Plasma Lipidomics Profiles Highlight the Associations of the Dual Antioxidant/Pro-oxidant Molecules Sphingomyelin and Phosphatidylcholine with Subclinical Atherosclerosis in Patients with Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical Assessment
2.3. Carotid and Femoral Ultrasound Imaging
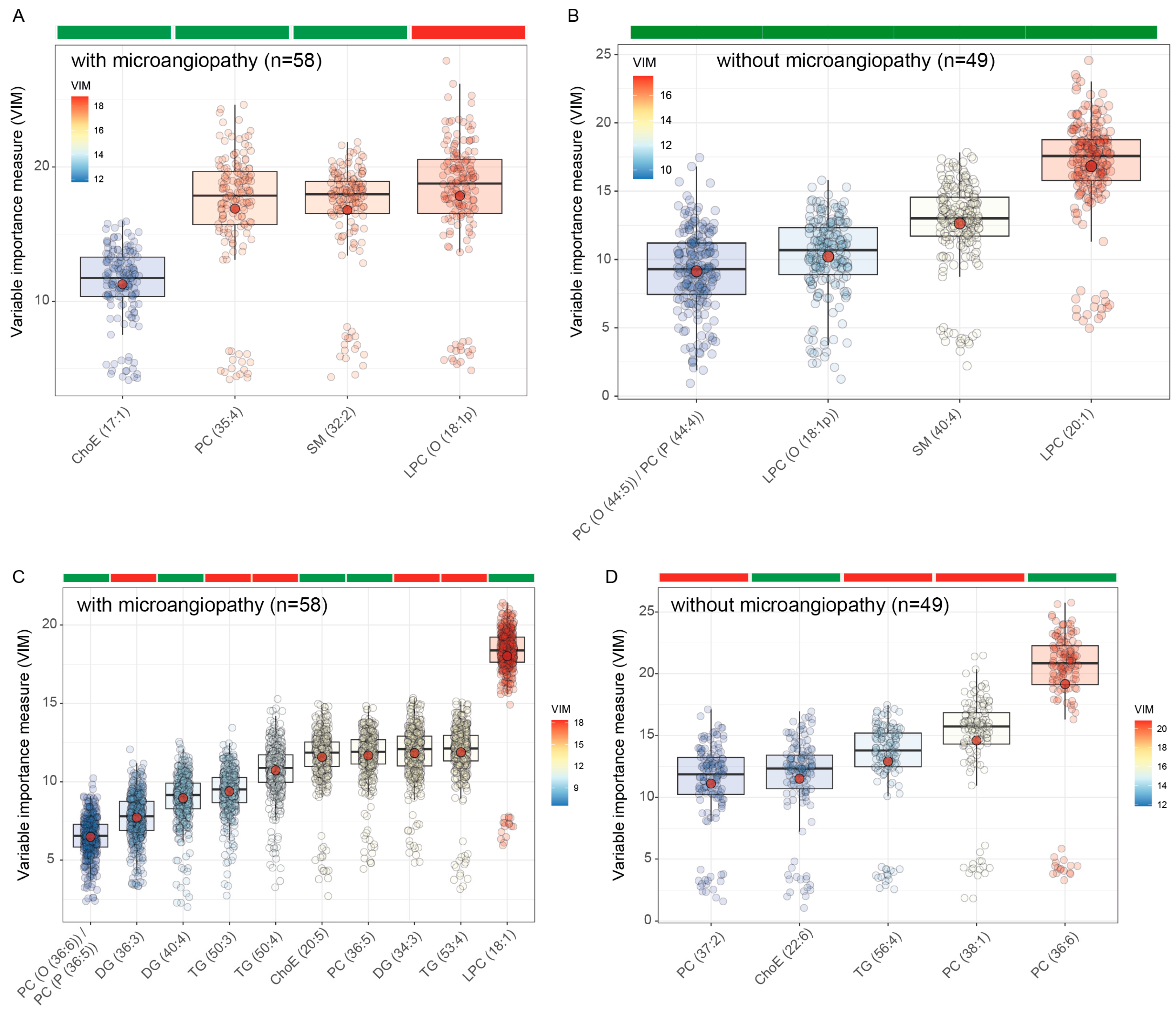
2.4. Microvascular Complications
2.5. Biochemical Measurements
2.6. Lipidomics Analysis Using UHPLC-qTOF/MS
2.7. Statistical Analyses
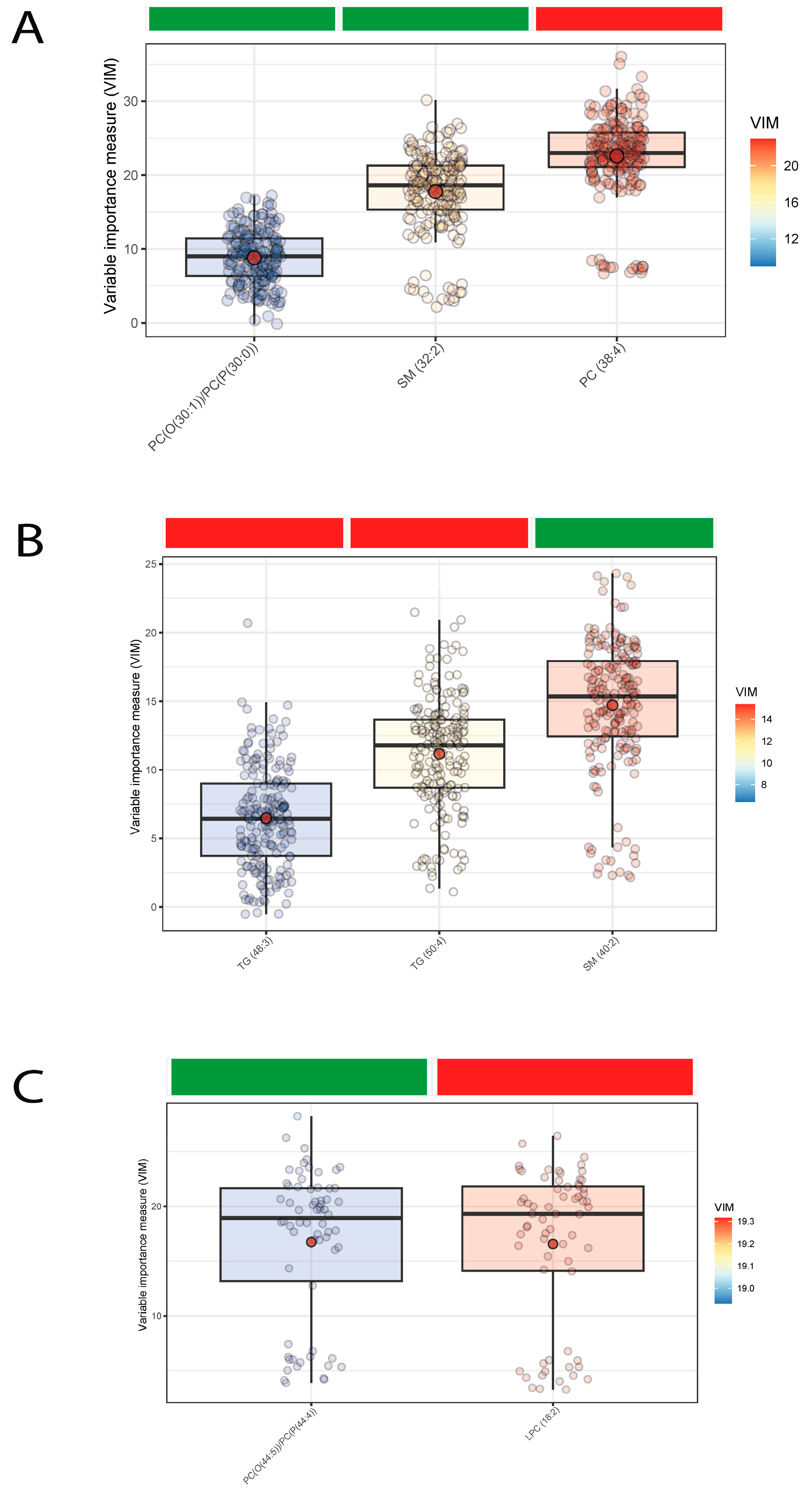
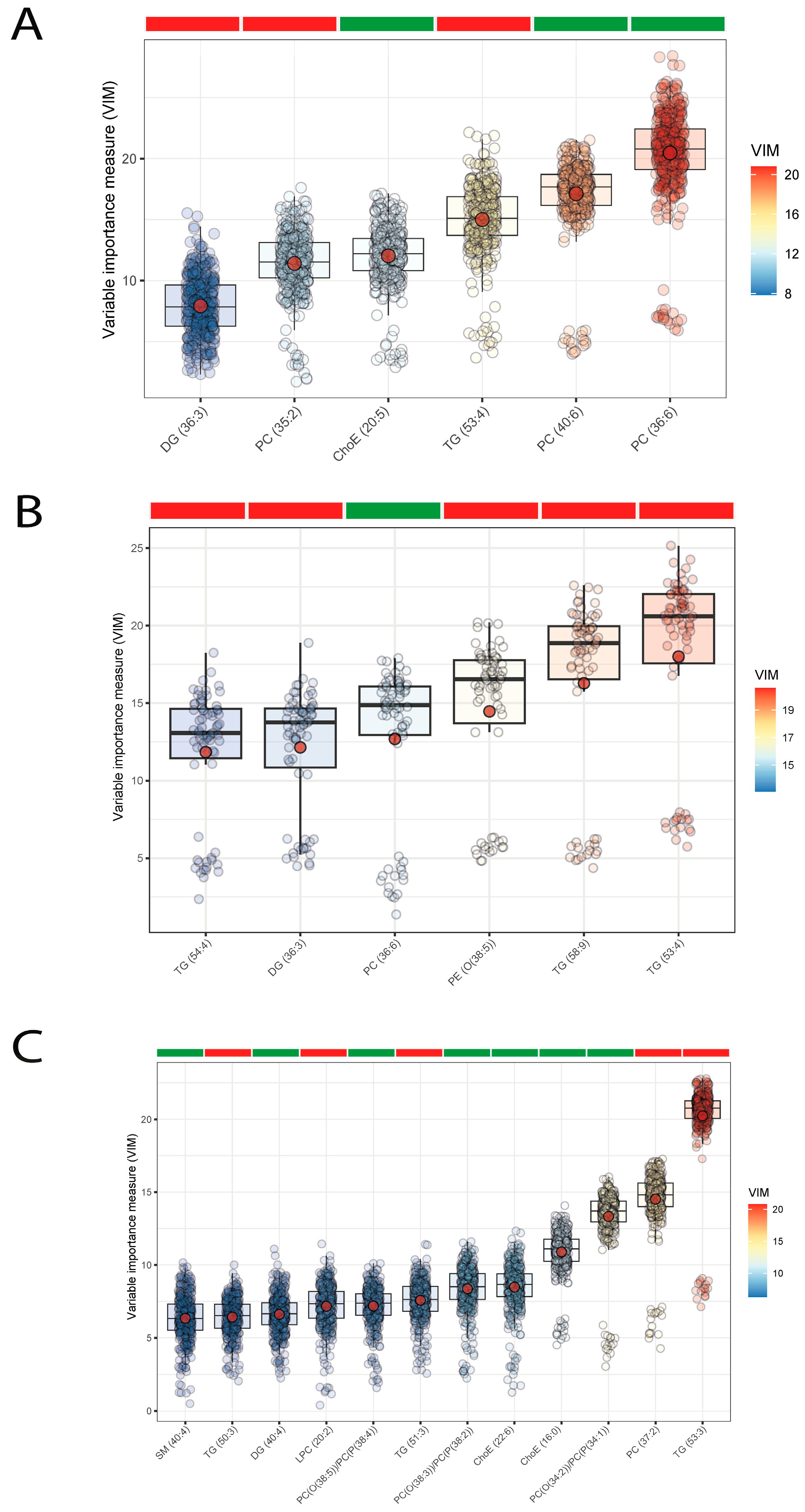
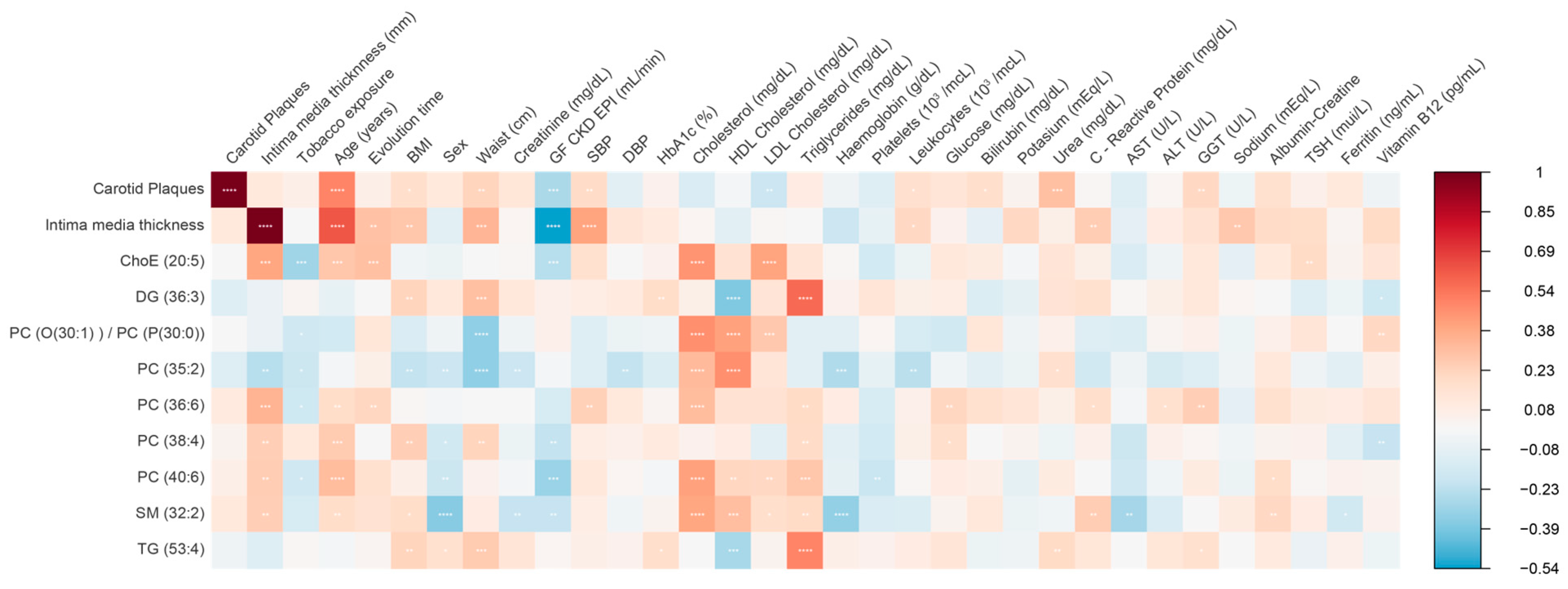
3. Results
4. Discussion
4.1. Findings Related to Sphingomyelins (SMs)
4.2. Findings Related to Ether Lipids
4.3. Findings Related to Phosphatidylcholines (PCs)
4.4. Findings Related to Lysophosphatidylcholines (LPCs)
4.5. Findings Related to Di- and Triglyceride Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S86–S104. [CrossRef]
- De Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 Diabetes Mellitus and Cardiovascular Disease. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef]
- Bimodal Pattern of Coronary Microvascular Involvement in Diabetes Mellitus. J. Am. Heart Assoc. 2017, 6, 2163. [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Rathsman, B.; Jensen-Urstad, K.; Nyström, T. Intensified Insulin Treatment Is Associated with Improvement in Skin Microcirculation and Ischaemic Foot Ulcer in Patients with Type 1 Diabetes Mellitus: A Long-Term Follow-up Study. Diabetologia 2014, 57, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef]
- Kupfer, R.; Larrúbia, M.R.; Bussade, I.; Pereira, J.R.D.; Lima, G.A.B.; Epifanio, M.A.; Schettino, C.D.S.; Momesso, D.P. Predictors of Subclinical Atherosclerosis Evaluated by Carotid Intima-Media Thickness in Asymptomatic Young Women with Type 1 Diabetes Mellitus. Arch. Endocrinol. Metab. 2017, 61, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef]
