Online Home-Based Physical Activity Counteracts Changes of Redox-Status Biomarkers and Fitness Profiles during Treatment Programs in Postsurgery Female Breast Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Randomization and Blinding Procedures
2.3. Exercise Training Program
2.4. Blood Sampling and Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.5. RNA Extraction and RT-qPCR
2.6. Multiplex Cytokine Assay
2.7. Total Antioxidant Capacity
2.8. Total Glutathione
2.9. Total Plasma Free Thiol
2.10. Thiobarbituric Acid-Reactive Substances (TBARs)
2.11. SOD Activity
2.12. CAT Activity
2.13. Physical Activity Level
2.14. Functional Capacity Evaluation and Patient-Reported Outcomes
2.15. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Subjects
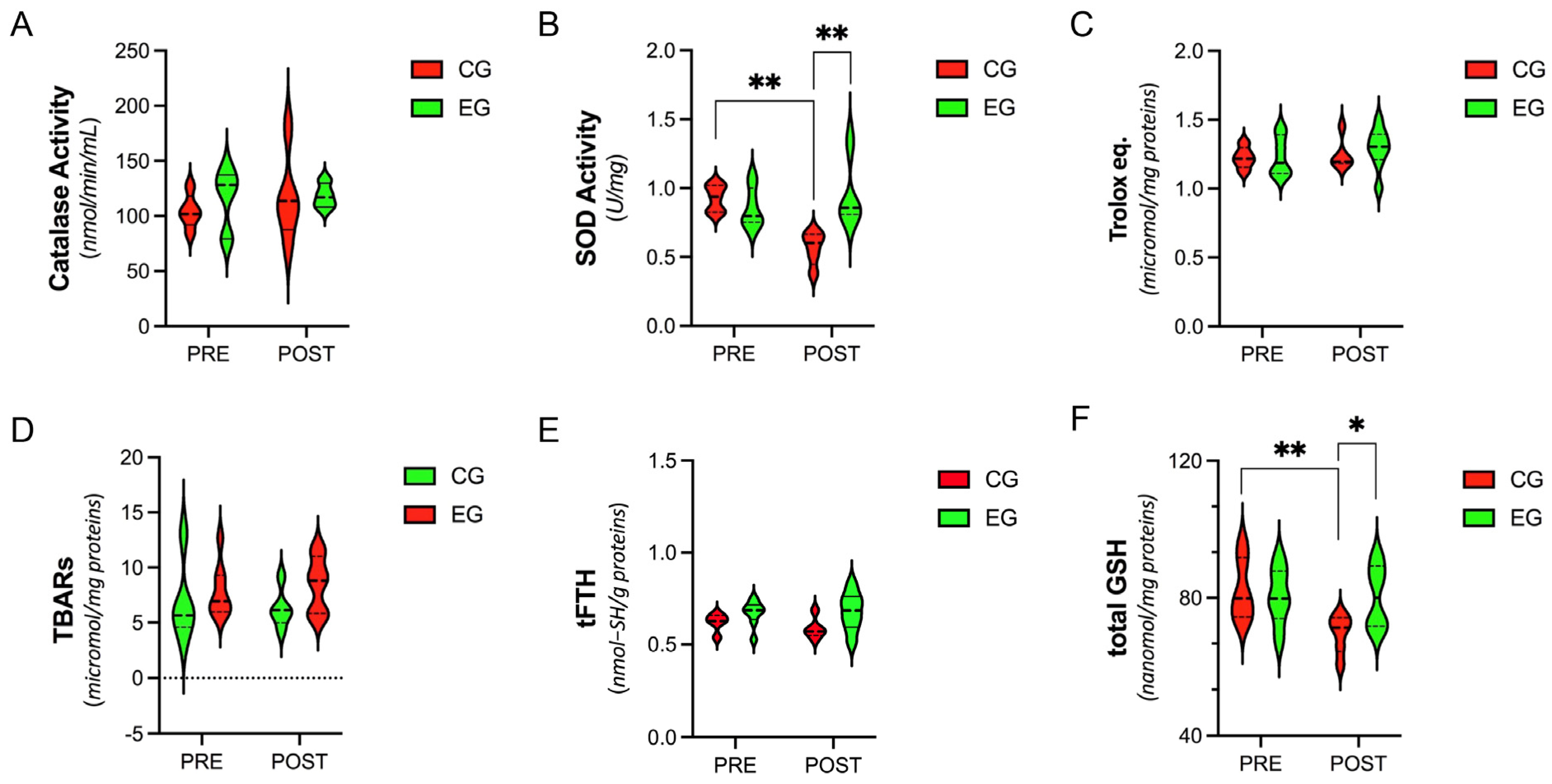
3.2. Systemic Level of Antioxidants and Oxidative-Stress Parameters
3.3. Plasma Levels of Cytokines
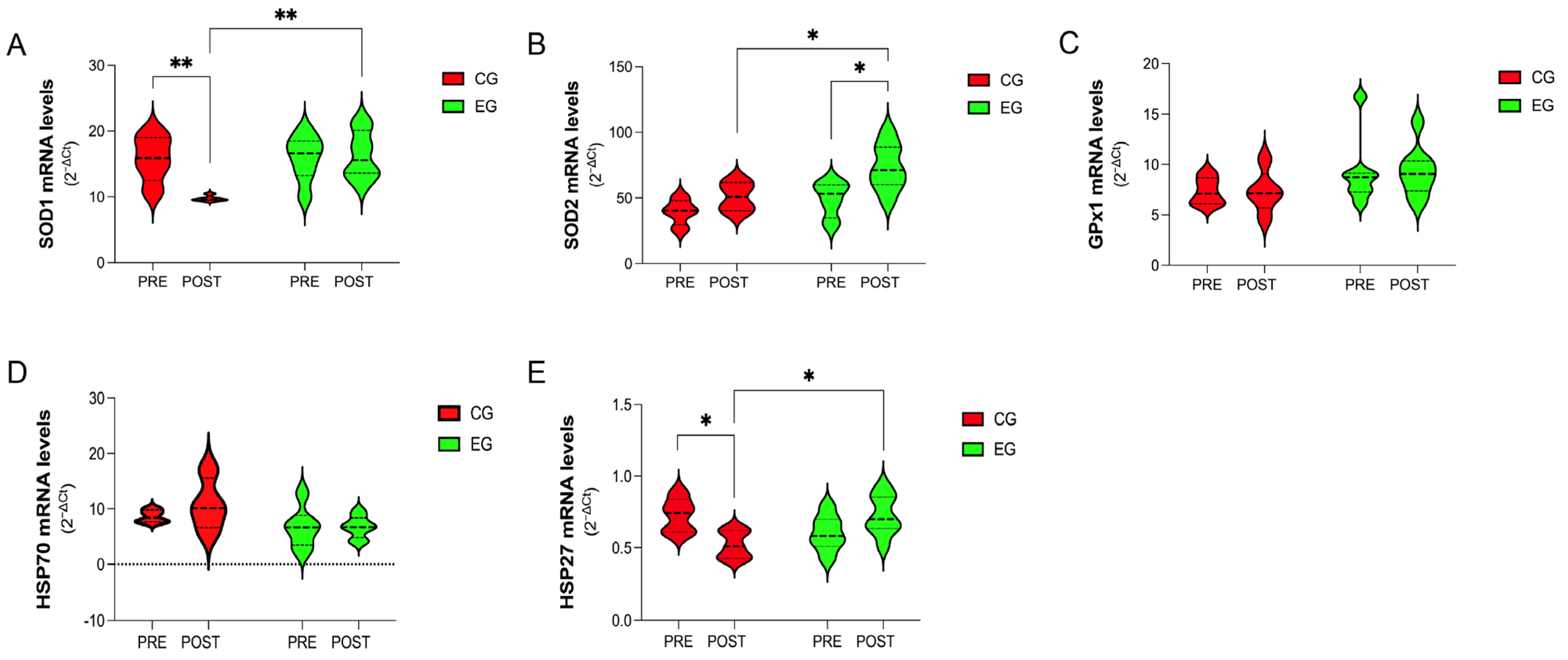
3.4. Gene-Expression Analysis of Antioxidants and Stress-Response Proteins in PBMCs
3.5. Functional Parameters
3.6. Body Composition
3.7. Health-Related Quality of Life and Fatigue Perception
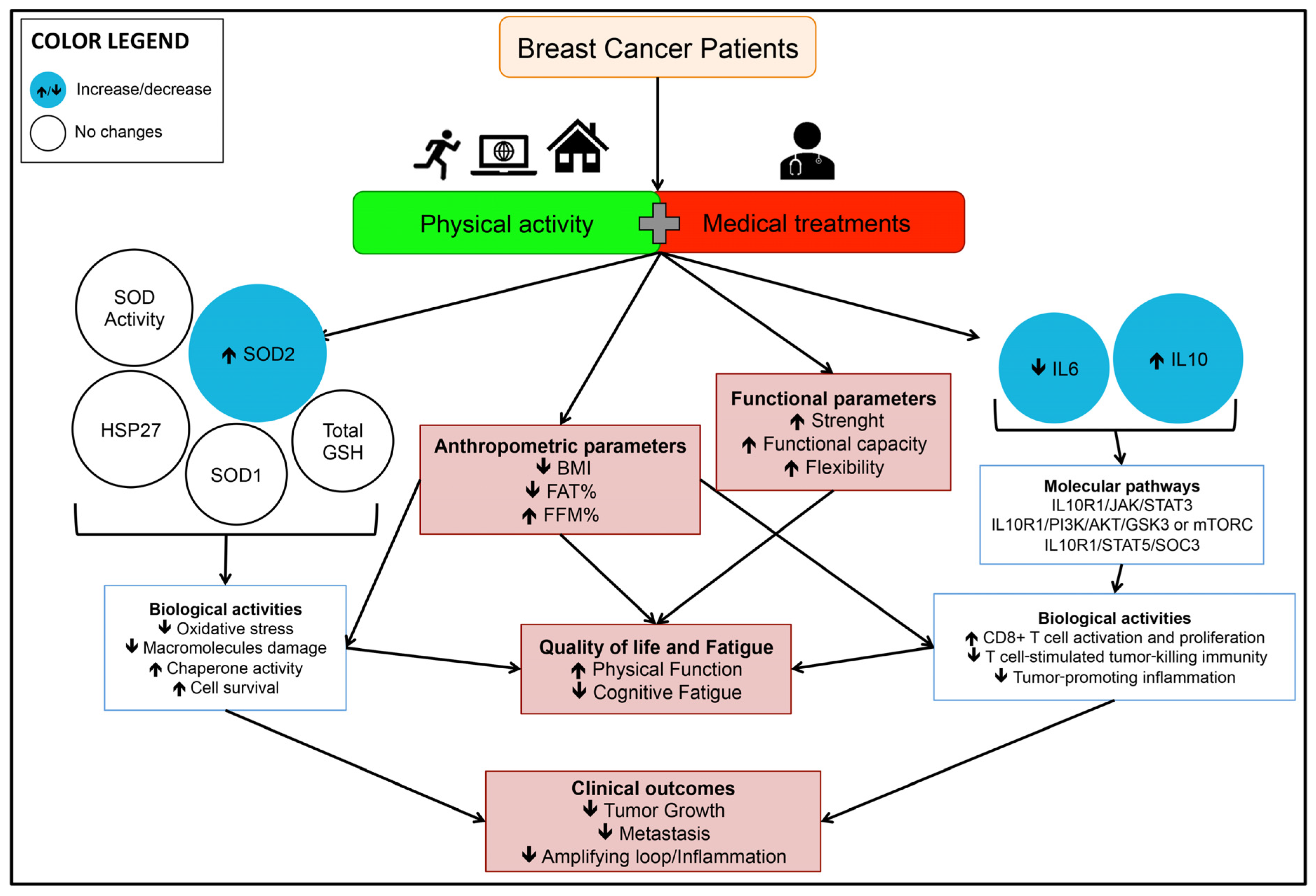
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of Oxidative Stress in the Development and Progression of Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J. Overweight, Obesity, Oxidative Stress and the Risk of Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9579–9586. [Google Scholar] [CrossRef]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent Oxidative Stress in Cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Sangeetha, P. Increase in Free Radical Generation and Lipid Peroxidation Following Chemotherapy in Patients with Cancer. Free Radic. Biol. Med. 1990, 8, 15–19. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Eustress: On Constant Alert for Redox Homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Behrend, L.; Henderson, G.; Zwacka, R.M. Reactive Oxygen Species in Oncogenic Transformation. Biochem. Soc. Trans. 2003, 31, 1441–1444. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer. Cochrane Database Syst. Rev. 2016, 9, CD005001. [Google Scholar] [CrossRef]
- Casla, S.; Hojman, P.; Márquez-Rodas, I.; López-Tarruella, S.; Jerez, Y.; Barakat, R.; Martín, M. Running Away from Side Effects: Physical Exercise as a Complementary Intervention for Breast Cancer Patients. Clin. Transl. Oncol. 2015, 17, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle Dysfunction in Cancer Patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Tranchita, E.; Murri, A.; Grazioli, E.; Cerulli, C.; Emerenziani, G.P.; Ceci, R.; Caporossi, D.; Dimauro, I.; Parisi, A. The Beneficial Role of Physical Exercise on Anthracyclines Induced Cardiotoxicity in Breast Cancer Patients. Cancers 2022, 14, 2288. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Peterson, B.; McBride, C.; Lipkus, I.; Clipp, E. Current Health Behaviors and Readiness to Pursue Life-Style Changes among Men and Women Diagnosed with Early Stage Prostate and Breast Carcinomas. Cancer 2000, 88, 674–684. [Google Scholar] [CrossRef]
- Armfield, N.R.; Bradford, M.; Bradford, N.K. The Clinical Use of Skype—For Which Patients, with Which Problems and in Which Settings? A Snapshot Review of the Literature. Int. J. Med. Inf. 2015, 84, 737–742. [Google Scholar] [CrossRef]
