Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Induction of Diabetes Mellitus
2.4. Intrathecal Catheterization and Osmotic Pump Infusion
2.5. Behavior Test for Tactile Allodynia
2.6. Spinal Cord Sample Preparation for Western Blotting Analysis
2.7. Quantitative Real-Time PCR
2.8. Immunofluorescence Studies
2.9. Statistical Analysis
3. Results
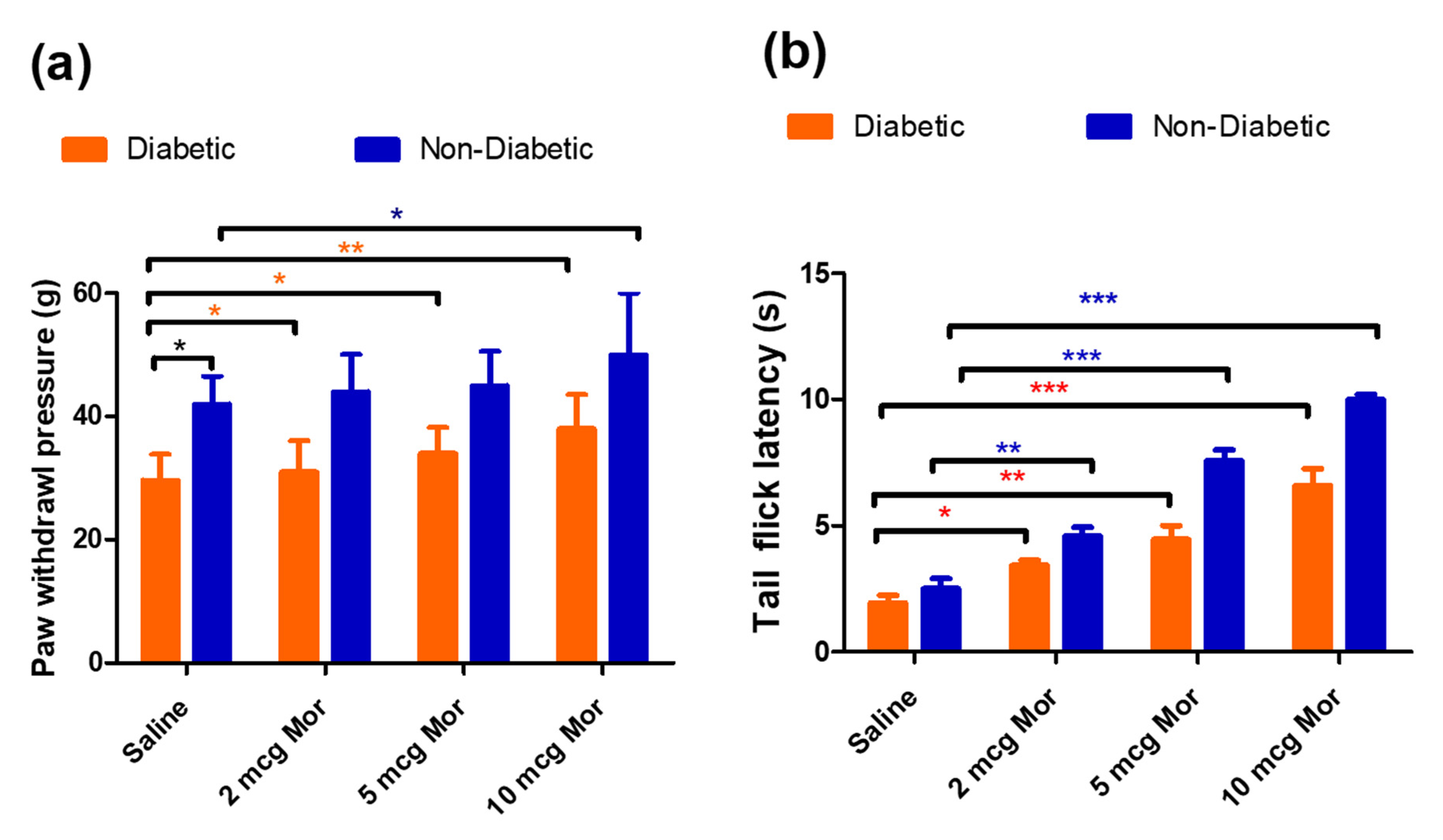
3.1. Body Weight, Blood Glucose Levels, and Paw Withdrawal Sensitivity in Diabetic Rats
3.2. The Antiallodynic and Anti-Hyperalgesic Effects of MOR in Diabetic and Non-Diabetic Rats
3.3. Effects of Spinal Infusion of MOR, TEN, and Their Combination on Tactile Allodynia and MAT
3.4. Effects of Spinal Infusion of MOR, TEN, and Their Combination on Blood Glucose Levels
3.5. Effects of Spinal Infusion of MOR, TEN, and Their Combination on Body Weight in Diabetic Rats
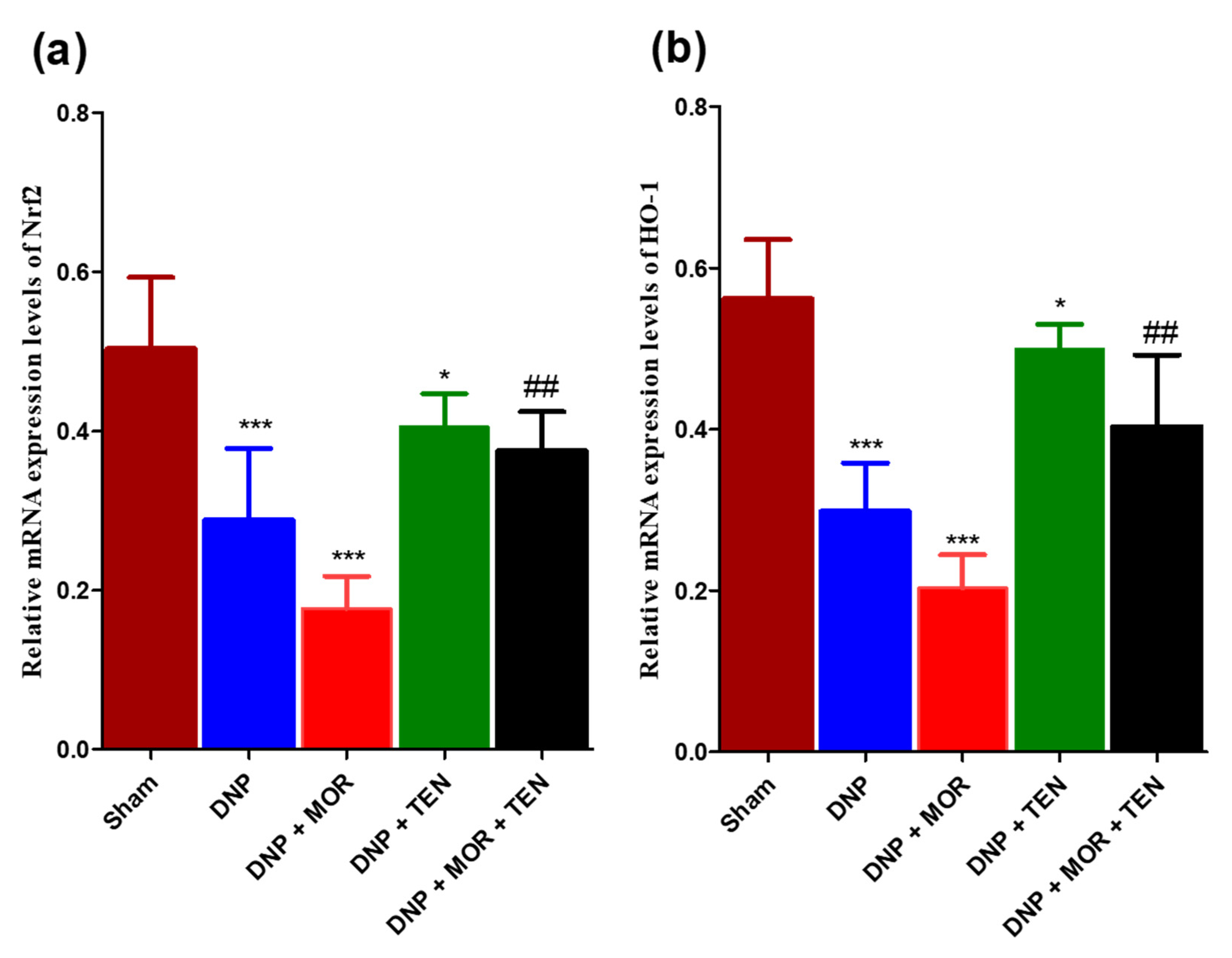
3.6. Teneligliptin Suppressed MOR-Induced Nrf-2 Inflammasome Activation and HO-1 Activation in the Dorsal Horn of Diabetic Rats
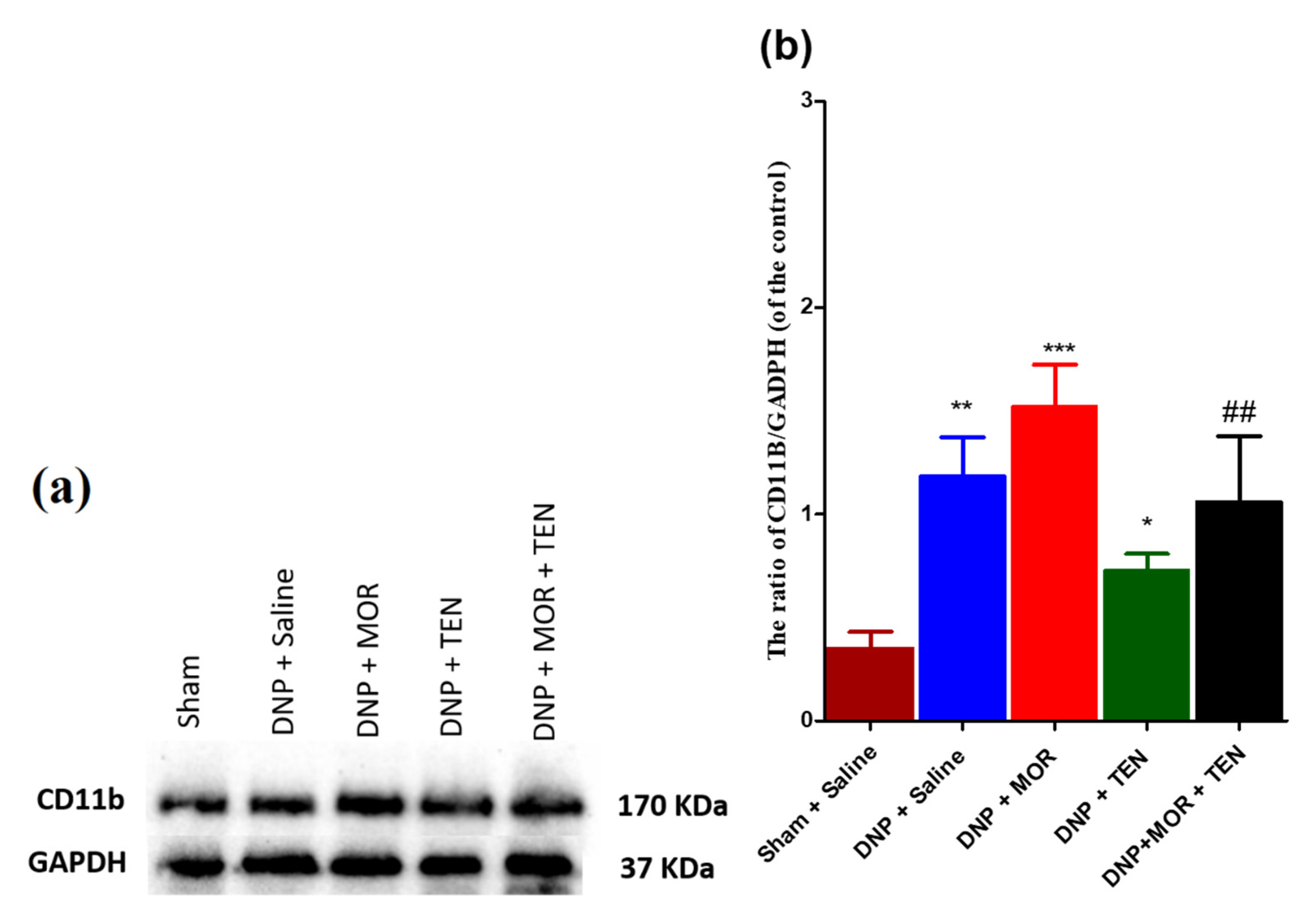
3.7. Teneligliptin Suppressed MOR-Induced CD-11b Expression in the Dorsal Horn of Diabetic Rats
3.8. TEN Inhibits Microglial Activation in the Dorsal Horn of DNP and MOR-Infused DNP Rats
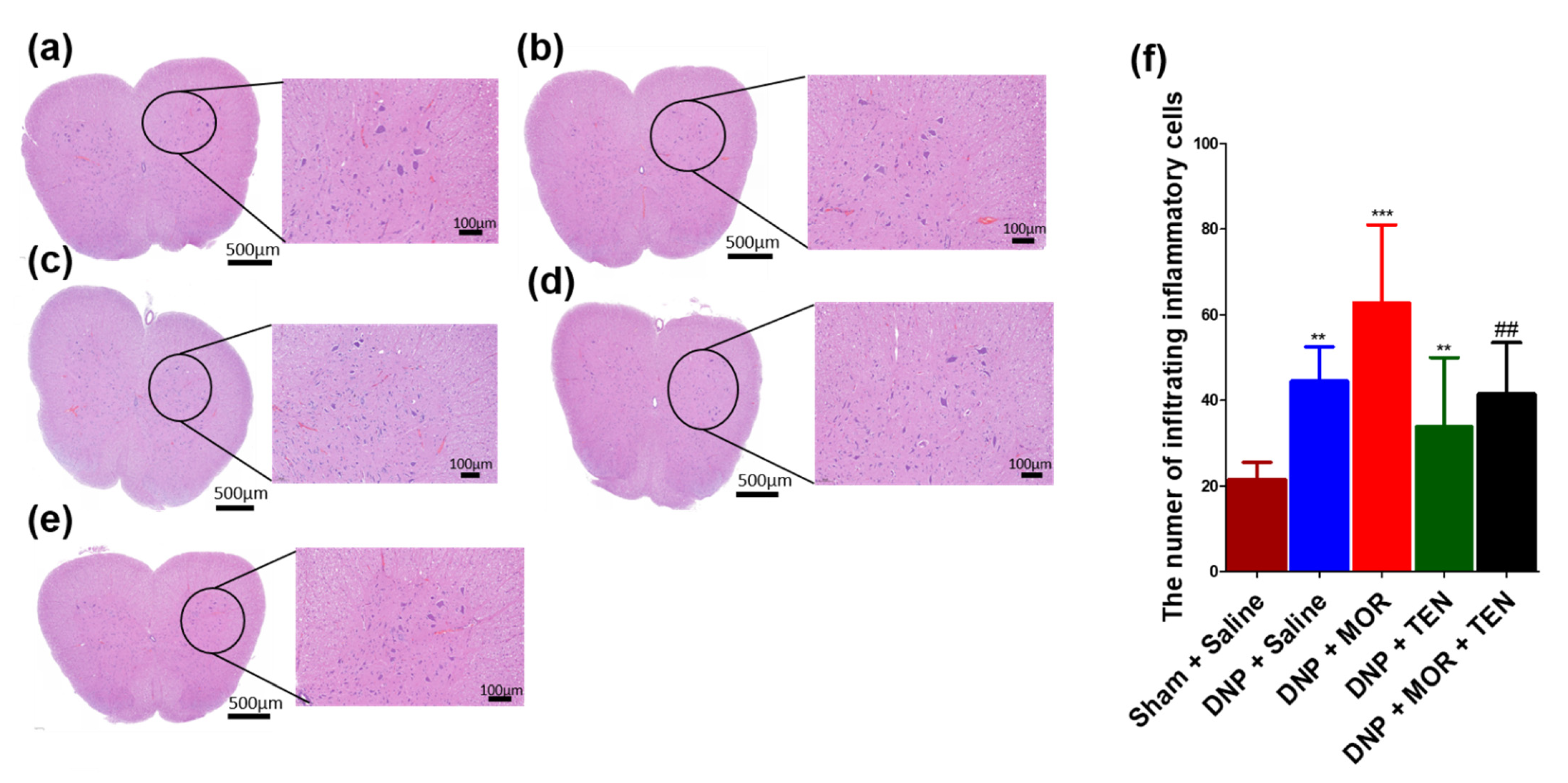
3.9. TEN Inhibits Mononucleate Cell Infiltration in the Dorsal Horn of DNP and MOR-Infused DNP Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef]
- Fischer, T.Z.; Waxman, S.G. Neuropathic pain in diabetes-evidence for a central mechanism. Nat. Rev. Neurol. 2010, 6, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Wang, L.; Kamaleldin, M.; Craigie, S.; Riva, J.J.; Montoya, L.; Mulla, S.M.; Lopes, L.C.; Vogel, N.; Chen, E.; et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 2018, 320, 2448–2460. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.R.; Wolfe, J.; Said, Q.; Thomas, J.; Martin, B.C. Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population. Clin. J. Pain 2015, 31, 414–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.R.; Tan, P.H.; Cheng, J.K.; Liu, Y.C.; Ji, R.R. Microglia: A promising target for treating neuropathic and postoperative pain, and morphine tolerance. J. Formos Med. Assoc. 2011, 110, 487–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, M.R.; Shavit, Y.; Grace, P.M.; Rice, K.C.; Maier, S.F.; Watkins, L.R. Exploring the neuroimmunopharmacology of opioids: An integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol. Rev. 2011, 63, 772–810. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Q.; Zhou, Y.Q.; Gao, F. Targeting Cytokines for Morphine Tolerance: A Narrative Review. Curr. Neuropharmacol. 2019, 17, 366–376. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, X.; Jiang, L.; Hu, L.; Kong, H.; Han, Y.; Qian, C.; Song, C.; Qian, Y.; Liu, W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflamm. 2016, 13, 294. [Google Scholar] [CrossRef] [Green Version]
- Mika, J.; Wawrzczak-Bargiela, A.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav. Immun. 2009, 23, 75–84. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, C.; Tang, J.; Wang, C.; Wu, P.; Zhang, G.; Liu, W.; Jamangulova, N.; Wu, X.; Song, X. Resveratrol reduces morphine tolerance by inhibiting microglial activation via AMPK signalling. Eur. J. Pain 2014, 18, 1458–1470. [Google Scholar] [CrossRef]
- Sheikholeslami, M.A.; Parvardeh, S.; Ghafghazi, S.; Moini Zanjani, T.; Sabetkasaei, M. The Attenuating Effect of Curcumin on Morphine Dependence in Rats: The Involvement of Spinal Microglial Cells and Inflammatory Cytokines. Iran. J. Pharm. Res. 2019, 18, 198–207. [Google Scholar] [CrossRef]
- Skrabalova, J.; Drastichova, Z.; Novotny, J. Morphine as a Potential Oxidative Stress-Causing Agent. Mini Rev. Org. Chem. 2013, 10, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.S.; Geng, W.S.; Wang, Z.Q.; Jia, J.J. Morphine Addiction and Oxidative Stress: The Potential Effects of Thioredoxin-1. Front. Pharmacol. 2020, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Alboghobeish, S.; Amirgholami, N.; Houshmand, G.; Cauli, O. Venlafaxine prevents morphine antinociceptive tolerance: The role of neuroinflammation and the l-arginine-nitric oxide pathway. Exp. Neurol. 2018, 303, 134–141. [Google Scholar] [CrossRef]
- Hamdy, M.M.; Elbadr, M.M.; Barakat, A. Fluoxetine uses in nociceptive pain management: A promising adjuvant to opioid analgesics. Fundam. Clin. Pharmacol. 2018, 32, 532–546. [Google Scholar] [CrossRef]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, F.; Szymusiak, M.; Liu, Y.; Wang, Z.J. Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II α activity. J. Pharmacol. Exp. Ther. 2015, 352, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, J.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Xu, A.; Xu, G.; Ng, C.F.; Yao, X.; Gao, Y.; et al. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid. Redox Signal. 2014, 21, 1571–1581. [Google Scholar] [CrossRef] [Green Version]
