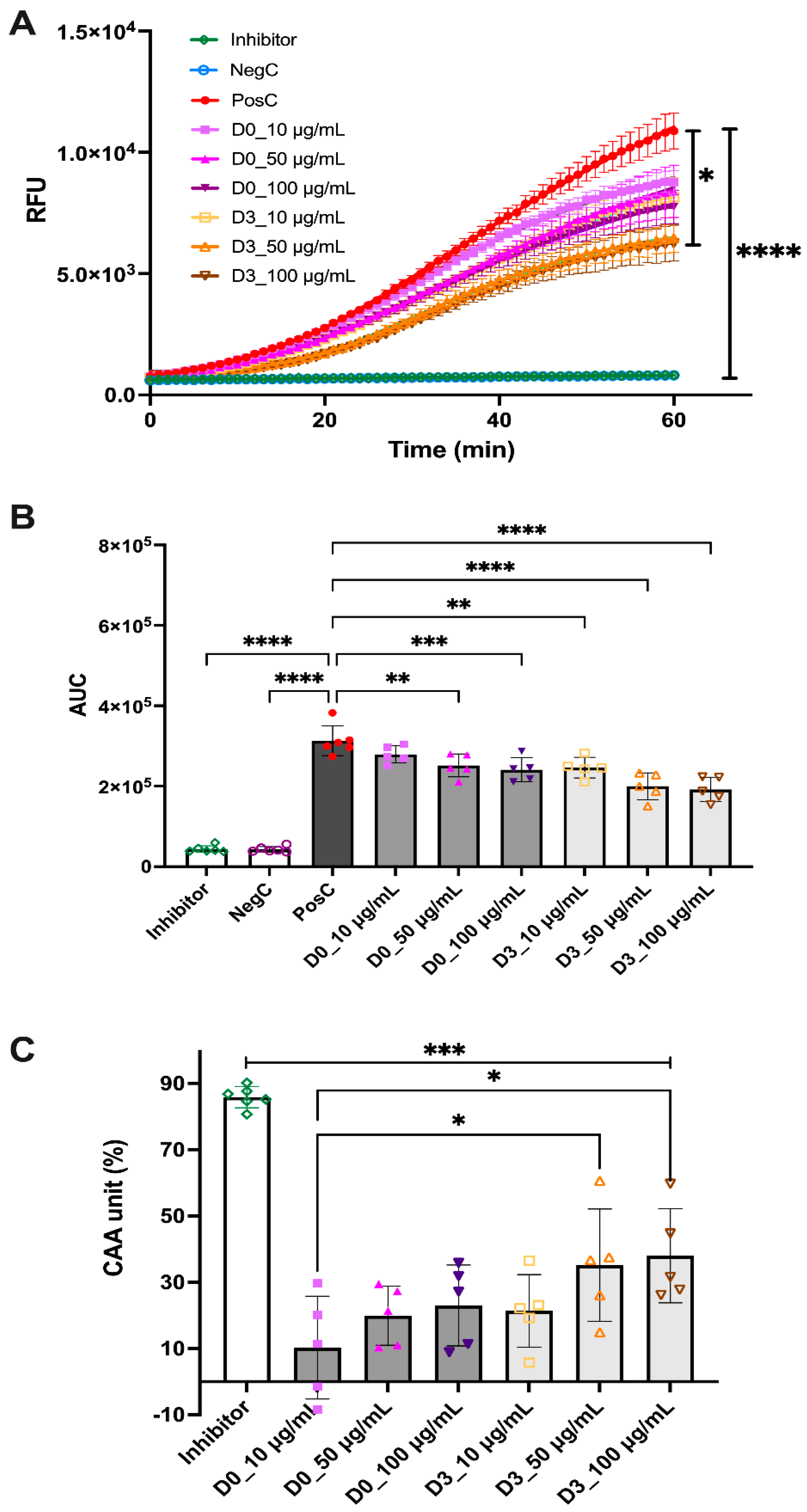
3.3. In Vitro Antioxidant Activity of D0-CD and D3-CD
3.4. Free Amino Acid and Small Molecule Identification of GCD
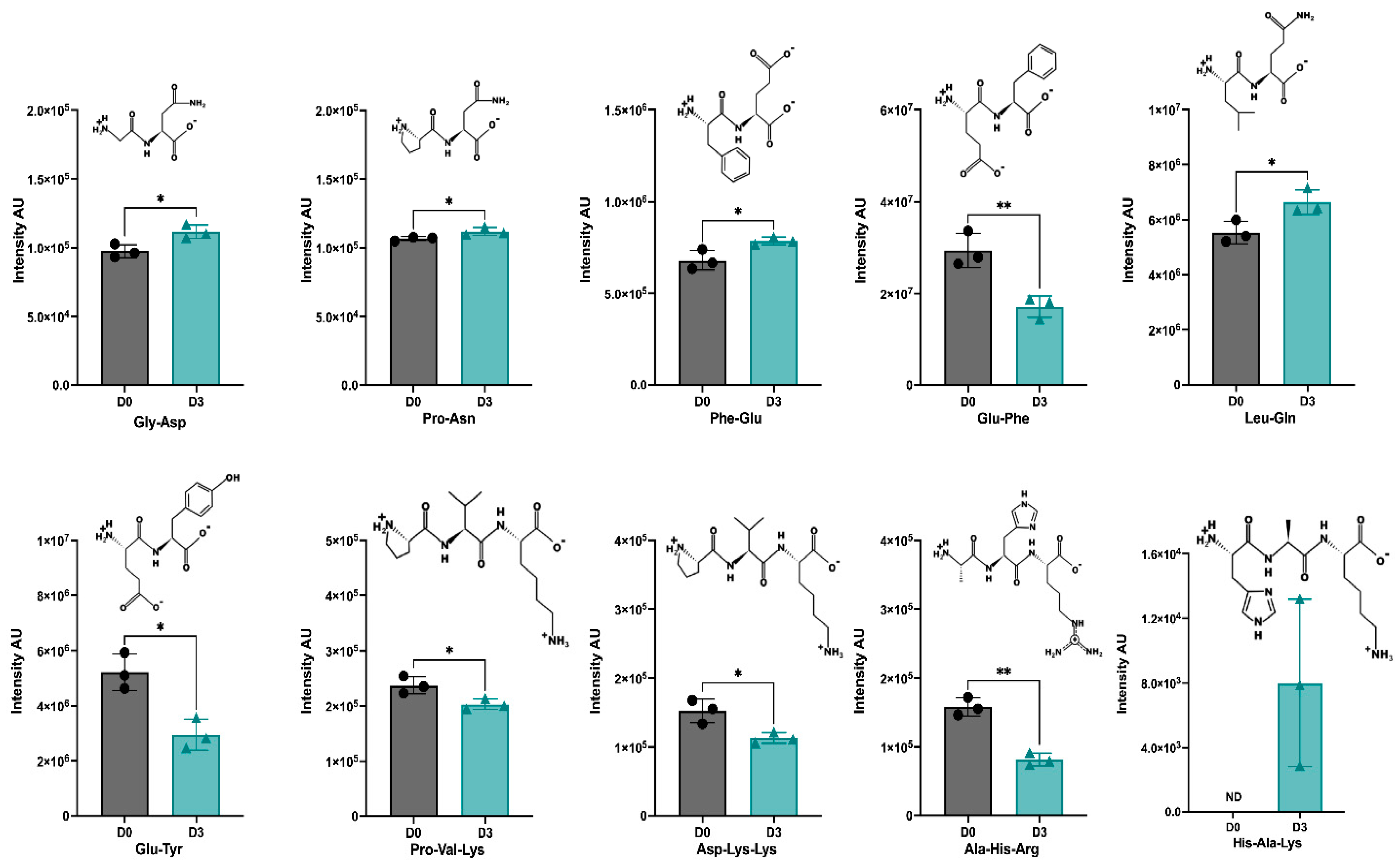
3.5. Identification of Peptides in D0-CD and D3-CD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse Proteins: Secondary Structure, Functionality and Applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Health Risks & Benefits of Chickpea (Cicer arietinum) Consumption. J. Agric. Food Chem. 2017, 65, 6–22. [Google Scholar] [PubMed]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas—Composition, Nutritional Value, Health Benefits, Application to Bread and Snacks: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive Proteins and Peptides in Pulse Crops: Pea, Chickpea and Lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of Bioactive Peptides during Food Processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.J. Food Proteins as a Source of Bioactive Peptides with Diverse Functions. Br. J. Nutr. 2012, 108, S149–S157. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-Based Antioxidants and the In Vitro Methods Used for Their Assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Pauls, K.P.; Deng, Z.; Tsao, R. Antioxidant and Anti-Inflammatory Polyphenols and Peptides of Common Bean (Phaseolus vulga L.) Milk and Yogurt in Caco-2 and HT-29 Cell Models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H. Bin; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure-Activity Relationship of Antioxidant Dipeptides: Dominant Role of Tyr, Trp, Cys and Met Residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Cargo-froom, C.; Shoveller, A.; Marinangeli, C.P.F.; Columbus, D.A. Methods for Processing Pulses to Optimize Nutritional Functionality and Maximize Amino Acid Availability in Foods and Feeds. Cereal Foods World 2020, 65, 1–10. [Google Scholar] [CrossRef]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.C.C.; Udenigwe, C.C. Germination as a Bioprocess for Enhancing the Quality and Nutritional Prospects of Legume Proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of Antinutritional Factors on Protein Digestibility and Amino Acid Availability in Foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef]
- Wanasundara, P.K.J.P.D.; Wanasundara, U.N.; Shahidi, F. Changes in Flax (Linum usitatissimum L.) Seed Lipids during Germination. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 41–48. [Google Scholar] [CrossRef]
