Potent Antioxidant and Anti-Tyrosinase Activity of Butein and Homobutein Probed by Molecular Kinetic and Mechanistic Studies †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inhibited Autoxidation Studies in Micelles
2.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
2.4. Tyrosnase Inbibition
2.5. Stability of Inhibitors in the Presence of Oxygen
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity in the Inhibited Autoxidation of Methyl Linoleate Micelles
3.2. EPR Spectroscopy
3.3. Explaining the Excellent Antioxidant Activity of Butein in MeLin Micelles
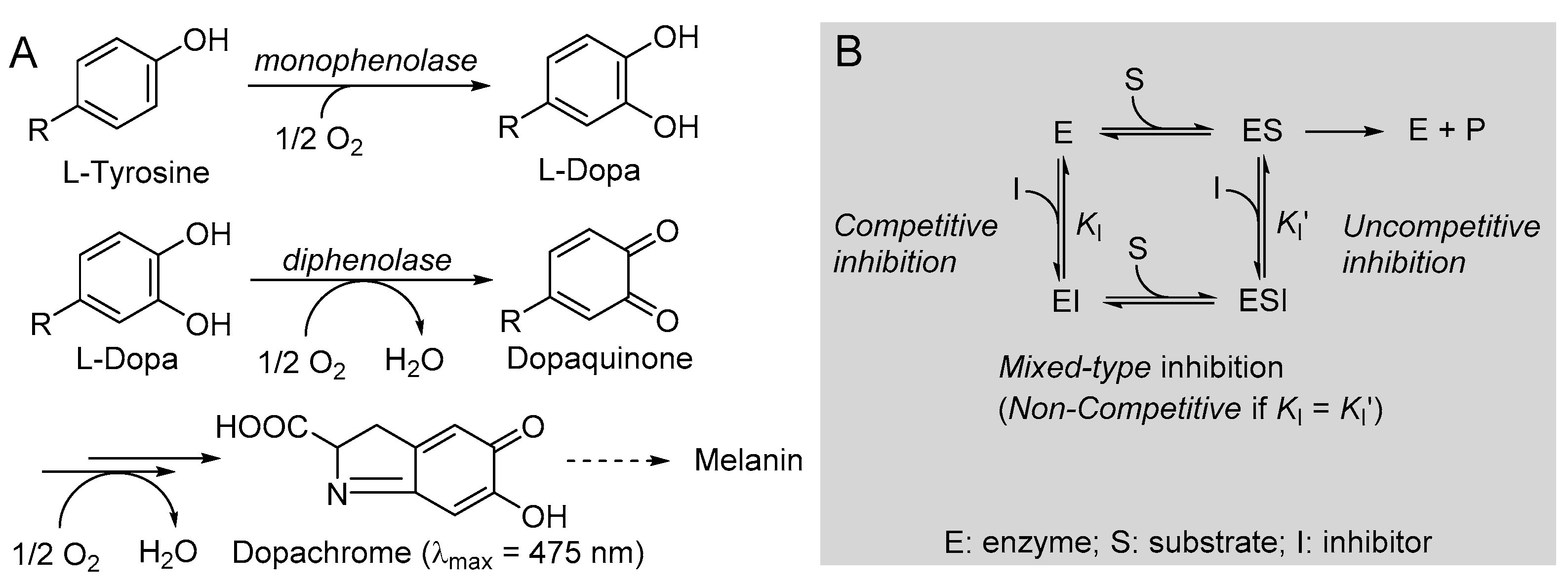
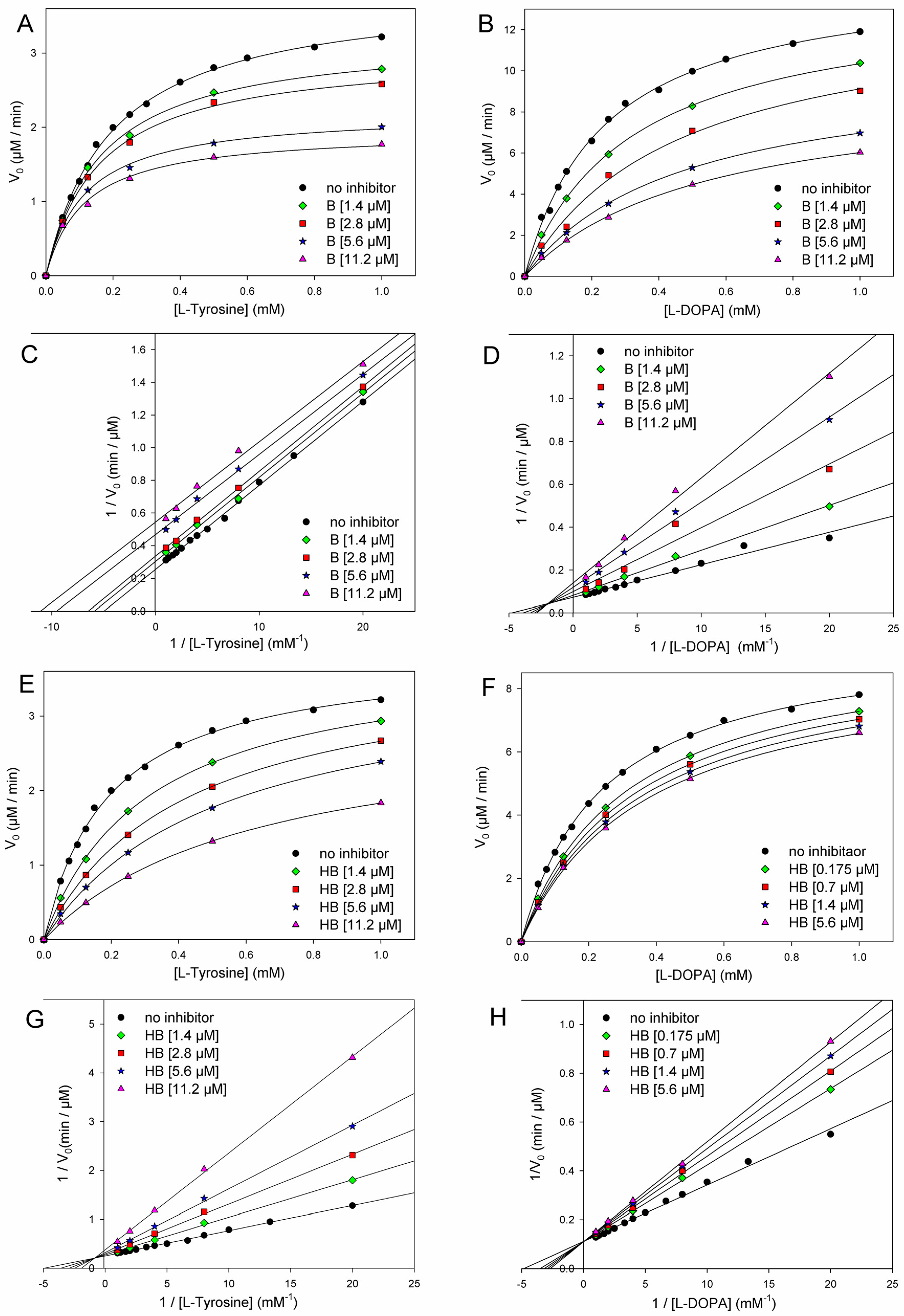
3.4. Kinetics of Inhibition of Mushroom Tyrosinase (mTYR)
3.5. Determination of IC50 against mTYR
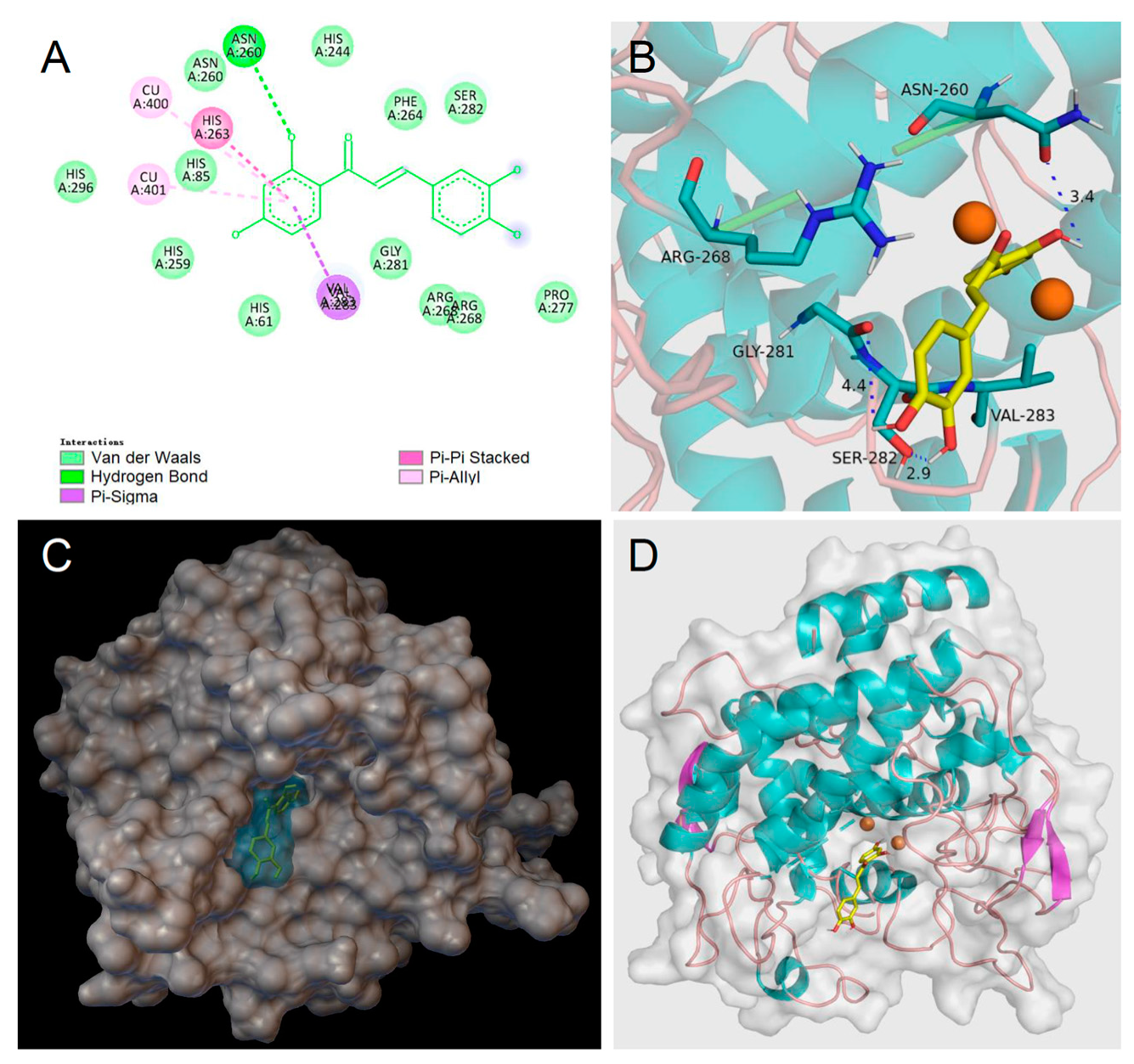
3.6. Molecular Docking
3.7. Stability of Butein and Homobutein toward Air and mTYR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Butein: From ancient traditional remedy to modern nutraceutical. Phytochem. Lett. 2015, 11, 188–201. [Google Scholar] [CrossRef]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Lee, S.H.; Seo, G.S.; Sohn, D.H. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW264.7 cells. Biochem. Biophys. Res. Commun. 2004, 323, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, W.-C.; Kim, K.-S.; Park, J.-W.; Lee, S.H.; Yoon, S.-W. Shrinkage of Gastric Cancer in an elderly patient who received Rhus verniciflua Stokes extract. J. Altern. Complement. Med. 2010, 16, 497–500. [Google Scholar] [CrossRef]
