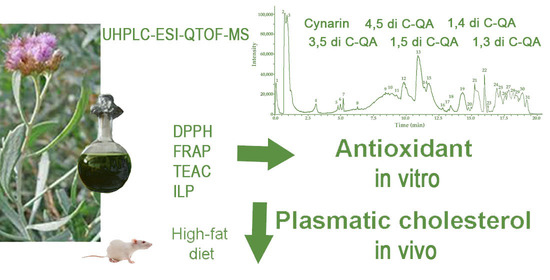
Ultra-High-Resolution Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Analysis of Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) and Antioxidant and Hypocholesterolemic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Botanical Description, Plant Material and Methanolic Extract of T. absinthioides (METa)
2.3. Ultra-High-Resolution Liquid Chromatography Analysis (UHPLC-ESI-QTOF-MS)
2.4. The Total Phenols Assay by Folin−Ciocalteu Reagent (FCR) and Flavonoids Content
2.5. In Vitro Antioxidant Activity
2.5.1. Radical Scavenging Capacity Assay of 2,2-Diphenyl-1-picrylhydrazyl (DPPH)
2.5.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.5.3. Trolox Equivalent Antioxidant Activity Assay (TEAC)
2.5.4. Inhibition of Lipid Peroxidation (ILP) in Erythrocytes
2.6. Animals, Diets and Experimental Procedure
2.6.1. Lipid Profile Parameters
2.6.2. LXRs Protein Expression
2.7. Statistical Analysis
3. Results
3.1. Metabolite Profiling: UHPLC-ESI-QTOF-MS Analysis of METa
3.2. In Vitro Assays: Total Phenolics and Flavonoids Contents and Antioxidant Activity of METa
3.3. In Vivo Assay
3.3.1. Animals’ BW Gain and Intake of Food
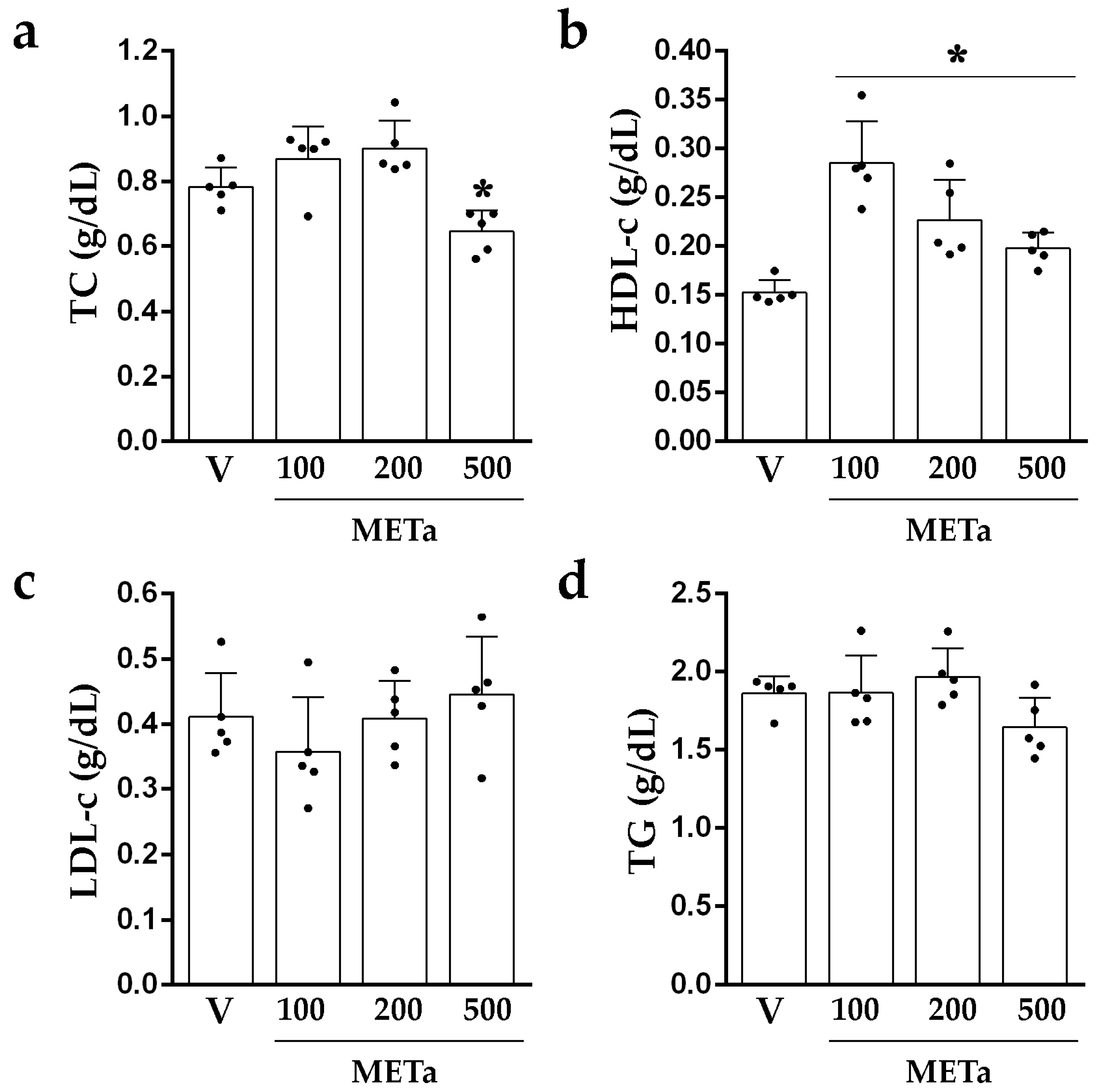
3.3.2. Levels of TC, HDL-c, LDL-c and TG
3.3.3. The Protein Expression of LXRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurina-Sanz, M.B.; Donadel, O.J.; Rossomando, P.C.; Tonn, C.E.; Guerreiro, E. Sesquiterpenes from Tessaria absinthioides. Phytochemistry 1997, 44, 897–900. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Isla, M.I.; Ríos, J.L.; Giner, R.M.; Alberto, M.R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Res. Int. 2015, 67, 230–237. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Ana, Y.; Rojas-Márquez, J.D.; Stempin, C.C.; Zampini, I.C.; Isla, M.I.; Alberto, M.R. Argentinean Puna plants with in vitro antioxidant and anti-inflammatory activities as a potential nutraceutical. J. Food Sci. 2019, 84, 3352–3363. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Gamarra-Luques, C.; Bórquez, J.; Caballero, D.; Feresin, G.E.; Tapia, A. UHPLC–Q/Orbitrap/MS/MS fingerprinting, free radical scavenging, and antimicrobial activity of Tessaria absinthiodes (Hook. & arn.) DC. (Asteraceae) lyophilized decoction from Argentina and Chile. Antioxidants 2019, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Feresin, G.E.; Coirini, H. Tessaria absinthioides (Hook. & arn.) dc. (asteraceae) decoction improves the hypercholesterolemia and alters the expression of lxrs in rat liver and hypothalamus. Metabolites 2021, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Danino, O.; Gottlieb, H.E.; Grossman, S.; Bergman, M. Antioxidant activity of 1, 3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res. Int. 2009, 42, 1273–1280. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.W. Antioxidant and anti-inflammatory activities of 3, 5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beveragesand spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Ghibaudi, L.; Cook, J.; Farley, C.; Van Heek, M.; Hwa, J.J. Fat intake affects adiposity, comorbidity factors, and energy metabolism of Sprague-Dawley rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clín. Engl. Ed. 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Krečman, V.; Škottová, N.; Walterová, D.; Ulrichová, J.; Šimének, V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998, 64, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, C. Natural modulators of liver X receptors. J. Integr. Med. 2014, 12, 76–85. [Google Scholar] [CrossRef]
- Komati, R.; Spadoni, D.; Zheng, S.; Sridhar, J.; Riley, K.E.; Wang, G. Ligands of therapeutic utility for the liver X Receptors. Molecules 2017, 22, 88. [Google Scholar] [CrossRef]
- Baranowski, M. Biological role of liver X receptors. J. Physiol. Pharmacol. 2008, 59, 31–55. [Google Scholar]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Investig. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Ulven, S.M.; Dalen, K.T.; Gustafsson, J.Å.; Nebb, H.I. LXR is crucial in lipid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 59–63. [Google Scholar] [CrossRef]
- Steffensen, K.R.; Nilsson, M.; Schuster, G.U.; Stulnig, T.M.; Dahlman-Wright, K.; Gustafsson, J.Å. Gene expression profiling in adipose tissue indicates different transcriptional mechanisms of liver X receptors α and β, respectively. Biochem. Biophys. Res. Commun. 2003, 310, 589–593. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Coirini, H. High-Fat Diets and LXRs Expression in Rat Liver and Hypothalamus. Cell. Mol. Neurobiol. 2019, 39, 963–974. [Google Scholar] [CrossRef]