- Vasankari, V.; Halonen, J.; Husu, P.; Vähä-Ypyä, H.; Tokola, K.; Suni, J.; Sievänen, H.; Anttila, V.; Airaksinen, J.; Vasankari, T.; et al. Personalised EHealth Intervention to Increase Physical Activity and Reduce Sedentary Behaviour in Rehabilitation after Cardiac Operations: Study Protocol for the PACO Randomised Controlled Trial (NCT03470246). BMJ Open Sport Exerc. Med. 2019, 5, e000539. [Google Scholar] [CrossRef]
- Schinköthe, T. Individualized EHealth Support for Oncological Therapy Management. Breast Care 2019, 14, 130–134. [Google Scholar] [CrossRef]
- Bertucci, F.; Le Corroller-Soriano, A.-G.; Monneur-Miramon, A.; Moulin, J.-F.; Fluzin, S.; Maraninchi, D.; Gonçalves, A. Outpatient Cancer Care Delivery in the Context of E-Oncology: A French Perspective on “Cancer Outside the Hospital Walls”. Cancers 2019, 11, 219. [Google Scholar] [CrossRef]
- Grazioli, E.; Cerulli, C.; Dimauro, I.; Moretti, E.; Murri, A.; Parisi, A. New Strategy of Home-Based Exercise during Pandemic COVID-19 in Breast Cancer Patients: A Case Study. Sustainability 2020, 12, 6940. [Google Scholar] [CrossRef]
- Sagarra-Romero, L.; Butragueño, J.; Gomez-Bruton, A.; Lozano-Berges, G.; Vicente-Rodríguez, G.; Morales, J.S. Effects of an Online Home-Based Exercise Intervention on Breast Cancer Survivors during COVID-19 Lockdown: A Feasibility Study. Support. Care Cancer 2022, 30, 6287–6297. [Google Scholar] [CrossRef]
- Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Ariza-García, A.; Díaz-Rodríguez, L.; Del-Moral-Ávila, R.; Arroyo-Morales, M. Telehealth System: A Randomized Controlled Trial Evaluating the Impact of an Internet-Based Exercise Intervention on Quality of Life, Pain, Muscle Strength, and Fatigue in Breast Cancer Survivors: Telehealth System in Breast Cancer. Cancer 2016, 122, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Cornette, T.; Vincent, F.; Mandigout, S.; Antonini, M.T.; Leobon, S.; Labrunie, A.; Venat, L.; Lavau-Denes, S.; Tubiana-Mathieu, N. Effects of Home-Based Exercise Training on VO2 in Breast Cancer Patients under Adjuvant or Neoadjuvant Chemotherapy (SAPA): A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 223–232. [Google Scholar] [PubMed]
- Spector, D.; Deal, A.M.; Amos, K.D.; Yang, H.; Battaglini, C.L. A Pilot Study of a Home-Based Motivational Exercise Program for African American Breast Cancer Survivors: Clinical and Quality-of-Life Outcomes. Integr. Cancer Ther. 2014, 13, 121–132. [Google Scholar] [CrossRef]
- Pinto, B.M.; Frierson, G.M.; Rabin, C.; Trunzo, J.J.; Marcus, B.H. Home-Based Physical Activity Intervention for Breast Cancer Patients. J. Clin. Oncol. 2005, 23, 3577–3587. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Dimauro, I.; Grazioli, E.; Palombo, R.; Guidotti, F.; Fantini, C.; Sgrò, P.; De Francesco, D.; Di Luigi, L.; Capranica, L.; et al. Exercise-Mediated Downregulation of MALAT1 Expression and Implications in Primary and Secondary Cancer Prevention. Free Radic. Biol. Med. 2020, 160, 28–39. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Beltran Valls, M.R.; Duranti, G.; Dimauro, I.; Quaranta, F.; Pittaluga, M.; Sabatini, S.; Caserotti, P.; Parisi, P.; Parisi, A.; et al. Oxidative Stress Responses to a Graded Maximal Exercise Test in Older Adults Following Explosive-Type Resistance Training. Redox Biol. 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Giuliani, S.; Rossi, M.N.; Dimauro, I.; Sabatini, S.; Mariottini, P.; Cervelli, M. The Impact of Spermidine on C2C12 Myoblasts Proliferation, Redox Status and Polyamines Metabolism under H2O2 Exposure. Int. J. Mol. Sci. 2022, 23, 10986. [Google Scholar] [CrossRef]
