Biomarkers of Oxidative Stress in Systemic Lupus Erythematosus Patients with Active Nephritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Measurements of Thiols, sRAGE and MDA
2.3. Statistical Analysis
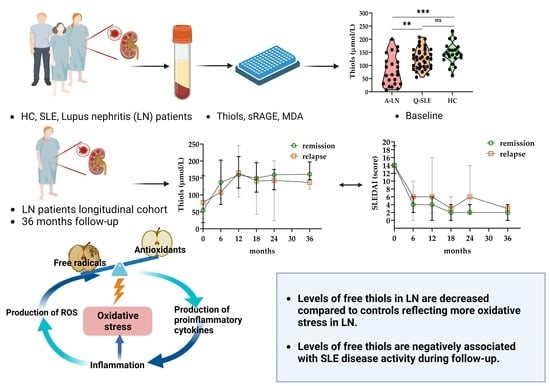
3. Results
3.1. Cohort Demographics and Characteristics
3.2. Levels of Biomarkers and Correlation of Biomarkers in Groups at Baseline
3.3. LN Cohort in Longitudinal Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahajan, A.; Herrmann, M.; Munoz, L.E. Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Front. Immunol. 2016, 7, 35. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef]
- Sherer, Y.; Gorstein, A.; Fritzler, M.J.; Shoenfeld, Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Semin. Arthritis Rheum. 2004, 34, 501–537. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Meliambro, K.; Campbell, K.N.; Chung, M. Therapy for Proliferative Lupus Nephritis. Rheum. Dis. Clin. N. Am. 2018, 44, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Fernandez, D.R.; Telarico, T.; Doherty, E.; Francis, L.; Phillips, P.E. T-cell and B-cell signaling biomarkers and treatment targets in lupus. Curr. Opin. Rheumatol. 2009, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Tsokos, G.C. Reactive oxygen species: The Yin and Yang in (auto-)immunity. Autoimmun. Rev. 2021, 20, 102869. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Wang, G.; Pierangeli, S.S.; Papalardo, E.; Ansari, G.A.; Khan, M.F. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: Correlation with disease activity. Arthritis Rheum. 2010, 62, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J. Biomed. Sci. 2014, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Abdulahad, D.A.; Westra, J.; Bijzet, J.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, R71. [Google Scholar] [CrossRef]
- Hardt, U.; Larsson, A.; Gunnarsson, I.; Clancy, R.M.; Petri, M.; Buyon, J.P.; Silverman, G.J.; Svenungsson, E.; Gronwall, C. Autoimmune reactivity to malondialdehyde adducts in systemic lupus erythematosus is associated with disease activity and nephritis. Arthritis Res. Ther. 2018, 20, 36. [Google Scholar] [CrossRef]
- Amara, A.; Constans, J.; Chaugier, C.; Sebban, A.; Dubourg, L.; Peuchant, E.; Pellegrin, J.L.; Leng, B.; Conri, C.; Geffard, M. Autoantibodies to malondialdehyde-modified epitope in connective tissue diseases and vasculitides. Clin. Exp. Immunol. 1995, 101, 233–238. [Google Scholar] [CrossRef]
- Arends, S.; Grootscholten, C.; Derksen, R.H.; Berger, S.P.; de Sevaux, R.G.; Voskuyl, A.E.; Bijl, M.; Berden, J.H.; on behalf of the Dutch Working Party on systemic lupus erythematosus. Long-term follow-up of a randomised controlled trial of azathioprine/methylprednisolone versus cyclophosphamide in patients with proliferative lupus nephritis. Ann. Rheum. Dis. 2012, 71, 966–973. [Google Scholar] [CrossRef]
- Grootscholten, C.; Bajema, I.M.; Florquin, S.; Steenbergen, E.J.; Peutz-Kootstra, C.J.; Goldschmeding, R.; Bijl, M.; Hagen, E.C.; Van Houwelingen, H.C.; Derksen, R.H.; et al. Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum. 2007, 56, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Grootscholten, C.; Ligtenberg, G.; Hagen, E.C.; van den Wall Bake, A.W.; de Glas-Vos, J.W.; Bijl, M.; Assmann, K.J.; Bruijn, J.A.; Weening, J.J.; van Houwelingen, H.C.; et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int. 2006, 70, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.; Berden, J.H.; Grootscholten, C.; Derksen, R.H.; Berger, S.P.; de Sevaux, R.G.; Voskuyl, A.E.; Bijl, M.; on behalf of the Dutch Working Party on SLE. Induction therapy with short-term high-dose intravenous cyclophosphamide followed by mycophenolate mofetil in proliferative lupus nephritis. Neth. J. Med. 2014, 72, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lambers, W.M.; de Leeuw, K.; Doornbos-van der Meer, B.; Diercks, G.F.H.; Bootsma, H.; Westra, J. Interferon score is increased in incomplete systemic lupus erythematosus and correlates with myxovirus-resistance protein A in blood and skin. Arthritis Res. Ther. 2019, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar]
- Twisk, J.W. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur. J. Epidemiol. 2004, 19, 769–776. [Google Scholar] [CrossRef]
- Rasmussen, J.L.; Dunlap, W.P. Dealing with Nonnormal Data: Parametric Analysis of Transformed Data vs Nonparametric Analysis. Educ. Psychol. Meas. 1991, 51, 809–820. [Google Scholar] [CrossRef]
- Hu, C.; Du, Y.; Xu, X.; Li, H.; Duan, Q.; Xie, Z.; Wen, C.; Han, X. Lipidomics Revealed Aberrant Metabolism of Lipids Including FAHFAs in Renal Tissue in the Progression of Lupus Nephritis in a Murine Model. Metabolites 2021, 11, 142. [Google Scholar] [CrossRef]
- Krata, N.; Foroncewicz, B.; Zagozdzon, R.; Moszczuk, B.; Zielenkiewicz, M.; Paczek, L.; Mucha, K. Peroxiredoxins as Markers of Oxidative Stress in IgA Nephropathy, Membranous Nephropathy and Lupus Nephritis. Arch. Immunol. Ther. Exp. 2021, 70, 3. [Google Scholar] [CrossRef]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced level of serum thiols in patients with a diagnosis of active disease. J. Anti Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Jespersen, B.; Birn, H.; Krogstrup, N.V.; Bourgonje, A.R.; Leuvenink, H.G.D.; van Goor, H.; Norregaard, R. Elevated plasma free thiols are associated with early and one-year graft function in renal transplant recipients. PLoS ONE 2021, 16, e0255930. [Google Scholar] [CrossRef]
- Ates, I.; Kaplan, M.; Yuksel, M.; Mese, D.; Alisik, M.; Erel, O.; Yilmaz, N.; Guler, S. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine 2016, 51, 47–51. [Google Scholar] [CrossRef]
- van Dijk, P.R.; Pasch, A.; van Ockenburg-Brunet, S.L.; Waanders, F.; Eman Abdulle, A.; Muis, M.J.; Hillebrands, J.L.; Bilo, H.J.G.; van Goor, H. Thiols as markers of redox status in type 1 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820903641. [Google Scholar] [CrossRef]
- Schoots, M.H.; Bourgonje, M.F.; Bourgonje, A.R.; Prins, J.R.; van Hoorn, E.G.M.; Abdulle, A.E.; Muller Kobold, A.C.; van der Heide, M.; Hillebrands, J.L.; van Goor, H.; et al. Oxidative stress biomarkers in fetal growth restriction with and without preeclampsia. Placenta 2021, 115, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ermurat, S.; Guler Kazanci, E.; Toka, D.I.; Ayar, K.; Eren, F.K.; Neselioglu, S. Evaluation of thiol/disulfide hemostasis and serum Ischemia modified albumin as oxidative stress biomarkers in systemic lupus erythematosus patients: Relationship with major organ involvement and disease activity. Lupus 2022, 31, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, P.; de Souza, G.K.; de Lima, D.S.; Passos, L.F.; Boechat, A.L.; Lima, E.S. Serum thiols as a biomarker of disease activity in lupus nephritis. PLoS ONE 2015, 10, e0119947. [Google Scholar] [CrossRef] [PubMed]