- Bueno, D.B.; da Silva Júnior, S.I.; Seriani Chiarotto, A.B.; Cardoso, T.M.; Neto, J.A.; Lopes dos Reis, G.C.; Glória, M.B.A.; Tavano, O.L. The Germination of Soybeans Increases the Water-Soluble Components and Could Generate Innovations in Soy-Based Foods. LWT 2020, 117, 108599. [Google Scholar] [CrossRef]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of Cooking and Germination on Phenolic Composition and Dietary Fibre Fractions in Dark Beans (Phaseolus vulgaris L.) and Lentils (Lens culinaris L.). LWT—Food Sci. Technol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Bamdad, F.; Dokhani, S.; Keramat, J.; Zareie, R. The Impact of Germination and In Vitro Digestion on the Formation of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Lentil Proteins Compared to Whey Proteins. Int. J. Nutr. Food Eng. 2009, 3, 109–119. [Google Scholar]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A Review of Food Safety Considerations along the Farm to Fork Continuum. Int. J. Food Microbiol. 2018, 290, 76–85. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, E.; Yang, J.; Ciftci, O.; Vu, D.C.; Alvarez, S.; Purdum, S.; Majumder, K. Evaluating the Effect of Cooking and Gastrointestinal Digestion in Modulating the Bio-Accessibility of Different Bioactive Compounds of Eggs. Food Chem. 2021, 344, 128623. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Mat, D.J.L.; Cattenoz, T.; Souchon, I.; Michon, C.; Le Feunteun, S. Monitoring Protein Hydrolysis by Pepsin Using PH-Stat: In Vitro Gastric Digestions in Static and Dynamic PH Conditions. Food Chem. 2018, 239, 268–275. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Rui, H.L. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In Vitro Digestion of Lipids in Real Foods: Influence of Lipid Organization Within the Food Matrix and Interactions with Nonlipid Components. J. Food Sci. 2018, 83, 2629–2637. [Google Scholar] [CrossRef]
- Hacham, Y.; Avraham, T.; Amir, R. The N-Terminal Region of Arabidopsis Cystathionine γ-Synthase Plays an Important Regulatory Role in Methionine Metabolism. Plant Physiol. 2002, 128, 454–462. [Google Scholar] [CrossRef]
- Rao, P.S.; Nolasco, E.; Handa, A.; Naldrett, M.J.; Alvarez, S.; Majumder, K. Effect of PH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated in-Vitro Gastrointestinal Digestion. Antioxidants 2020, 9, 1114. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Nutritional Composition and Antinutritional Factors of Chickpeas (Cicer arietinum L.) Undergoing Different Cooking Methods and Germination. Plant Foods Hum. Nutr. 2002, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of Germination and Enzymatic Hydrolysis of Cowpea Bean (Vigna unguiculata) on the Generation of Peptides Capable of Inhibiting Dipeptidyl Peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Menacho, L.M.; Berhow, M.A.; Mandarino, J.M.G.; Chang, Y.K.; Mejia, E.G. de Effect of Time and Temperature on Bioactive Compounds in Germinated Brazilian Soybean Cultivar BRS 258. Food Res. Int. 2010, 43, 1856–1865. [Google Scholar] [CrossRef]