- Luna, L.; Simirgiotis, M.J.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-MS metabolome fingerprinting: The isolation of main compounds and antioxidant activity of the andean species Tetraglochin ameghinoi (Speg.) Speg. Molecules 2018, 23, 793. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Simirgiotis, M.J.; Manrique, S.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-HESI-OT-MS-MS biomolecules profiling, antioxidant and antibacterial activity of the “orange-yellow resin” from Zuccagnia punctata Cav. Antioxidants 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Varzakas, T.; Oreopoulou, V. In vitro activity of vitamins, flavanoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Arantes, A.A.; Falé, P.L.; Costa, L.C.; Pacheco, R.; Ascensão, L.; Serralheiro, M.L. Inhibition of HMG-CoA reductase activity and cholesterol permeation through Caco-2 cells by caffeoylquinic acids from Vernonia condensata leaves. Rev. Bras. Farmacogn. 2016, 26, 738–743. [Google Scholar] [CrossRef]
- Gezer, C. Potential health effects of the popular compounds of antichoke: Cynarin. Prog. Nutr. 2017, 19 (Suppl. S1), 5–9. [Google Scholar] [CrossRef]
- Weggemans, R.M.; Trautwein, E.A. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: A meta-analysis. Eur. J. Clin. Nutr. 2003, 57, 940–946. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Ose, L.; Marais, A.D. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: A multicenter, randomized, placebo-controlled trial. J. Pediatr. 2003, 143, 74–80. [Google Scholar] [CrossRef]
- Topping, D.L.; Storer, G.B.; Calvert, G.D.; Illman, R.J.; Oakenfull, D.G.; Weller, R.A. Effects of dietary saponins on fecal bile acids and neutral sterols, plasma lipids, and lipoprotein turnover in the pig. Am. J. Clin. Nutr. 1980, 33, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Thompson, P.D. Phytosterols and vascular disease. Atherosclerosis 2006, 186, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Traves, P.G.; Hortelano, S.; Zeini, M.; Chao, T.-H.; Lam, T.; Neuteboom, S.T.; Theodorakis, E.A.; Palladino, M.A.; Castrillo, A.; Bosca, L. Selective Activation of Liver X Receptors by Acanthoic Acid-related Diterpenes. Mol. Pharmacol. 2007, 71, 1545–1553. [Google Scholar] [CrossRef]