- Ceci, R.; Maldini, M.; Olson, M.E.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Duranti, G. Moringa Oleifera Leaf Extract Protects C2C12 Myotubes against H2O2-Induced Oxidative Stress. Antioxidants 2022, 11, 1435. [Google Scholar] [CrossRef]
- Colamartino, M.; Duranti, G.; Ceci, R.; Sabatini, S.; Testa, A.; Cozzi, R. A Multi-Biomarker Analysis of the Antioxidant Efficacy of Parkinson’s Disease Therapy. Toxicol. Vitr. 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Di Filippo, E.S.; Bondi, D.; Verratti, V.; Doria, C.; Caporossi, D.; Sabatini, S.; Dimauro, I.; Pietrangelo, T. Endurance Training Improves Plasma Superoxide Dismutase Activity in Healthy Elderly. Mech. Ageing Dev. 2020, 185, 111190. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.D.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.; Tomaszewski, K.A.; Hammerlid, E.; Ignacio Arraras, J.; Conroy, T.; Lanceley, A.; Schmidt, H.; Wirtz, M.; Singer, S.; Pinto, M.; et al. International Psychometric Validation of an EORTC Quality of Life Module Measuring Cancer Related Fatigue (EORTC QLQ-FA12). JNCI J. Natl. Cancer Inst. 2017, 109, djw273. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.; Chiavarina, B.; Pavlides, S.; Wang, C.; Whitaker-Menezes, D.; Daumer, K.; Lin, Z.; Witkiewicz, A.; et al. Oxidative Stress in Cancer Associated Fibroblasts Drives Tumor-Stroma Co-Evolution: A New Paradigm for Understanding Tumor Metabolism, the Field Effect and Genomic Instability in Cancer Cells. Cell Cycle 2010, 9, 3276–3296. [Google Scholar] [CrossRef]
- Feng, J.-F.; Lu, L.; Zeng, P.; Yang, Y.-H.; Luo, J.; Yang, Y.-W.; Wang, D. Serum Total Oxidant/Antioxidant Status and Trace Element Levels in Breast Cancer Patients. Int. J. Clin. Oncol. 2012, 17, 575–583. [Google Scholar] [CrossRef]
- Danesh, H.; Ziamajidi, N.; Mesbah-Namin, S.A.; Nafisi, N.; Abbasalipourkabir, R. Association between Oxidative Stress Parameters and Hematological Indices in Breast Cancer Patients. Int. J. Breast Cancer 2022, 2022, 1459410. [Google Scholar] [CrossRef]
- Seth, L.R.K.; Kharb, S.; Kharb, D.P. Serum Biochemical Markers in Carcinoma Breast. Indian J. Med. Sci. 2003, 57, 350–354. [Google Scholar]
- Rajneesh, C.P.; Manimaran, A.; Sasikala, K.R.; Adaikappan, P. Lipid Peroxidation and Antioxidant Status in Patients with Breast Cancer. Singapore Med. J. 2008, 49, 640–643. [Google Scholar]
- Punnonen, K.; Ahotupa, M.; Asaishi, K.; Hyöty, M.; Kudo, R.; Punnonen, R. Antioxidant Enzyme Activities and Oxidative Stress in Human Breast Cancer. J. Cancer Res. Clin. Oncol. 1994, 120, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Šimůnek, T.; Štěrba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Geršl, V. Anthracycline-Induced Cardiotoxicity: Overview of Studies Examining the Roles of Oxidative Stress and Free Cellular Iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Workman, P. Hsp90 Molecular Chaperone Inhibitors: Are We There Yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Lai, W.