- Oates, J.C.; Mashmoushi, A.K.; Shaftman, S.R.; Gilkeson, G.S. NADPH oxidase and nitric oxide synthase-dependent superoxide production is increased in proliferative lupus nephritis. Lupus 2013, 22, 1361–1370. [Google Scholar] [CrossRef]
- Qing, X.; Pitashny, M.; Thomas, D.B.; Barrat, F.J.; Hogarth, M.P.; Putterman, C. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: Involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol. Lett. 2008, 121, 61–73. [Google Scholar] [CrossRef]
- Watanabe, H.; Son, M. The Immune Tolerance Role of the HMGB1-RAGE Axis. Cells 2021, 10, 564. [Google Scholar] [CrossRef]
- Mohamed, A.K.; Bierhaus, A.; Schiekofer, S.; Tritschler, H.; Ziegler, R.; Nawroth, P.P. The role of oxidative stress and NF-kappaB activation in late diabetic complications. Biofactors 1999, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Georgescu, S.R.; Tampa, M.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Penescu, M.N.; Nicolae, I. Cellular Response against Oxidative Stress, a Novel Insight into Lupus Nephritis Pathogenesis. J. Pers. Med. 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of RAGE and Oxidative Stress in Inflammatory and Infectious Skin Diseases. Antioxidants 2021, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Ma, J.L.; Jiao, Y.L.; Li, J.F.; Wang, L.C.; Yang, Q.R.; You, L.; Cui, B.; Chen, Z.J.; Zhao, Y.R. The plasma level of soluble receptor for advanced glycation end products is decreased in patients with systemic lupus erythematosus. Scand. J. Immunol. 2012, 75, 614–622. [Google Scholar] [CrossRef]
- Nowak, A.; Przywara-Chowaniec, B.; Damasiewicz-Bodzek, A.; Blachut, D.; Nowalany-Kozielska, E.; Tyrpien-Golder, K. Advanced Glycation End-Products (AGEs) and Their Soluble Receptor (sRAGE) in Women Suffering from Systemic Lupus Erythematosus (SLE). Cells 2021, 10, 3523. [Google Scholar] [CrossRef]
- Bobek, D.; Grcevic, D.; Kovacic, N.; Lukic, I.K.; Jelusic, M. The presence of high mobility group box-1 and soluble receptor for advanced glycation end-products in juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatr. Rheumatol. Online J. 2014, 12, 50. [Google Scholar] [CrossRef]
- Shah, D.; Sah, S.; Wanchu, A.; Wu, M.X.; Bhatnagar, A. Altered redox state and apoptosis in the pathogenesis of systemic lupus erythematosus. Immunobiology 2013, 218, 620–627. [Google Scholar] [CrossRef]
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2011, 163, 29–43. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Pettersson, A.; Wichert, S.; Gullstrand, B.; Hansson, M.; Hellmark, T.; Johansson, A.C. Low production of reactive oxygen species in granulocytes is associated with organ damage in systemic lupus erythematosus. Arthritis Res. Ther. 2014, 16, R120. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, 16, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.T.; Caster, D.J. The Potential of Nrf2 Activation as a Therapeutic Target in Systemic Lupus Erythematosus. Metabolites 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | LN (n = 23) | SLE (n = 47) | HC (n = 23) | p1 | p2 |
|---|---|---|---|---|---|
| Age (years) | 34 (28–49) | 43 (29–54) | 47 (28–61) | 0.324 | N.A. |
| Gender females, n (%) | 18 (78%) | 37 (79%) | 18 (82%) | 0.947 | N.A. |