- Civantos, E.; Bosch, E.; Ramirez, E.; Zhenyukh, O.; Egido, J.; Lorenzo, O.; Mas, S. Sitagliptin ameliorates oxidative stress in experimental diabetic nephropathy by diminishing the miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab. Syndr. Obes. 2017, 10, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, R.M.; Safar, M.M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015, 133, 700–707. [Google Scholar] [CrossRef]
- Ideta, T.; Shirakami, Y.; Miyazaki, T.; Kochi, T.; Sakai, H.; Moriwaki, H.; Shimizu, M. The Dipeptidyl Peptidase-4 Inhibitor Teneligliptin Attenuates Hepatic Lipogenesis via AMPK Activation in Non-Alcoholic Fatty Liver Disease Model Mice. Int. J. Mol. Sci. 2015, 16, 29207–29218. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sharma, R.; Hatware, K.; Patil, K. Review on Chemistry, Analysis and Pharmacology of Teneligliptin: A Novel DPP-4 Inhibitor. Mini Rev. Med. Chem. 2020, 20, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Akahoshi, F.; Sakashita, H.; Kitajima, H.; Nakamura, M.; Sonda, S.; Takeuchi, M.; Tanaka, Y.; Ueda, N.; Sekiguchi, S.; et al. Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): A highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2012, 20, 5705–5719. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Haneda, M.; Ito, H.; Sasaki, K.; Matsukawa, M.; Yamada, Y. Long-Term, Real-World Safety and Efficacy of Teneligliptin: A Post-Marketing Surveillance of More Than 10,000 Patients with Type 2 Diabetes in Japan. Adv. Ther. 2020, 37, 1065–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butul, M.; Pingali, U.; Nutalapati, C. Evaluation of the analgesic activity of single and multiple oral doses of teneligliptin (20 mg/day), using hot air analgesiometer in healthy human volunteers: A randomized, double blind, placebo controlled, cross over study. Int. J. Basic Clin. Pharmacol. 2020, 9, 729. [Google Scholar] [CrossRef]
- Nakagami, H.; Pang, Z.; Shimosato, T.; Moritani, T.; Kurinami, H.; Koriyama, H.; Tenma, A.; Shimamura, M.; Morishita, R. The dipeptidyl peptidase-4 inhibitor teneligliptin improved endothelial dysfunction and insulin resistance in the SHR/NDmcr-cp rat model of metabolic syndrome. Hypertens. Res. 2014, 37, 629–635. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, X.; Yang, C.; Li, Y. Teneligliptin protects against hypoxia/reoxygenation-induced endothelial cell injury. Biomed. Pharmacother. 2019, 109, 468–474. [Google Scholar] [CrossRef]