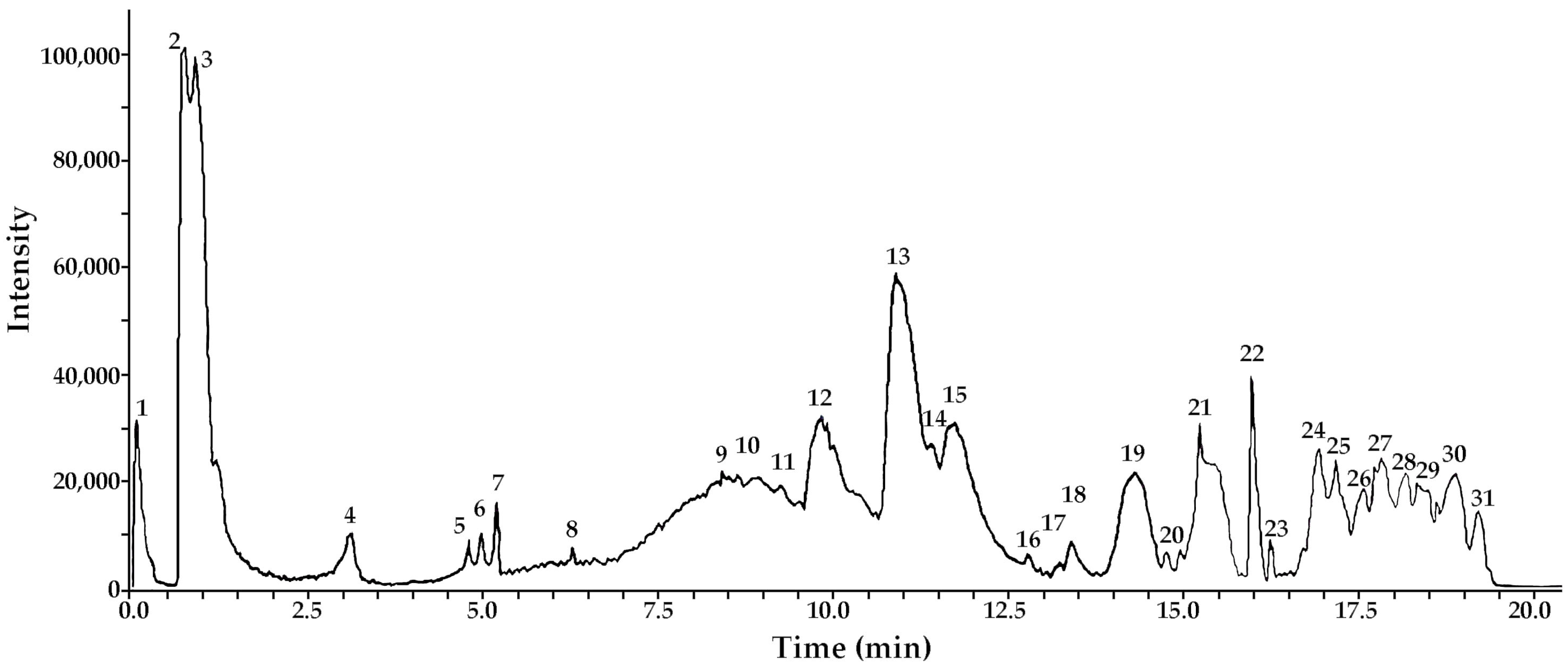

| Peak | Tentative Identification | [M-H]− | Retention Time (min) | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (ppm) | Metabolite Type | MS Ions (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Naformiate (internal standard) | C4H2O4 | 0.22 | 112.98293 | 112.98562 | 3.1 | Standard acid | 588.8964, 656.8829, 724.8745 |
| 2 | Di-caffeoyl quinic acid (cynarin) 1,5 di C-QA) | C25H23O12 | 0.72 | 515.11952 | 515.12013 | 1.99 | Phenolic acid | 353.0883, (CA) 191.0571 |
| 3 | Di-caffeoyl quinic acid (3,5 di C-QA) | C25H23O12 | 0.72 | 515.11952 | 515.12113 | 3.26 | Phenolic acid | 353.0881, (CA) 191.0573 |
| 4 | Di-caffeoyl quinic acid (4,5 di C-QA) | C25H23O12 | 3.16 | 515.11952 | 515.12212 | 5.17 | Phenolic acid | 353.0872, 191.0571 (QA) |
| 5 | Di-caffeoyl quinic acid (1,3 di C-QA) | C25H23O12 | 5.15 | 515.11952 | 515.12095 | 2.83 | Phenolic acid | 353.0873, 191.0572 (QA) |
| 6 | Di-caffeoyl quinic acid (1,4 di C-QA) | C25H23O12 | 5.17 | 515.11952 | 515.12101 | 2.95 | Phenolic acid | 353.0874, 191.0574 (QA) |
| 7 | Di-caffeoyl quinic acid (1,5 di C-QA) | C25H23O12 | 5.21 | 515.11952 | 515.11957 | 0.16 | Phenolic acid | 353.0872, 191.0575 (QA) |
| 8 | Tessaric acid derivative | C15H19O2 | 3.16 | 231.13981 | 231.13905 | 9.86 | Phenolic acid | 187.14872 |
| 9 | Tessaric acid | C15H19O3 | 8.45 | 247.13397 | 247.13383 | −0.45 | Terpene | 205.15975 |
| 10 | 5-Acetyl, 3-hydroxy-4 dihydrocostic acid | C34H47O7 | 8.75 | 309.17090 | 309.16962 | 3.95 | Terpene | 291.16019 |
| 11 | Axillarin | C17H13O8 | 9.32 | 345.06068 | 345.0615 | −2.6 | Flavone | 315.0507, 691.13021 (2M-H), 179.9921, 151.00349 |
| 12 | 3-O-Caffeoyl-5-O-malonylquinic acid | C19H19O12 | 9.94 | 439.08233 | 439.08363 | 3.0 | Phenolic acid | 341.12645 |
| 13 | gamma Costic acid | C15H21O2 | 11.02 | 233.15470 | 233.15516 | 1.93 | Terpene | |