-L.; Wong, N.-S. ROS Mediates 4HPR-Induced Posttranscriptional Expression of the Gadd153 Gene. Free Radic. Biol. Med. 2005, 38, 1585–1593. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Su, Y. Peroxiredoxins, a Novel Target in Cancer Radiotherapy. Cancer Lett. 2009, 286, 154–160. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Dürken, M.; Herrnring, C.; Finckh, B.; Nagel, S.; Nielsen, P.; Fischer, R.; Berger, H.M.; Moison, R.M.W.; Pichlmeier, U.; Kohlschütter, B.; et al. Impaired Plasma Antioxidative Defense and Increased Nontransferrin-Bound Iron during High-Dose Chemotherapy and Radiochemotherapy Preceding Bone Marrow Transplantation. Free Radic. Biol. Med. 2000, 28, 887–894. [Google Scholar] [CrossRef]
- Crohns, M.; Liippo, K.; Erhola, M.; Kankaanranta, H.; Moilanen, E.; Alho, H.; Kellokumpu-Lehtinen, P. Concurrent Decline of Several Antioxidants and Markers of Oxidative Stress during Combination Chemotherapy for Small Cell Lung Cancer. Clin. Biochem. 2009, 42, 1236–1245. [Google Scholar] [CrossRef]
- Singh, R.J. Glutathione: A Marker and Antioxidant for Aging. J. Lab. Clin. Med. 2002, 140, 380–381. [Google Scholar] [CrossRef]
- Sen, P.; Kemppainen, E.; Orešič, M. Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front. Mol. Biosci. 2018, 4, 96. [Google Scholar] [CrossRef]
- Dimauro, I.; Grazioli, E.; Lisi, V.; Guidotti, F.; Fantini, C.; Antinozzi, C.; Sgrò, P.; Antonioni, A.; Di Luigi, L.; Capranica, L.; et al. Systemic Response of Antioxidants, Heat Shock Proteins, and Inflammatory Biomarkers to Short-Lasting Exercise Training in Healthy Male Subjects. Oxid. Med. Cell. Longev. 2021, 2021, 1938492. [Google Scholar] [CrossRef]
- Abaspour, A.R.; Taghikhani, M.; Parizadeh, S.M.R.; Seyedi, S.M.R.; Ghazizadeh, H.; Kazemi, E.; Moohebati, M.; Ghafoori, F.; Mardannik, M.; Avan, A.; et al. HSP27 Expression in the Human Peripheral Blood Mononuclear Cells as an Early Prognostic Biomarker in Coronary Artery Disease Patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Verhaar, M.C.; Koomans, H.A.; Joles, J.A.; Braam, B. Role of Circulating Karyocytes in the Initiation and Progression of Atherosclerosis. Hypertension 2006, 47, 803–810. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Bibiloni, M.; Martorell, M.; Ferrer, M.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.; Pons, A.; et al. Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Roshan, V.D. Change in Adiponectin and Oxidative Stress after Modifiable Lifestyle Interventions in Breast Cancer Cases. Asian Pac. J. Cancer Prev. 2013, 14, 2845–2850. [Google Scholar] [CrossRef]
- Leaf, D.A.; Kleinman, M.T.; Hamilton, M.; Deitrick, R.W. The Exercise-Induced Oxidative Stress Paradox: The Effects of Physical Exercise Training. Am. J. Med. Sci. 1999, 317, 295. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Strazzanti, A.; Gangi, S.; Di Giacomo, C.; Basile, F.; Renis, M. Effects of Physical Activity on Systemic Oxidative/DNA Status in Breast Cancer Survivors. Oncol. Lett. 2017, 13, 441–448. [Google Scholar] [CrossRef]
- Hojan, K.; Gerreth, K.; Procyk, D.; Mania, K.; Zalewska, A.; Maciejczyk, M. Redox Status Response of Physical Exercise Training in Women with Breast Cancer during Trastuzumab Therapy. Healthcare 2022, 10, 2039. [Google Scholar] [CrossRef]