- Kimura, S.; Inoguchi, T.; Yamasaki, T.; Yamato, M.; Ide, M.; Sonoda, N.; Yamada, K.; Takayanagi, R. A novel DPP-4 inhibitor teneligliptin scavenges hydroxyl radicals: In vitro study evaluated by electron spin resonance spectroscopy and in vivo study using DPP-4 deficient rats. Metabolism 2016, 65, 138–145. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vascul. Pharmacol. 2017, 96–98, 19–25. [Google Scholar] [CrossRef]
- Peng, W.; Rao, D.; Zhang, M.; Shi, Y.; Wu, J.; Nie, G.; Xia, Q. Teneligliptin prevents doxorubicin-induced inflammation and apoptosis in H9c2 cells. Arch. Biochem. Biophys. 2020, 683, 108238. [Google Scholar] [CrossRef]
- De Nigris, V.; Prattichizzo, F.; Iijima, H.; Ceriello, A. DPP-4 Inhibitors Have Different Effects on Endothelial Low-Grade Inflammation and on the M1-M2 Macrophage Polarization Under Hyperglycemic Conditions. Diabetes Metab. Syndr. Obes. 2021, 14, 1519–1531. [Google Scholar] [CrossRef]
- Morishita, R.; Nakagami, H. Teneligliptin: Expectations for its pleiotropic action. Expert Opin. Pharmacother. 2015, 16, 417–426. [Google Scholar] [CrossRef]
- Király, K.; Kozsurek, M.; Lukácsi, E.; Barta, B.; Alpár, A.; Balázsa, T.; Fekete, C.; Szabon, J.; Helyes, Z.; Bölcskei, K.; et al. Glial cell type-specific changes in spinal dipeptidyl peptidase 4 expression and effects of its inhibitors in inflammatory and neuropatic pain. Sci. Rep. 2018, 8, 3490. [Google Scholar] [CrossRef] [Green Version]
- Kuthati, Y.; Rao, V.N.; Busa, P.; Wong, C.-S. Teneligliptin Exerts Antinociceptive Effects in Rat Model of Partial Sciatic Nerve Transection Induced Neuropathic Pain. Antioxidants 2021, 10, 1438. [Google Scholar] [CrossRef]
- Lv, J.; Li, Z.; She, S.; Xu, L.; Ying, Y. Effects of intrathecal injection of rapamycin on pain threshold and spinal cord glial activation in rats with neuropathic pain. Neurol. Res. 2015, 37, 739–743. [Google Scholar] [CrossRef]
- Kuthati, Y.; Goutham Davuluri, V.N.; Yang, C.P.; Chang, H.C.; Chang, C.P.; Wong, C.S. Melatonin MT2 receptor agonist IIK-7 produces antinociception by modulation of ROS and suppression of spinal microglial activation in neuropathic pain rats. J. Pain Res. 2019, 12, 2473–2485. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.R.; Sweigart, K.L.; Lakoski, J.M.; Pan, H.L. Functional mu opioid receptors are reduced in the spinal cord dorsal horn of diabetic rats. Anesthesiology 2002, 97, 1602–1608. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, L.; Shi, T.J.; Ju, G.; Elde, R.; Hökfelt, T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience 1998, 82, 223–240. [Google Scholar] [CrossRef]