- Hornemann, T.; Worgall, T.S. Sphingolipids and Atherosclerosis. Atherosclerosis 2013, 226, 16–28. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Ekroos, K.; Jänis, M.; Tarasov, K.; Hurme, R.; Laaksonen, R. Lipidomics: A Tool for Studies of Atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Kraaijenhof, J.M.; Mayr, M.; Nicholls, S.J.; Koenig, W.; Catapano, A.L.; Stroes, E.S.G. Proteomics and Lipidomics in Atherosclerotic Cardiovascular Disease Risk Prediction. Eur. Heart J. 2023, 44, 1594–1607. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Q.; Huang, Y. Potential Therapeutic Targets for Atherosclerosis in Sphingolipid Metabolism. Clin. Sci. 2019, 133, 763–776. [Google Scholar] [CrossRef]
- Serés-Noriega, T.; Ortega, E.; Perea, V.; Giménez, M.; Boswell, L.; Mariaca, K.; Font, C.; Mesa, A.; Viñals, C.; Blanco, J.; et al. Nuclear Magnetic Resonance-Based Lipidomics in the Assessment of Cardiometabolic Risk in Type 1 Diabetes: An Exploratory Analysis. Diabetes Ther. 2023, 14, 553–567. [Google Scholar] [CrossRef]
- Subbaiah, P.V.; Subramanian, V.S.; Wang, K. Novel Physiological Function of Sphingomyelin in Plasma. J. Biol. Chem. 1999, 274, 36409–36414. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Subbaiah, P.V. Protection of Membrane Cholesterol by Sphingomyelin against Free Radical-Mediated Oxidation. Free Radic. Biol. Med. 2006, 40, 2092–2102. [Google Scholar] [CrossRef]
- Frey, B.; Haupt, R.; Alms, S.; Holzmann, G.; König, T.; Kern, H.; Kox, W.; Rüstow, B.; Schlame, M. Increase in Fragmented Phosphatidylcholine in Blood Plasma by Oxidative Stress. J. Lipid Res. 2000, 41, 1145–1153. [Google Scholar] [CrossRef]