- Delrieu, L.; Touillaud, M.; Pérol, O.; Morelle, M.; Martin, A.; Friedenreich, C.M.; Mury, P.; Dufresne, A.; Bachelot, T.; Heudel, P.-E.; et al. Impact of Physical Activity on Oxidative Stress Markers in Patients with Metastatic Breast Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 6694594. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, L.; Martin, A.; Touillaud, M.; Pérol, O.; Morelle, M.; Febvey-Combes, O.; Freyssenet, D.; Friedenreich, C.; Dufresne, A.; Bachelot, T.; et al. Sarcopenia and Serum Biomarkers of Oxidative Stress after a 6-Month Physical Activity Intervention in Women with Metastatic Breast Cancer: Results from the ABLE Feasibility Trial. Breast Cancer Res. Treat. 2021, 188, 601–613. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative Stress in Biological Systems and Its Relation with Pathophysiological Functions: The Effect of Physical Activity on Cellular Redox Homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.H.; Caiozzo, V.J.; Zaldivar, F.; Nemet, D.; Larson, J.; Hung, S.; Heck, J.D.; Hatfield, G.W.; Cooper, D.M. Effects of Exercise on Gene Expression in Human Peripheral Blood Mononuclear Cells. J. Appl. Physiol. 2004, 97, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Dimauro, I.; Sgrò, P.; Emerenziani, G.P.; Magi, F.; Baldari, C.; Guidetti, L.; Di Luigi, L.; Parisi, P.; Caporossi, D. Acute Exercise Modulates BDNF and Pro-BDNF Protein Content in Immune Cells. Med. Sci. Sports Exerc. 2012, 44, 1871–1880. [Google Scholar] [CrossRef]
- Beltran Valls, M.R.; Dimauro, I.; Brunelli, A.; Tranchita, E.; Ciminelli, E.; Caserotti, P.; Duranti, G.; Sabatini, S.; Parisi, P.; Parisi, A.; et al. Explosive Type of Moderate-Resistance Training Induces Functional, Cardiovascular, and Molecular Adaptations in the Elderly. AGE 2014, 36, 759–772. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Castelao, J.E.; Pike, M.C.; Sevanian, A.; Haile, R.W. Role of Lipid Peroxidation in the Epidemiology and Prevention of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Victorino, V.J.; Herrera, A.C.S.A.; Freitas, L.F.; De Rossi, T.; Campos, F.C.; Simão, A.N.C.; Barbosa, D.S.; Pinge-Filho, P.; Cecchini, R.; et al. Differential Oxidative Status and Immune Characterization of the Early and Advanced Stages of Human Breast Cancer. Breast Cancer Res. Treat. 2012, 133, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Silva, L.C.; Fernandes, J.R.; Benard, G. Preventing or Reversing Immunosenescence: Can Exercise Be an Immunotherapy? Immunotherapy 2013, 5, 879–893. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr. Rev. 2008, 65, S140–S146. [Google Scholar] [CrossRef]
- Nigro, E.; Polito, R.; Elce, A.; Signoriello, G.; Iacotucci, P.; Carnovale, V.; Gelzo, M.; Zarrilli, F.; Castaldo, G.; Daniele, A. Physical Activity Regulates TNFα and IL-6 Expression to Counteract Inflammation in Cystic Fibrosis Patients. Int. J. Environ. Res. Public. Health 2021, 18, 4691. [Google Scholar] [CrossRef] [PubMed]