- Chen, S.R.; Pan, H.L. Antinociceptive effect of morphine, but not mu opioid receptor number, is attenuated in the spinal cord of diabetic rats. Anesthesiology 2003, 99, 1409–1414. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Li, Y.-Q. The Downregulation of Opioid Receptors and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 5981. [Google Scholar] [CrossRef]
- Kou, Z.Z.; Wan, F.P.; Bai, Y.; Li, C.Y.; Hu, J.C.; Zhang, G.T.; Zhang, T.; Chen, T.; Wang, Y.Y.; Li, H.; et al. Decreased Endomorphin-2 and μ-Opioid Receptor in the Spinal Cord Are Associated with Painful Diabetic Neuropathy. Front. Mol. Neurosci. 2016, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.S.; Morley, J.E.; Wilcox, G.; Brown, D.M.; Handwerger, B.S. Tail pinch behavior and analgesia in diabetic mice. Physiol. Behav. 1982, 28, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.S.; Dewey, W.L. Narcotics and diabetes. I. The effects of streptozotocin-induced diabetes on the antinociceptive potency of morphine. J. Pharmacol. Exp. Ther. 1981, 218, 318–323. [Google Scholar] [PubMed]
- Raz, I.; Hasdai, D.; Seltzer, Z.; Melmed, R.N. Effect of hyperglycemia on pain perception and on efficacy of morphine analgesia in rats. Diabetes 1988, 37, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, S.; Gurumoorthy, K.; Kaul, C.L.; Ramarao, P. Role of L-type Ca2+ channels in attenuated morphine antinociception in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2002, 435, 187–194. [Google Scholar] [CrossRef]
- Courteix, C.; Bourget, P.; Caussade, F.; Bardin, M.; Coudore, F.; Fialip, J.; Eschalier, A. Is the reduced efficacy of morphine in diabetic rats caused by alterations of opiate receptors or of morphine pharmacokinetics? J. Pharmacol. Exp. Ther. 1998, 285, 63–70. [Google Scholar]
- Ayoub, B.M.; Mowaka, S.; Safar, M.M.; Ashoush, N.; Arafa, M.G.; Michel, H.E.; Tadros, M.M.; Elmazar, M.M.; Mousa, S.A. Repositioning of Omarigliptin as a once-weekly intranasal Anti-parkinsonian Agent. Sci. Rep. 2018, 8, 8959. [Google Scholar] [CrossRef] [Green Version]