- Cho, S.-G.; Woo, S.-M.; Ko, S.-G. Butein suppresses breast cancer growth by reducing a production of intracellular reactive oxygen species. J. Exp. Clin. Cancer Res. 2014, 33, 51. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Mizerska-Dudka, M.; Daniluk, J.; Kandefer-Szerszen, M. Butein inhibits ethanol-induced activation of liver stellate cells through TGF-beta, NFkappaB, p38, and JNK signaling pathways and inhibition of oxidative stress. J. Gas-troenterol. 2013, 48, 222–237. [Google Scholar] [CrossRef]
- Tungalag, T.; Park, K.W.; Yang, D.K. Butein Ameliorates Oxidative Stress in H9c2 Cardiomyoblasts through Activation of the NRF2 Signaling Pathway. Antioxidants 2022, 11, 1430. [Google Scholar] [CrossRef]
- Cheng, Z.-J.; Kuo, S.-C.; Chan, S.-C.; Ko, F.-N.; Teng, C.-M. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim. Biophys. Acta Lipids Lipid Metab. 1998, 1392, 291–299. [Google Scholar] [CrossRef]
- Serobatse, K.R.N.; Kabanda, M.M. Antioxidant and antimalarial properties of butein and homobutein based on their ability to chelate iron (II and III) cations: A DFT study in vacuo and in solution. Eur. Food Res. Technol. 2016, 242, 71–90. [Google Scholar] [CrossRef]
- Orlikova, B.; Schnekenburger, M.; Zloh, M.; Golais, F.; Diederich, M.; Tasdemir, D. Natural chalcones as dual inhibitors of HDACs and NF-κB. Oncol. Rep. 2012, 28, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Pedulli, G.F.; Cabrini, L.; Zambonin, L.; Landi, L. Solvent and pH Effects on the Antioxidant Activity of Caffeic and Other Phenolic Acids. J. Agric. Food Chem. 2006, 54, 2932–2937. [Google Scholar] [CrossRef]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hering, A.; Stefanowicz-Hajduk, J.; Dziomba, S.; Halasa, R.; Krzemieniecki, R.; Sappati, S.; Baginski, M.; Ochocka, J.R. Mangiferin Affects Melanin Synthesis by an Influence on Tyrosinase: Inhibition, Mechanism of Action and Molecular Docking Studies. Antioxidants 2023, 12, 1016. [Google Scholar] [CrossRef]