- Noz, M.P.; Hartman, Y.A.W.; Hopman, M.T.E.; Willems, P.H.G.M.; Tack, C.J.; Joosten, L.A.B.; Netea, M.G.; Thijssen, D.H.J.; Riksen, N.P. Sixteen-Week Physical Activity Intervention in Subjects With Increased Cardiometabolic Risk Shifts Innate Immune Function Towards a Less Proinflammatory State. J. Am. Heart Assoc. 2019, 8, e013764. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G.; et al. Response of Oxidative Stress and Inflammatory Biomarkers to a 12-Week Aerobic Exercise Training in Women with Metabolic Syndrome. Sports Med.-Open 2015, 1, 19. [Google Scholar] [CrossRef]
- Masjedi, A.; Hashemi, V.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M.; Jadidi-Niaragh, F. The Significant Role of Interleukin-6 and Its Signaling Pathway in the Immunopathogenesis and Treatment of Breast Cancer. Biomed. Pharmacother. 2018, 108, 1415–1424. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Højfeldt, G.; Hojman, P. The Role of Intratumoral and Systemic IL-6 in Breast Cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, O.; Yoshiuchi, K.; Inagaki, M.; Matsuoka, Y.; Yoshikawa, E.; Sugawara, Y.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; et al. Association between Adjuvant Regional Radiotherapy and Cognitive Function in Breast Cancer Patients Treated with Conservation Therapy. Cancer Med. 2014, 3, 702–709. [Google Scholar] [CrossRef]
- Starkweather, A.R.; Lyon, D.E.; Schubert, C.M. Pain and Inflammation in Women With Early-Stage Breast Cancer Prior to Induction of Chemotherapy. Biol. Res. Nurs. 2013, 15, 234–241. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Wu, X.; Spitz, M.; Kurzrock, R.; Fisch, M.; Bruera, E.; Shete, S. Molecular Epidemiology, Cancer-Related Symptoms, and Cytokines Pathway. Lancet Oncol. 2008, 9, 777–785. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 Signaling Pathway in Targeted Therapy for Cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Wolk, K.; Kunz, S.; Asadullah, K.; Sabat, R. Cutting Edge: Immune Cells as Sources and Targets of the IL-10 Family Members? J. Immunol. 2002, 168, 5397–5402. [Google Scholar] [CrossRef] [PubMed]
- Macatonia, S.E.; Doherty, T.M.; Knight, S.C.; O’Garra, A. Differential Effect of IL-10 on Dendritic Cell-Induced T Cell Proliferation and IFN-Gamma Production. J. Immunol. 1993, 150, 3755–3765. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-M.; Lam, H.P.; Hsu, H.-J.; Jiang, S.-J. Interleukin-10: A Double-Edged Sword in Breast Cancer. Tzu Chi Med. J. 2021, 33, 203. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Beaty, T.L.; Jackson, M.J.; Fulton, A.M. Antimetastatic and Antitumor Activities of Interleukin 10 in a Murine Model of Breast Cancer. JNCI J. Natl. Cancer Inst. 1996, 88, 536–541. [Google Scholar] [CrossRef]
- Dorsey, R.; Kundu, N.; Yang, Q.; Tannenbaum, C.S.; Sun, H.; Hamilton, T.A.; Fulton, A.M. Immunotherapy with Interleukin-10 Depends on the CXC Chemokines Inducible Protein-10 and Monokine Induced by IFN-Gamma. Cancer Res. 2002, 62, 2606–2610. [Google Scholar]
- Kozłowski, L.; Zakrzewska, I.; Tokajuk, P.; Wojtukiewicz, M.Z. Concentration of Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Interleukin-10 (IL-10) in Blood Serum of Breast Cancer Patients. Rocz. Akad. Med. Bialymstoku 2003, 48, 82–84. [Google Scholar]
- van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. High Prevalence of Pain in Patients with Cancer in a Large Population-Based Study in The Netherlands. Pain 2007, 132, 312–320. [Google Scholar] [CrossRef]
- Schwartz, A.L. Exercise and Weight Gain in Breast Cancer Patients Receiving Chemotherapy. Cancer Pract. 2000, 8, 231–237. [Google Scholar] [CrossRef]