- Lipsy, R.J. The National Cholesterol Education Program Adult Treatment Panel III Guidelines. J. Manag. Care Pharm. 2003, 9, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of Variable Selection Methods for Random Forests and Omics Data Sets. Brief Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Hannun, Y.A. Translational Aspects of Sphingolipid Metabolism. Trends Mol. Med. 2007, 13, 327–336. [Google Scholar] [CrossRef]
- Kang, S.-C.; Kim, B.-R.; Lee, S.-Y.; Park, T.-S. Sphingolipid Metabolism and Obesity-Induced Inflammation. Front. Endocrinol. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Guyton, J.R.; Klemp, K.F. Development of the Lipid-Rich Core in Human Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Paultre, F.; Pearson, T.A.; Reed, R.G.; Francis, C.K.; Lin, M.; Berglund, L.; Tall, A.R. Plasma Sphingomyelin Level as a Risk Factor for Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C.; Jiang, X.-C.; Tabas, I.; Tall, A.; Shea, S. Plasma Sphingomyelin and Subclinical Atherosclerosis: Findings from the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2006, 163, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, E.H.; et al. Elevation of Ceramide and Activation of Secretory Acid Sphingomyelinase in Patients with Acute Coronary Syndromes. Coron. Artery Dis. 2014, 25, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Peter Slotte, J. Molecular Properties of Various Structurally Defined Sphingomyelins -- Correlation of Structure with Function. Prog. Lipid Res. 2013, 52, 206–219. [Google Scholar] [CrossRef]
- Liu, J.; Huan, C.; Chakraborty, M.; Zhang, H.; Lu, D.; Kuo, M.-S.; Cao, G.; Jiang, X.-C. Macrophage Sphingomyelin Synthase 2 Deficiency Decreases Atherosclerosis in Mice. Circ. Res. 2009, 105, 295–303. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, F.; Liu, J.; Dong, J.; Bui, H.H.; Peake, D.A.; Kuo, M.-S.; Cao, G.; Jiang, X.-C. Selective Reduction in the Sphingomyelin Content of Atherogenic Lipoproteins Inhibits Their Retention in Murine Aortas and the Subsequent Development of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2114–2120. [Google Scholar] [CrossRef]