- Choi, J.; Neupane, T.; Baral, R.; Jee, J.-G. Hydroxamic Acid as a Potent Metal-Binding Group for Inhibiting Tyrosinase. Antioxidants 2022, 11, 280. [Google Scholar] [CrossRef]
- Guo, Y.; Cariola, A.; Matera, R.; Gabbanini, S.; Valgimigli, L. Real-time oxygen sensing as a powerful tool to investigate tyrosinase kinetics allows revising mechanism and activity of inhibition by glabridin. Food Chem. 2022, 393, 133423. [Google Scholar] [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chem. 2023, 405, 134953. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enz. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamira, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef]
- Fenoll, L.G.; Rodriguez-Lopez, J.N.; Garcia-Molina, F.; Garcìa-Canovas, F.; Tudela, J. Unification for the Expression of the Monophenolase and Diphenolase Activities of Tyrosinase. IUBMB Life 2002, 54, 137–141. [Google Scholar] [CrossRef]
- Konopko, A.; Litwinienko, G. Unexpected Role of pH and Microenvironment on the Antioxidant and Synergistic Activity of Resveratrol in Model Micellar and Liposomal Systems. J. Org. Chem. 2022, 87, 1698–1709. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef]
- Johansson, H.; Shanks, D.; Engman, L.; Amorati, R.; Pedulli, G.F.; Valgimigli, L. Long-lasting antioxidant protection: A re-generable BHA analogue. J. Org. Chem. 2010, 75, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Doba, T.; Gabe, E.; Hughes, L.; Lee, F.L.; Prasad, L.; Ingold, K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985, 107, 7053–7065. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Locke, S.J.; Schaffer, T.D. Benzophenone-photosensitized autoxidation of linoleate in solution and sodium dodecyl sulfate micelles. Can. J. Chem. 1987, 65, 2229–2240. [Google Scholar] [CrossRef]