- Kendall, A.R.; Mahue-Giangreco, M.; Carpenter, C.L.; Ganz, P.A.; Bernstein, L. Influence of Exercise Activity on Quality of Life in Long-Term Breast Cancer Survivors. Qual. Life Res. 2005, 14, 361–371. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Mori, M.; Gao, R.; Nail, L.M.; King, M.E. Exercise Reduces Daily Fatigue in Women with Breast Cancer Receiving Chemotherapy. Med. Sci. Sports Exerc. 2001, 33, 718–723. [Google Scholar] [CrossRef]
- Segal, R.; Evans, W.; Johnson, D.; Smith, J.; Colletta, S.; Gayton, J.; Woodard, S.; Wells, G.; Reid, R. Structured Exercise Improves Physical Functioning in Women With Stages I and II Breast Cancer: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2001, 19, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Murri, A.; Vitucci, D.; Tranchita, E.; Grazioli, E.; Gori, S.; Modena, A.; Turazza, M.; Filippini, R.; Galeazzi, S.; Verzè, M.; et al. “OPERATION PHALCO”—Adapted Physical Activity for Breast Cancer Survivors: Is It Time for a Multidisciplinary Approach? Cancers 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Fasano, G.A.; Bayard, S.; Johnson, J.; Gordon, A.; Harris, M.; Taiwo, E.; Yeung, J.; Zenilman, M.; Newman, L.; Bea, V.J. Breast Cancer and Obesity: A Qualitative Analysis of a Diverse Population of Breast Cancer Patients’ Perspectives on Weight Management. J. Racial Ethn. Health Disparities, 2023; ahead of print. [Google Scholar] [CrossRef]

| EG (n = 10) | CG (n = 10) | p-Value * | |
|---|---|---|---|
| Age (Years) | 50.55 ± 5.69 | 45.15 ± 5.54 | 0.874 |
| Weight (Kg) | 60.30 ± 5.55 | 55.30 ± 1.05 | 0.404 |
| Height (m) | 161.68 ± 8.61 | 157.51 ± 3.55 | 0.349 |
| BMI (Kg/m2) | 23.10 ± 2.53 | 21.38 ± 0.50 | 0.248 |
| Type of Intervention | |||
| Quadrantectomy | 7 | 6 | N.A. |
| Mastectomy | 3 | 4 | N.A. |
| Treatments | |||
| Chemo + Hormonal + Radio | 2 | 2 | N.A. |
| Hormonal + Radio | 4 | 5 | N.A. |
| Hormonal | 4 | 3 | N.A. |
| Physical Activity Level | |||
| IPAQ (MET-min/week) | 898.3 ± 988.5 | 885.6 ± 666.0 | 0.695 |
| PRE (Mean ± SD) | POST (Mean ± SD) | % Change | p-Value * | |
|---|---|---|---|---|
| 6MWT (m) | ||||
| EG | 564.42 ± 50.78 | 601.1 ± 52.11 | +6.50 | 0.007 |
| CG | 530.41 ± 48.78 | 480.1 ± 32.09 | −9.43 | 0.044 |
| Borg (0–10) | ||||
| EG | 2.75 ± 0.43 | 1.12 ± 0.95 | −59.18 | 0.003 |
| CG | 1.19 ± 0.28 | 1.74 ± 0.50 | +42.00 | 0.172 |
| HGR (Kg) | ||||
| EG | 29.91 ± 3.75 | 28.90 ± 4.62 | −3.49 | 0.422 |
| CG | 29.90 ± 3.69 | 28.90 ± 4.64 | −3.87 | 0.138 |
| HGL (Kg) | ||||
| EG | 27.25 ± 3.14 | 26.04 ± 4.04 | −4.46 | 0.425 |
| CG | 23.14 ± 4.07 | 20.10 ± 3.19 | −13.50 | 0.002 |
| 30′ STS (n) | ||||
| EG | 18.04 ± 4.20 | 22.17 ± 5.51 | +22.84 | 0.060 |
| CG | 19.04 ± 3.18 | 18.13 ± 5.52 | −5.21 | 0.355 |
| Sit-and-Reach (cm) | ||||
| EG | 1.77 ± 8.61 | 6.24 ± 8.75 | +250.00 | 0.004 |
| CG | 1.71 ± 8.64 | 2.87 ± 3.72 | +57.53 | 0.821 |
| Scratch R (cm) | ||||
| EG | 24.81 ± 10.59 | 18.80 ± 6.73 | −24.12 | 0.009 |
| CG | 23.2 ± 10 | 14.1 ± 6.8 | −4.32 | 0.731 |
| Scratch L (cm) | ||||
| EG | 27.50 ± 7.11 | 22.31 ± 7.41 | −18.81 | 0.005 |
| CG | 25.4 ± 6.1 | 26.3 ± 8.4 | −4.00 | 0.869 |
| Tandem (s) | ||||
| EG | 10 ± 0 | 10 ± 0 | 0 | N.A. |
| CG | 10 ± 0 | 10 ± 0 | 0 | N.A. |