- Kosaraju, J.; Holsinger, R.M.D.; Guo, L.; Tam, K.Y. Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6074–6084. [Google Scholar] [CrossRef]
- Kosaraju, J.; Gali, C.C.; Khatwal, R.B.; Dubala, A.; Chinni, S.; Holsinger, R.M.; Madhunapantula, V.S.; Muthureddy Nataraj, S.K.; Basavan, D. Saxagliptin: A dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology 2013, 72, 291–300. [Google Scholar] [CrossRef]
- Kosaraju, J.; Murthy, V.; Khatwal, R.B.; Dubala, A.; Chinni, S.; Muthureddy Nataraj, S.K.; Basavan, D. Vildagliptin: An anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J. Pharm. Pharmacol. 2013, 65, 1773–1784. [Google Scholar] [CrossRef]
- Kosaraju, J.; Madhunapantula, S.V.; Chinni, S.; Khatwal, R.B.; Dubala, A.; Muthureddy Nataraj, S.K.; Basavan, D. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav. Brain Res. 2014, 267, 55–65. [Google Scholar] [CrossRef]
- Listos, J.; Listos, P.; Baranowska-Bosiacka, I.; Karpiuk, A.; Filarowska, J.; Łupina, M.; Słowik, T.; Zawiślak, S.; Kotlińska, J. Linagliptin, a Selective Dipeptidyl Peptidase-4 Inhibitor, Reduces Physical and Behavioral Effects of Morphine Withdrawal. Molecules 2022, 27, 2478. [Google Scholar] [CrossRef]
- Łupina, M.; Talarek, S.; Kotlińska, J.; Gibuła-Tarłowska, E.; Listos, P.; Listos, J. The role of linagliptin, a selective dipeptidyl peptidase-4 inhibitor, in the morphine rewarding effects in rats. Neurochem. Int. 2020, 133, 104616. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Fu, M.; Dong, R.; Yang, Y.; Luo, J.; Hu, S.; Li, W.; Xu, X.; Tu, L. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem. Pharmacol. 2020, 177, 113951. [Google Scholar] [CrossRef]
- Guo, K.; Jin, F. Dipeptidyl Peptidase-4 (DPP-4) Inhibitor Saxagliptin Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Regulating the Nrf-2/HO-1 and NF-κB Pathways. J. Investig. Surg. 2021, 34, 695–702. [Google Scholar] [CrossRef]
- Abdel-Gaber, S.A.; Geddawy, A.; Moussa, R.A. The hepatoprotective effect of sitagliptin against hepatic ischemia reperfusion-induced injury in rats involves Nrf-2/HO-1 pathway. Pharmacol. Rep. 2019, 71, 1044–1049. [Google Scholar] [CrossRef]