- Valgimigli, L.; Valgimigli, M.; Gaiani, S.; Pedulli, G.F.; Bolondi, L. Measurement of oxidative stress in human liver by EPR spin-probe technique. Free Radic. Res. 2000, 33, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Solvent effects on the reactivity and free spin distribution of 2,2-diphenyl-1-picrylhydrazyl radicals. J. Org. Chem. 1996, 61, 7947–7950. [Google Scholar] [CrossRef]
- Brigati, G.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F. Determination of the Substituent Effect on the O−H Bond Dissociation Enthalpies of Phenolic Antioxidants by the EPR Radical Equilibration Technique. J. Org. Chem. 2002, 67, 4828–4832. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati, R.; Minisci, F. Thermochemical and kinetic studies of a bisphenol anti-oxidant. J. Org. Chem. 2001, 66, 5456–5462. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Computat. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Santos-Martins, D.; Solis-Vasquez, L.; Tillack, A.F.; Sanner, M.F.; Koch, A.; Forli, S. Accelerating AutoDock4 with GPUs and Gradient-Based Local Search. J. Chem. Theory Comput. 2021, 17, 1060–1073. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 2010, 39, 2106–2119. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Guerra, M. A Critical Evaluation of the Factors Determining the Effect of Intramolecular Hydrogen Bonding on the O-H Bond Dissociation Enthalpy of Catechol and of Flavonoid Antioxidants. Chem. Eur. J. 2004, 10, 933–939. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; D’Ischia, M. 5-S-lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Jin, Z.; Amorati, R. Hydroperoxyl radical (HOO•) as a reducing agent: Unexpected synergy with antioxidants. A review. Free Radic. Res. 2023, 57, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.A.; Haidasz, E.A.; Griesser, M.; Pratt, D.A. Inhibition of hydrocarbon autoxidation by nitroxide-catalyzed cross-dismutation of hydroperoxyl and alkylperoxyl radicals. Chem. Sci. 2018, 9, 6068–6079. [Google Scholar] [CrossRef]
- Guo, Y.; Amorati, R. The Underrecognized Role of the Hydroperoxyl (HOO•) Radical in Chain Propagation of Lipids and its Implication in Antioxidant Activity. In Lipid Oxidation in Food and Biological Systems; Bravo-Diaz, C., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO• to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef] [PubMed]
- Mollica, F.; Lucernati, R.; Amorati, R. Expanding the spectrum of polydopamine antioxidant activity by nitroxide conjugation. J. Mater. Chem. B 2021, 9, 9980–9988. [Google Scholar] [CrossRef]
- Song, X.; Ni, M.; Zhang, Y.; Zhang, G.; Pan, J.; Gong, D. Comparing the inhibitory abilities of epigallocatechin-3-gallate and gallocatechin gallate against tyrosinase and their combined effects with kojic acid. Food Chem. 2021, 349, 129172. [Google Scholar] [CrossRef]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef]
- Mayr, F.; Sturm, S.; Ganzera, M.; Waltenberger, B.; Martens, S.; Schwaiger, S.; Schuster, D.; Stuppner, H. Mushroom Tyrosinase-Based Enzyme Inhibition Assays Are Not Suitable for Bioactivity-Guided Fractionation of Extracts. J. Nat. Prod. 2019, 82, 136–147. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef] [PubMed]


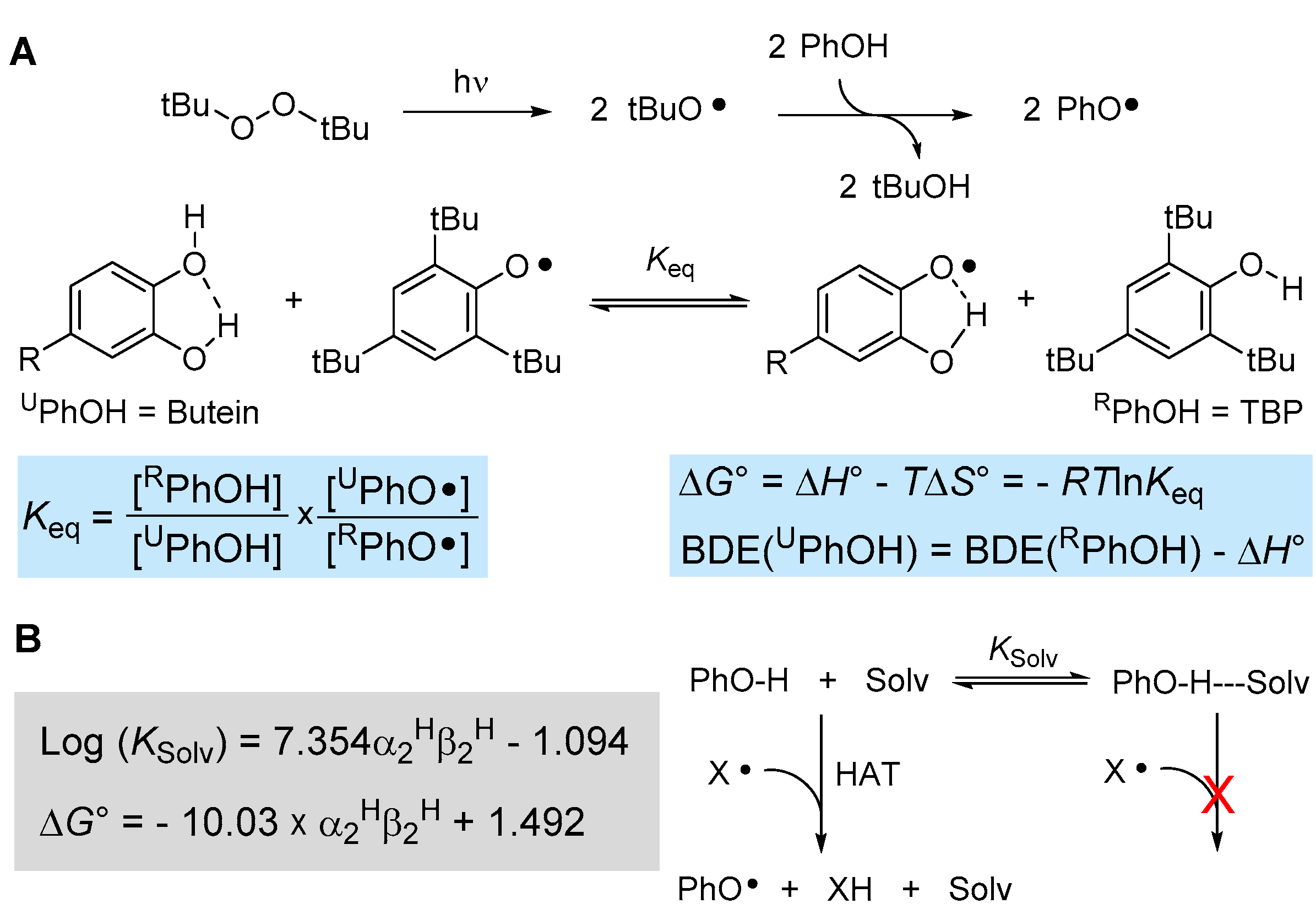

| Antioxidant | kinh/103 M−1s−1 | n | BDEOH/kcal/mol |
|---|---|---|---|
| Butein | 29.8 ± 9.2 | 3.7 ± 1.1 | 78.4 ± 0.2 |
| Homobutein | 2.8 ± 0.9 | -- | 82.6 1 |
| α-TOH | 22.4 ± 5.8 | 2 2 | 77.1 3 |