| PRE (Mean ± SD) | POST (Mean ± SD) | % Change | p-Value * | |
|---|---|---|---|---|
| Weight (kg) | ||||
| EG | 60.30 ± 5.55 | 59.72 ± 6.81 | −0.95 | 0.560 |
| CG | 55.30 ± 1.90 | 58.54 ± 4.87 | +4.51 | 0.214 |
| BMI (Kg/m2) | ||||
| EG | 23.10 ± 2.53 | 22.71 ± 2.85 | −1.35 | 0.214 |
| CG | 21.38 ± 0.50 | 22.74 ± 2.84 | +6.55 | 0.045 |
| FFM (%) | ||||
| EG | 72.57 ± 5.25 | 74.60 ± 6.50 | +2.80 | 0.049 |
| CG | 60.67 ± 5.32 | 60.61 ± 3.5 | −0.67 | 0.243 |
| FAT (%) | ||||
| EG | 27.43 ± 5.25 | 25.53 ± 6.65 | −6.93 | 0.043 |
| CG | 30.18 ± 4.05 | 34.04 ± 4.43 | +12.71 | 0.032 |
| TBW (%) | ||||
| EG | 54.14 ± 5.63 | 56.23 ± 7.21 | +3.86 | 0.072 |
| CG | 41.14 ± 5.31 | 40.22 ± 3.20 | −2.11 | 0.560 |
| BCM (%) | ||||
| EG | 37.95 ± 3.74 | 38.21 ± 4.05 | +0.85 | 0.328 |
| CG | 36.12 ± 2.17 | 32.41 ± 2.12 | +3.86 | 0.124 |
| PRE (Mean ± SD) | POST (Mean ± SD) | % Change | p-Value * | |
|---|---|---|---|---|
| EORTC QLQ C-30 | ||||
| Physical Function | ||||
| EG | 88.12 ± 8.01 | 93.32 ± 4.71 | +5.78 | 0.042 |
| CG | 90.47 ± 3.55 | 91.52 ± 4.52 | +1.06 | 0.345 |
| Emotional Function | ||||
| EG | 76.85 ± 11.62 | 87.03 ± 22.19 | +13.23 | 0.242 |
| CG | 90.43 ± 8.92 | 89.91 ± 8.92 | −0.69 | 0.438 |
| Cognitive Function | ||||
| EG | 87.03 ± 16.19 | 88.81 ± 14.43 | +2.12 | 0.782 |
| CG | 90.47 ± 13.11 | 88.30 ± 11.30 | −2.70 | 0.367 |
| Social Function | ||||
| EG | 81.48 ± 19.44 | 92.59 ± 14.69 | +13.65 | 0.169 |
| CG | 88.02 ± 15.85 | 85.70 ± 14.99 | −2.68 | 0.388 |
| Global Health | ||||
| EG | 62.96 ± 9.42 | 69.54 ± 15.02 | +10.24 | 0.300 |
| CG | 72.99 ± 11.40 | 66.64 ± 22.22 | −10.71 | 0.285 |
| FA-12 | ||||
| Physical Fatigue | ||||
| EG | 18.51 ± 16.25 | 14.81 ± 14.44 | −20.01 | 0.532 |
| CG | 12.38 ± 8.92 | 13.32 ± 8.60 | +7.15 | 0.843 |
| Emotional Fatigue | ||||
| EG | 12.39 ± 11.75 | 8.6 ± 22.05 | −30.32 | 0.707 |
| CG | 7.94 ± 13.92 | 9.5 ± 13.41 | +16.57 | 0.832 |
| Cognitive Fatigue | ||||
| EG | 18.51 ± 21.15 | 7.40 ± 14.59 | −60.00 | 0.044 |
| CG | 4.71 ± 12.54 | 7.94 ± 13.92 | +40.00 | 0.662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulton, C.; Grazioli, E.; Antinozzi, C.; Fantini, C.; Cerulli, C.; Murri, A.; Duranti, G.; Ceci, R.; Vulpiani, M.C.; Pellegrini, P.; et al. Online Home-Based Physical Activity Counteracts Changes of Redox-Status Biomarkers and Fitness Profiles during Treatment Programs in Postsurgery Female Breast Cancer Patients. Antioxidants 2023, 12, 1138. https://doi.org/10.3390/antiox12051138
Moulton C, Grazioli E, Antinozzi C, Fantini C, Cerulli C, Murri A, Duranti G, Ceci R, Vulpiani MC, Pellegrini P, et al. Online Home-Based Physical Activity Counteracts Changes of Redox-Status Biomarkers and Fitness Profiles during Treatment Programs in Postsurgery Female Breast Cancer Patients. Antioxidants. 2023; 12(5):1138. https://doi.org/10.3390/antiox12051138
Chicago/Turabian StyleMoulton, Chantalle, Elisa Grazioli, Cristina Antinozzi, Cristina Fantini, Claudia Cerulli, Arianna Murri, Guglielmo Duranti, Roberta Ceci, Maria Chiara Vulpiani, Patrizia Pellegrini, and et al. 2023. "Online Home-Based Physical Activity Counteracts Changes of Redox-Status Biomarkers and Fitness Profiles during Treatment Programs in Postsurgery Female Breast Cancer Patients" Antioxidants 12, no. 5: 1138. https://doi.org/10.3390/antiox12051138