- Si, J.; Meng, R.; Gao, P.; Hui, F.; Li, Y.; Liu, X.; Yang, B. Linagliptin protects rat carotid artery from balloon injury and activates the NRF2 antioxidant pathway. Exp. Anim. 2019, 68, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Pujadas, G.; De Nigris, V.; Prattichizzo, F.; La Sala, L.; Testa, R.; Ceriello, A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine 2017, 56, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leánez, S.; Pol, O. Treatment with Sulforaphane Produces Antinociception and Improves Morphine Effects during Inflammatory Pain in Mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Castany, S.; Carcolé, M.; Leánez, S.; Pol, O. The Induction of Heme Oxygenase 1 Decreases Painful Diabetic Neuropathy and Enhances the Antinociceptive Effects of Morphine in Diabetic Mice. PLoS ONE 2016, 11, e0146427. [Google Scholar] [CrossRef] [Green Version]
- Gault, V.A.; Lennox, R.; Flatt, P.R. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves recognition memory, oxidative stress and hippocampal neurogenesis and upregulates key genes involved in cognitive decline. Diabetes Obes. Metab. 2015, 17, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lietzau, G.; Davidsson, W.; Östenson, C.G.; Chiazza, F.; Nathanson, D.; Pintana, H.; Skogsberg, J.; Klein, T.; Nyström, T.; Darsalia, V.; et al. Type 2 diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor Linagliptin. Acta Neuropathol. Commun. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Röhnert, P.; Schmidt, W.; Emmerlich, P.; Goihl, A.; Wrenger, S.; Bank, U.; Nordhoff, K.; Täger, M.; Ansorge, S.; Reinhold, D.; et al. Dipeptidyl peptidase IV, aminopeptidase N and DPIV/APN-like proteases in cerebral ischemia. J. Neuroinflamm. 2012, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariyar, R.; Bastola, T.; Lee, D.H.; Seo, J. Neuroprotective Effects of the DPP4 Inhibitor Vildagliptin in In Vivo and In Vitro Models of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 2388. [Google Scholar] [CrossRef] [PubMed]
- Pahan, P.; Xie, J.Y. Microglial inflammation modulates opioid analgesic tolerance. J. Neurosci. Res. 2023. [Google Scholar] [CrossRef]
- Rajchgot, T.; Thomas, S.C.; Wang, J.C.; Ahmadi, M.; Balood, M.; Crosson, T.; Dias, J.P.; Couture, R.; Claing, A.; Talbot, S. Neurons and Microglia; A Sickly-Sweet Duo in Diabetic Pain Neuropathy. Front. Neurosci. 2019, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Tu, H.; Chu, H.; Guan, S.; Hao, F.; Xu, N.; Zhao, Z.; Liang, Y. The role of the M1/M2 microglia in the process from cancer pain to morphine tolerance. Tissue Cell 2021, 68, 101438. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Cai, Y.; Kong, H.; Pan, Y.-B.; Jiang, L.; Pan, X.-X.; Hu, L.; Qian, Y.-N.; Jiang, C.-Y.; Liu, W.-T. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J. Neuroinflamm. 2016, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Gao, L.; Zhang, N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419866379. [Google Scholar] [CrossRef] [Green Version]