| Inhibition of Monophenolase Reaction (Substrate = l-Tyrosine) | ||||
| Butein (µM) | Km or Kmapp (mM) | Vmax or Vmaxapp (µM/min) | KI′ (µM) Calc. from Vmax | KI′ (µM) Calc. from Km |
| 0 | 0.19 ± 0.01 | 3.85 ± 0.04 | - | - |
| 1.4 | 0.16 ± 0.02 | 3.24 ± 0.07 | 7.44 | 7.47 |
| 2.8 | 0.15 ± 0.01 | 3.02 ± 0.05 | 10.19 | 10.50 |
| 5.6 | 0.12 ± 0.02 | 2.21 ± 0.04 | 7.55 | 9.60 |
| 11.2 | 0.11 ± 0.01 | 1.95 ± 0.07 | 11.49 | 15.40 |
| Average | 9.17 ± 2.01 | 10.74 ± 3.36 | ||
| Inhibition of Diphenolase Reaction (Substrate = l-Dopa) | ||||
| Butein (µM) | Km or Kmapp (mM) | Vmax or Vmaxapp (µM/min) | KI (µM) Competitive | KI′ (µM) Uncompetitive |
| 0 | 0.24 ± 0.01 | 11.78 ± 0.22 | - | - |
| 1.4 | 0.32 ± 0.01 | 10.93 ± 0.22 | 3.21 | 18.04 |
| 2.8 | 0.45 ± 0.06 | 10.60 ± 0.88 | 2.59 | 25.24 |
| 5.6 | 0.47 ± 0.02 | 8.17 ± 0.21 | 3.07 | 12.68 |
| 11.2 | 0.54 ± 0.03 | 7.42 ± 0.26 | 4.35 | 19.05 |
| Average | 3.30 ± 0.75 | 18.75 ± 5.15 | ||
| Inhibition of Monophenolase Reaction (Substrate = l-Tyrosine) | ||||
| Homobutein (µM) | Km or Kmapp (mM) | Vmax or Vmaxapp (µM/min) | KI (µM) Competitive | KI′ (µM) Uncompetitive |
| 0 | 0.19 ± 0.01 | 3.85 ± 0.04 | - | - |
| 1.4 | 0.31 ± 0.01 | 3.83 ± 0.04 | 2.19 | 268.10 |
| 2.8 | 0.42 ± 0.02 | 3.78 ± 0.05 | 2.24 | 151.20 |
| 5.6 | 0.52 ± 0.02 | 3.63 ± 0.06 | 2.94 | 92.40 |
| 11.2 | 0.63 ± 0.01 | 3.15 ± 0.03 | 3.67 | 50.40 |
| Average | 2.76 ± 0.70 | 140.53 ± 94.57 | ||
| Inhibition of Diphenolase Reaction (Substrate = l-Dopa) | ||||
| Homobutein (µM) | Km or Kmapp (mM) | Vmax or Vmaxapp (µM/min) | KI (µM) Competitive | KI′ (µM) Uncompetitive |
| 0 | 0.24 ± 0.01 | 11.60 ± 0.22 | - | - |
| 0.175 | 0.27 ± 0.01 | 11.48 ± 0.22 | 1.28 | 16.75 |
| 0.70 | 0.34 ± 0.05 | 11.30 ± 0.88 | 1.54 | 26.38 |
| 1.4 | 0.36 ± 0.02 | 11.10 ± 0.21 | 2.46 | 30.83 |