| Systolic blood pressure (mmHg) | 120 (110–130) | 120 (110–130) | 120 (110–120) | 0.77 | N.A. |
| Diastolic blood pressure (mmHg) | 80 (70–80) | 75 (70–80) | 75 (70–80) | 0.867 | N.A. |
| Weight (Kg) | 67 (63–86) | 72 (60–85) | 69 (62–80) | 0.859 | N.A. |
| Thrombocytes (10^9/L) | 303 (263–341) | 230 (198–281) | 236 (212–273) | 0.006 | AB = 0.007 AC = 0.036 |
| Creatinine (umol/L) | 81 (76–92) | 72 (62–81) | 71 (62–76) | <0.001 | AB = 0.002 AC = 0.001 |
| ALAT (U/L) | 19 (12–29) | 19 (15–23) | 19 (14–28) | 0.952 | N.A. |
| Hemoglobin (mmol/L) | 8.1 (7.4–8.3) | 8.0 (7.7–8.5) | 8.3 (8.0–8.9) | 0.068 | N.A. |
| Leukocytes (10^9/L) | 9.2 (7.6–12.4) | 5.4 (4.4–7.1) | 5.6 (4.8–6.2) | <0.001 | AB < 0.001 AC < 0.001 |
| Complement 3 (g/L) | 0.79 (0.7–1.0) | 1.0 (0.8–1.1) | 1.06 (0.9–1.2) | 0.013 | AB = 0.044 AC = 0.018 |
| Complement 4 (g/L) | 0.18 (0.1–0.3) | 2.0 (2.0–4.0) | 0.19 (0.2–0.3) | 0.257 | N.A. |
| SLEDAI, score | 14 (12–19) | 2 (2–4) | N.A. | N.A. | <0.001 |
| Anti-dsDNA positive, n (%) | 14 (61%) | 21(45%) | N.A. | N.A. | 0.203 |
| Prednisone use, n (%) | 23 (100%) | 13 (28%) | N.A. | N.A. | <0.001 |
| Azathioprine use, n (%) | 13 (57%) | 6 (13%) | N.A. | N.A. | <0.001 |
| Thiols | sRAGE | MDA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% Confidence Interval | p-Value | B | 95% Confidence Interval | p-Value | B | 95% Confidence Interval | p-Value | ||||
| SLEDAI | −4.72 | −5.98 | −3.46 | <0.001 * | 20.69 | 1.47 | 39.91 | 0.035 * | 0.84 | 0.15 | 1.52 | 0.016 * |
| Hemoglobin | −0.23 | −11.64 | 11.17 | 0.968 | −54.45 | −207.14 | 98.24 | 0.485 | 2.19 | −4.03 | 8.41 | 0.490 |
| Leukocytes | −0.81 | −5.20 | 3.58 | 0.718 | −5.58 | −32.20 | 20.99 | 0.680 | −1.71 | −3.36 | 0 | 0.044 * |
| Thrombocytes | −0.10 | −0.23 | 0.02 | 0.113 | 0.09 | −1.74 | 1.92 | 0.923 | 0 | −0.05 | 0.05 | 0.902 |
| Complement 3 | −17.06 | −72.92 | 38.80 | 0.550 | 256.06 | −258.55 | 770.68 | 0.329 | 16.64 | −24.49 | 57.78 | 0.428 |
| Complement 4 | −130.99 | −281.09 | 19.12 | 0.087 | 1490.08 | −334.02 | 3314.19 | 0.109 | 40.20 | −62.58 | 142.98 | 0.443 |
| Creatinine | 0.17 | 0 | 0.33 | 0.048 * | 0.46 | −2.42 | 3.33 | 0.756 | −0.10 | −0.21 | 0 | 0.048 * |
| urine protein/24 h | −1.28 | −4.84 | 2.28 | 0.481 | −5.21 | −46.52 | 36.11 | 0.805 | 0.26 | −1.10 | 1.61 | 0.713 |
| Anti-dsDNA (pos vs. neg) | −12.64 | −40.14 | 14.85 | 0.367 | −184.13 | −459.76 | 91.50 | 0.190 | −6.63 | −18.24 | 4.97 | 0.263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; de Leeuw, K.; Arends, S.; Doornbos-van der Meer, B.; Bulthuis, M.L.C.; van Goor, H.; Westra, J., on behalf of the Dutch LN Studies. Biomarkers of Oxidative Stress in Systemic Lupus Erythematosus Patients with Active Nephritis. Antioxidants 2023, 12, 1627. https://doi.org/10.3390/antiox12081627
Liu L, de Leeuw K, Arends S, Doornbos-van der Meer B, Bulthuis MLC, van Goor H, Westra J on behalf of the Dutch LN Studies. Biomarkers of Oxidative Stress in Systemic Lupus Erythematosus Patients with Active Nephritis. Antioxidants. 2023; 12(8):1627. https://doi.org/10.3390/antiox12081627
Chicago/Turabian StyleLiu, Lu, Karina de Leeuw, Suzanne Arends, Berber Doornbos-van der Meer, Marian L. C. Bulthuis, Harry van Goor, and Johanna Westra on behalf of the Dutch LN Studies. 2023. "Biomarkers of Oxidative Stress in Systemic Lupus Erythematosus Patients with Active Nephritis" Antioxidants 12, no. 8: 1627. https://doi.org/10.3390/antiox12081627