- Deleo, J.A.; Tanga, F.Y.; Tawfik, V.L. Neuroimmune Activation and Neuroinflammation in Chronic Pain and Opioid Tolerance/Hyperalgesia. Neuroscientist 2004, 10, 40–52. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Niemi, J.P.; Howarth, M.A.; Liu, K.W.; Moore, C.Z.; Mahajan, D.; Zigmond, R.E. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J. Neuroinflamm. 2018, 15, 192. [Google Scholar] [CrossRef]
- Newton, V.L.; Guck, J.D.; Cotter, M.A.; Cameron, N.E.; Gardiner, N.J. Neutrophils Infiltrate the Spinal Cord Parenchyma of Rats with Experimental Diabetic Neuropathy. J. Diabetes Res. 2017, 2017, 4729284. [Google Scholar] [CrossRef] [Green Version]
- Terminel, M.N.; Bassil, C.; Rau, J.; Trevino, A.; Ruiz, C.; Alaniz, R.; Hook, M.A. Morphine-induced changes in the function of microglia and macrophages after acute spinal cord injury. BMC Neurosci. 2022, 23, 58. [Google Scholar] [CrossRef]
- Trivedi, A.; Olivas, A.D.; Noble-Haeusslein, L.J. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clin. Neurosci. Res. 2006, 6, 283–292. [Google Scholar] [CrossRef] [Green Version]





| Gene | Sequence of Primers |
|---|---|
| Nrf-2 | Forward: 5′-TTGGCAGAGACATTCCCATTTGTA-3′ Reverse: 5′-GAGCTATCGAGTGACTGAGCCTGA-3′ |
| HO-1 | Forward: 5′-AGGTGCACATCCGTGCAGAG-3′ Reverse: 5′-CTTCCAGGGCCGTATAGATATGGTA-3′ |
| GAPDH | Forward: 5′-GGCACAGTCAAGGCTGAGAATG-3′ Reverse: 5′-ATGGTGGTGAAGACGCCAGTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuthati, Y.; Rao, V.N.; Huang, W.-H.; Busa, P.; Wong, C.-S. Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats. Antioxidants 2023, 12, 1478. https://doi.org/10.3390/antiox12071478
Kuthati Y, Rao VN, Huang W-H, Busa P, Wong C-S. Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats. Antioxidants. 2023; 12(7):1478. https://doi.org/10.3390/antiox12071478
Chicago/Turabian StyleKuthati, Yaswanth, Vaikar Navakanth Rao, Wei-Hsiu Huang, Prabhakar Busa, and Chih-Shung Wong. 2023. "Teneligliptin Co-Infusion Alleviates Morphine Tolerance by Inhibition of Spinal Microglial Cell Activation in Streptozotocin-Induced Diabetic Rats" Antioxidants 12, no. 7: 1478. https://doi.org/10.3390/antiox12071478