- Dida Bulbula, D.; Urga, K. Study on the Effect of Traditional Processing Methods on Nutritional Composition and Anti Nutritional Factors in Chickpea (Cicer arietinum). Cogent Food Agric. 2018, 4, 1422370. [Google Scholar] [CrossRef]
- Dawson, J.M.; Heatlie, P.L. Lowry Method of Protein Quantification: Evidence for Photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
- Knight, M.I.; Chambers, P.J. Problems Associated with Determining Protein Concentration: A Comparison of Techniques for Protein Estimations. Appl. Biochem. Biotechnol.—Part B Mol. Biotechnol. 2003, 23, 19–28. [Google Scholar] [CrossRef]
- Księżak, J.; Bojarszczuk, J. Evaluation of the Variation of the Contents of Anti-Nutrients and Nutrients in the Seeds of Legumes. Biotechnol. Anim. Husb. 2014, 30, 153–166. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Clemente, A.; Vioque, J.; Bautista, J.; Millán, F. Protein Isolates from Chickpea (Cicer arietinum L.): Chemical Composition, Functional Properties and Protein Characterization. Food Chem. 1999, 64, 237–243. [Google Scholar] [CrossRef]
- Magaña, M.D.; Segura-Campos, M.; Dávila-Ortiz, G.; Betancur-Ancona, D.; Chel-Guerrero, L. ACE-I Inhibitory Properties of Hydrolysates from Germinated and Ungerminated Phaseolus Lunatus Proteins. Food Sci. Technol. 2015, 35, 167–174. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Muzaffar, K.; Dar, B.N. Effect of Germination Time on Physicochemical, Electrophoretic, Rheological, and Functional Performance of Chickpea Protein Isolates. ACS Food Sci. Technol. 2021, 1, 802–812. [Google Scholar] [CrossRef]
- Di Stefano, E.; Tsopmo, A.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. Bioprocessing of Common Pulses Changed Seed Microstructures, and Improved Dipeptidyl Peptidase-IV and α-Glucosidase Inhibitory Activities. Sci. Rep. 2019, 9, 15308. [Google Scholar] [CrossRef] [PubMed]
- López-Barrios, L.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Changes in Antioxidant and Antiinflammatory Activity of Black Bean (Phaseolus vulgaris L.) Protein Isolates Due to Germination and Enzymatic Digestion. Food Chem. 2016, 203, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Díaz, M.F.; Jiménez, T.; Benítez, V.; Herrera, T.; Cuadrado, C.; Martín-Pedrosa, M.; Martín-Cabrejas, M.A. Changes in Nonnutritional Factors and Antioxidant Activity during Germination of Nonconventional Legumes. J. Agric. Food Chem. 2013, 61, 8120–8125. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Qian, B.; Zhao, S.; Qi, X.; Ye, L.; Guisti, M.M.; Wang, X. Effect of glycosylation patterns of Chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines. Food Chem. 2015, 172, 183–189. [Google Scholar] [CrossRef]