- Otsuka, F.; Finn, A.V.; Yazdani, S.K.; Nakano, M.; Kolodgie, F.D.; Virmani, R. The Importance of the Endothelium in Atherothrombosis and Coronary Stenting. Nat. Rev. Cardiol. 2012, 9, 439–453. [Google Scholar] [CrossRef]
- Zhang, P.; Hua, L.; Hou, H.; Du, X.; He, Z.; Liu, M.; Hu, X.; Yan, N. Sphingomyelin Synthase 2 Promotes H2O2-Induced Endothelial Dysfunction by Activating the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Med. 2018, 42, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Sandin, M.; Sampaio, J.L.; Almgren, P.; Narkiewicz, K.; Hoffmann, M.; Hedner, T.; Wahlstrand, B.; Simons, K.; Shevchenko, A.; et al. Plasma Lipid Composition and Risk of Developing Cardiovascular Disease. PLoS ONE 2013, 8, e71846. [Google Scholar] [CrossRef] [PubMed]
- Nieddu, G.; Michelucci, E.; Formato, M.; Ciampelli, C.; Obino, G.; Signore, G.; Di Giorgi, N.; Rocchiccioli, S.; Lepedda, A.J. Molecular Characterization of Plasma HDL, LDL, and VLDL Lipids Cargos from Atherosclerotic Patients with Advanced Carotid Lesions: A Preliminary Report. Int. J. Mol. Sci. 2022, 23, 12449. [Google Scholar] [CrossRef]
- Djekic, D.; Pinto, R.; Repsilber, D.; Hyotylainen, T.; Henein, M. Serum Untargeted Lipidomic Profiling Reveals Dysfunction of Phospholipid Metabolism in Subclinical Coronary Artery Disease. Vasc. Health Risk Manag. 2019, 15, 123–135. [Google Scholar] [CrossRef]