| 5.6 | 0.49 ± 0.08 | 10.84 ± 0.26 | 4.72 | 79.01 |
| Average | 2.50 ± 1.56 | 38.24 ± 27.81 | ||
| Butein IC50 (µM) | Homoutein IC50 (µM) | Kojic Acid IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| Substrate (mM) | Monophenolase | Diphenolase | Monophenolase | Diphenolase | Monophenolase | Diphenolase |
| 0.05 | 45.05 ± 18.31 | 3.76 ± 0.96 | 4.01 ± 0.66 | 3.04 ± 0.84 | 15.51 ± 3.52 | 4.81 ± 0.52 |
| 0.125 | 35.60 ± 28.42 | 4.12 ± 1.39 | 4.58 ± 0.88 | 3.83 ± 0.94 | 18.64 ± 3.85 | 8.23 ± 1.05 |
| 0.25 | 12.87 ± 3.22 | 5.38 ± 0.90 | 6.06 ± 0.95 | 5.60 ± 1.07 | 20.52 ± 2.71 | 9.31 ± 1.75 |
| 0.50 | 12.26 ± 2.52 | 9.31 ± 0.85 | 8.75 ± 1.18 | 7.57 ± 1.11 | 24.91 ± 2.92 | 12.03 ± 2.12 |
| 1.00 | 10.88 ± 2.19 | 15.20 ± 1.25 | 14.78 ± 1.05 | 12.36 ± 2.00 | 33.14 ± 5.03 | 18.27 ± 3.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Giovanardi, I.; Sagynova, T.; Cariola, A.; Bresciani, V.; Masetti, M.; Valgimigli, L. Potent Antioxidant and Anti-Tyrosinase Activity of Butein and Homobutein Probed by Molecular Kinetic and Mechanistic Studies. Antioxidants 2023, 12, 1763. https://doi.org/10.3390/antiox12091763
Pan W, Giovanardi I, Sagynova T, Cariola A, Bresciani V, Masetti M, Valgimigli L. Potent Antioxidant and Anti-Tyrosinase Activity of Butein and Homobutein Probed by Molecular Kinetic and Mechanistic Studies. Antioxidants. 2023; 12(9):1763. https://doi.org/10.3390/antiox12091763
Chicago/Turabian StylePan, Wenkai, Ilaria Giovanardi, Tomiris Sagynova, Alice Cariola, Veronica Bresciani, Matteo Masetti, and Luca Valgimigli. 2023. "Potent Antioxidant and Anti-Tyrosinase Activity of Butein and Homobutein Probed by Molecular Kinetic and Mechanistic Studies" Antioxidants 12, no. 9: 1763. https://doi.org/10.3390/antiox12091763