- Eum, H.L.; Park, Y.; Yi, T.G.; Lee, J.W.; Ha, K.S.; Choi, I.Y.; Park, N. Il Effect of Germination Environment on the Biochemical Compounds and Anti-Inflammatory Properties of Soybean Cultivars. PLoS ONE 2020, 15, e0232159. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Y.L.; Chen, J.; Zhou, L. Synergistic Inhibition of Lipid Oxidation by Pea Protein Hydrolysate Coupled with Licorice Extract in a Liposomal Model System. J. Agric. Food Chem. 2013, 61, 8452–8461. [Google Scholar] [CrossRef]
- Siow, H.L.; Gan, C.Y. Extraction of Antioxidative and Antihypertensive Bioactive Peptides from Parkia Speciosa Seeds. Food Chem. 2013, 141, 3435–3442. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino Acids, Peptides, and Proteins as Antioxidants for Food Preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Ltd.: Cambridge, UK, 2015; pp. 105–140. ISBN 9781782420972. [Google Scholar]
- Peña-Ramos, E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and Characterisation for Antioxidant Activity of Hydrolysed Whey Protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]


| Compound | Intensity of Day 0 (D0-CD) | Intensity of Day 3 (D3-CD) | m/z | Retention Time (min) |
|---|---|---|---|---|
| o-Coumaric acid # | 1,633,790 ± 63,865 | 1,509,267 ± 35,909 | 165.0546 | 8.91 |
| Isoliqueritigenin * | 290 ± 118 | 12,898 ± 1233 | 257.0807 | 2.96 |
| 3-Hydroxy-3′-methoxyflavone * | 57 ± 50 | 3086 ± 950 | 269.0805 | 4.38 |
| Isodalbergin * | 16,751 ± 7693 | 4,670,146 ± 1,343,553 | 269.0807 | 2.84 |
| Isoliquiritigenin 4-methyl ether * | 0 | 6226 ± 779 | 271.0964 | 2.73 |
| 3-Methoxy-4,2′,5′-trihydroxychalcone * | 0 | 13,566 ± 746 | 287.0911 | 2.92 |
| Geraldol * | 610 ± 712 | 32,031 ± 1400 | 301.0705 | 2.89 |
| 2′,4′-Dihydroxy-3,4-dimethoxychalcone * | 0 | 12,082 ± 2568 | 301.1070 | 2.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newton, A.; Majumder, K. Germination and Simulated Gastrointestinal Digestion of Chickpea (Cicer arietinum L.) in Exhibiting In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells. Antioxidants 2023, 12, 1114. https://doi.org/10.3390/antiox12051114
Newton A, Majumder K. Germination and Simulated Gastrointestinal Digestion of Chickpea (Cicer arietinum L.) in Exhibiting In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells. Antioxidants. 2023; 12(5):1114. https://doi.org/10.3390/antiox12051114
Chicago/Turabian StyleNewton, Ashley, and Kaustav Majumder. 2023. "Germination and Simulated Gastrointestinal Digestion of Chickpea (Cicer arietinum L.) in Exhibiting In Vitro Antioxidant Activity in Gastrointestinal Epithelial Cells" Antioxidants 12, no. 5: 1114. https://doi.org/10.3390/antiox12051114