- Sakamoto, H.; Yoshida, T.; Sanaki, T.; Shigaki, S.; Morita, H.; Oyama, M.; Mitsui, M.; Tanaka, Y.; Nakano, T.; Mitsutake, S.; et al. Possible Roles of Long-Chain Sphingomyelines and Sphingomyelin Synthase 2 in Mouse Macrophage Inflammatory Response. Biochem. Biophys. Res. Commun. 2017, 482, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in Cardiovascular Diseases and Metabolic Disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef]
- Sasset, L.; Zhang, Y.; Dunn, T.M.; Di Lorenzo, A. Sphingolipid De Novo Biosynthesis: A Rheostat of Cardiovascular Homeostasis. Trends Endocrinol. Metab. 2016, 27, 807–819. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Baker, N.L.; Hunt, K.J.; Hammad, S.M.; Arthur, J.; Virella, G.; Klein, R.L. Glycosylated Sphingolipids and Progression to Kidney Dysfunction in Type 1 Diabetes. J. Clin. Lipidol. 2019, 13, 481–491.e1. [Google Scholar] [CrossRef]
- Klein, R.L.; Hammad, S.M.; Baker, N.L.; Hunt, K.J.; Al Gadban, M.M.; Cleary, P.A.; Virella, G.; Lopes-Virella, M.F. Decreased Plasma Levels of Select Very Long Chain Ceramide Species Are Associated with the Development of Nephropathy in Type 1 Diabetes. Metabolism 2014, 63, 1287–1295. [Google Scholar] [CrossRef]
- Shiwani, H.A.; Elfaki, M.Y.; Memon, D.; Ali, S.; Aziz, A.; Egom, E.E. Updates on Sphingolipids: Spotlight on Retinopathy. Biomed. Pharmacother. 2021, 143, 112197. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and Functional Roles of Ether Lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Barr, J.; Caballería, J.; Martínez-Arranz, I.; Domínguez-Díez, A.; Alonso, C.; Muntané, J.; Pérez-Cormenzana, M.; García-Monzón, C.; Mayo, R.; Martín-Duce, A.; et al. Obesity-Dependent Metabolic Signatures Associated with Nonalcoholic Fatty Liver Disease Progression. J. Proteome Res. 2012, 11, 2521–2532. [Google Scholar] [CrossRef]
- Wallner, S.; Orsó, E.; Grandl, M.; Konovalova, T.; Liebisch, G.; Schmitz, G. Phosphatidylcholine and Phosphatidylethanolamine Plasmalogens in Lipid Loaded Human Macrophages. PLoS ONE 2018, 13, e0205706. [Google Scholar] [CrossRef]
- Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging Roles of Lysophospholipids in Health and Disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.; Sun, W.; Polfus, L.M.; Folsom, A.R.; Heiss, G.; Sharrett, A.R.; Boerwinkle, E.; Ballantyne, C.M.; Hoogeveen, R.C. Lipoprotein Associated Phospholipase A2 Activity, Apolipoprotein C3 Loss-of-Function Variants and Cardiovascular Disease: The Atherosclerosis Risk In Communities Study. Atherosclerosis 2015, 241, 641–648. [Google Scholar] [CrossRef]
- Wilensky, R.L.; Shi, Y.; Mohler, E.R.; Hamamdzic, D.; Burgert, M.E.; Li, J.; Postle, A.; Fenning, R.S.; Bollinger, J.G.; Hoffman, B.E.; et al. Inhibition of Lipoprotein-Associated Phospholipase A2 Reduces Complex Coronary Atherosclerotic Plaque Development. Nat. Med. 2008, 14, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhu, Q.; Lai, W.; Ma, Q.; Liu, C.; Chen, X.; Zhang, Y.; Wang, Z.; Chen, H.; Yan, H.; et al. Insights into the Prognosis of Lipidomic Dysregulation for Death Risk in Patients with Coronary Artery Disease. Clin. Transl. Med. 2020, 10, e189. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma Lipidomic Analysis of Stable and Unstable Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics Profiling and Risk of Cardiovascular Disease in the Prospective Population-Based Bruneck Study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.A.; Kim, Y.R.; Lee, I.S.; Sung, C.K.; Hong, J.; Sim, C.J.; Im, K.S.; Jung, J.H. Lyso-PAF Analogues and Lysophosphatidylcholines from the Marine Sponge Spirastrella Abata as Inhibitors of Cholesterol Biosynthesis. J. Nat. Prod. 1999, 62, 1554–1557. [Google Scholar] [CrossRef]
- Rozenberg, O.; Shih, D.M.; Aviram, M. Human Serum Paraoxonase 1 Decreases Macrophage Cholesterol Biosynthesis: Possible Role for Its Phospholipase-A2-like Activity and Lysophosphatidylcholine Formation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, R.; Holleboom, A.G.; van den Bogaard, B.; Nederveen, A.J.; de Groot, E.; Hutten, B.A.; Schimmel, A.W.; Hovingh, G.K.; Kastelein, J.J.P.; Kuivenhoven, J.A.; et al. Carriers of Lecithin Cholesterol Acyltransferase Gene Mutations Have Accelerated Atherogenesis as Assessed by Carotid 3.0-T Magnetic Resonance Imaging [Corrected]. J. Am. Coll. Cardiol. 2011, 58, 2481–2487. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Rajab, A.; Rabizadeh, S.; Bitaraf, M.; Ghanei, A.; Esteghamati, A.; Mirmiranpour, H. Lecithin–Cholesterol Acyltransferase (LCAT) Activity Is Lower in Patients with Type 2 Diabetes in the Presence of Metabolic Syndrome. Bioact. Compd. Health Dis. 2020, 3, 66. [Google Scholar] [CrossRef]
- Lair, B.; Laurens, C.; Van Den Bosch, B.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
| Total (n = 107) | Subclinical Atherosclerosis (n = 52) | Non Subclinical Atherosclerosis (n = 55) | p | |
|---|---|---|---|---|
| Age (years) | 52.8 (12.9) | 60.1 (11.1) | 45 (9.81) | <0.001 |
| Diabetes duration (years) | 24.5 (11.8) | 26.3 (13) | 22.6 (10.3) | 0.107 |
| BMI (kg/m2) | 26.7 (4.48) | 27.6 (4.49) | 25.7 (4.29) | 0.027 |
| Central obesity | 49.5% (n = 52) | 64.2% (n = 34) | 34.6% (n = 18) | 0.002 |
| HbA1c (%) | 7.87 (0.95) | 7.87 (0.81) | 7.88 (1.10) | 0.944 |
| Microvascular complications | 54.2% (n = 58) | 58.2% (n = 32) | 50% (n = 26) | 0.396 |
| Hypertension | 52.3% (n = 56) | 65.5% (n = 36) | 38.5% (n = 20) | 0.005 |
| Tobacco exposure | 41.1% (n = 44) | 47.3% (n = 26) | 34.6% (n = 18) | 0.184 |
| Current smoking | 18.7% (n = 20) | 21.8% (n = 12) | 15.45 (n = 8) | 0.394 |
| Tobacco Packs/year | 9 (16.4) | 11.5 (17.7) | 6.37 (14.6) | 0.105 |
| Total cholesterol mg/dL | 173 (30.9) | 170 (31.3) | 176 (30.4) | 0.291 |
| c-LDL mg/dL | 90.2 (24) | 86.7 (23.8) | 93.9 (24.8) | 0.132 |
| c-HDL mg/dL | 65.8 (19.2) | 65.7 (19.7) | 65.9 (18.8) | 0.962 |
| Triglycerides | 82.6 (43.2) | 85.4 (40.3) | 79.5 (46.3) | 0.483 |
| Glomerular filtrate ml/min | 92.3 (17) | 86.3 (17.4) | 98.6 (14.1) | <0.001 |
| Lipoprotein a | 30.9 (29.7) | 33.2 (33) | 28.4 (25.8) | 0.411 |
| CRP | 2.26 (3.33) | 2.79 (4.11) | 1.68 (2.10) | 0.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sojo, L.; Santos-González, E.; Riera, L.; Aguilera, A.; Barahona, R.; Pellicer, P.; Buxó, M.; Mayneris-Perxachs, J.; Fernandez-Balsells, M.; Fernández-Real, J.-M. Plasma Lipidomics Profiles Highlight the Associations of the Dual Antioxidant/Pro-oxidant Molecules Sphingomyelin and Phosphatidylcholine with Subclinical Atherosclerosis in Patients with Type 1 Diabetes. Antioxidants 2023, 12, 1132. https://doi.org/10.3390/antiox12051132
Sojo L, Santos-González E, Riera L, Aguilera A, Barahona R, Pellicer P, Buxó M, Mayneris-Perxachs J, Fernandez-Balsells M, Fernández-Real J-M. Plasma Lipidomics Profiles Highlight the Associations of the Dual Antioxidant/Pro-oxidant Molecules Sphingomyelin and Phosphatidylcholine with Subclinical Atherosclerosis in Patients with Type 1 Diabetes. Antioxidants. 2023; 12(5):1132. https://doi.org/10.3390/antiox12051132
Chicago/Turabian StyleSojo, Lidia, Elena Santos-González, Lídia Riera, Alex Aguilera, Rebeca Barahona, Paula Pellicer, Maria Buxó, Jordi Mayneris-Perxachs, Mercè Fernandez-Balsells, and José-Manuel Fernández-Real. 2023. "Plasma Lipidomics Profiles Highlight the Associations of the Dual Antioxidant/Pro-oxidant Molecules Sphingomyelin and Phosphatidylcholine with Subclinical Atherosclerosis in Patients with Type 1 Diabetes" Antioxidants 12, no. 5: 1132. https://doi.org/10.3390/antiox12051132