| 14 | Rhamnacin | C17H13O7 | 11.48 | 329.06574 | 329.0666 | −2.91 | Flavone | 315.0462, (M-CH3), 277.1075, 300.05554, 151.0020, 256.03405 |
| 15 | Hesperetin | C16H13O6 | 11.93 | 301.07142 | 301.07162 | −0.42 | Flavone | 263.12825, 201.05187 |
| 16 | Isorhamnetin | C16H11O7 | 12.62 | 315.05113 | 315.05153 | −2.6 | Flavone | 299.05616 (M-CH3), 287.0555 |
| 17 | Irigenin | C18H15O8 | 13.32 | 359.07724 | 359.07665 | −1.63 | Flavone | 341.06717, 317.0666, 299.0643, 112.9847, 179.0761, |
| 18 | Europetin 7-O-methylmyricetin | C16H11O8 | 13.84 | 331.04573 | 331.04574 | 0.0 | Flavone | 329.0656, 315.0513, (M-CH3), 301.0351 (M-2CH3) |
| 19 | Cirsiliol | C17H13O7 | 14.39 | 329.06664 | 329.06692 | 0.92 | Flavone | 315.0465, (M-CH3), 271.0260, 300.05554, 151.0020, 256.03405 |
| 20 | Arcapillin | C18H15O8 | 14.95 | 359.07754 | 359.07618 | −3.19 | Flavone | 277.1078, 112.9847, 179.0761, |
| 21 | Sakuranetin | C16H13O5 | 15.35 | 285.07682 | 285.07593 | −2.96 | Flavone | 247.0972, |
| 22 | Genkwanin | C16H12O5 | 16.11 | 283.06123 | 283.06023 | −2.92 | Flavone | 247.1515, 269.0407, 201.1487 |
| 23 | Eupatorin (3′,5-Dihydroxy-4′,6,7-trimethoxyflavone) | C18H15O7 | 16.32 | 343.08258 | 343.08117 | −3.58 | Flavone | 329.06662, 271.0255, 165.0193 |
| 24 | 2-Hydroxyoctadecanoic acid | C18H35O3 | 17.21 | 299.26085 | 299.25917 | −2.71 | Fatty acids | 643.3434 |
| 25 | Eupatorin 5-methyl eter | C19H17O7 | 17.46 | 357.08234 | 357.08232 | 0.03 | Flavone | 321.1702 |
| 26 | Eudesma-2,4(15),11(13)-trien-12-oic acid | C15H19O2 | 17.55 | 231.13905 | 231.13974 | 12.2 | Terpene | 161.04478 |
| 27 | Palmitic acid | C16H31O2 | 17.72 | 255.23475 | 255.23295 | 7.04 | Fatty acids | 231.13511 |
| 28 | Cryptocaryol D | C40H63O8 | 18.41 | 585.40081 | 585.39821 | −4.43 | Terpene | 460.35166 |
| 29 | 4 alpha-Phorbol 12,13-didecanoate | C40H63O8 | 18.53 | 671.47877 | 671.47151 | −3.67 | Phorbolester | 586.40293, 584.44304, 491.32338 |
| 30 | 4 alpha-Phorbol 2,13-didecanoate | C40H63O8 | 18.77 | 671.47397 | 671.46911 | −7.23 | Phorbolester | 586.40293, 491.32338 |
| 31 | 4alpha-Phorbol2,12-didecanoate | C40H63O8 | 18.98 | 671.47397 | 671.45284 | −5.5 | Phorbolester | 586.40293, 491.32338 |
| Content of Phenols | |
|---|---|
| Total phenolics (mg GAE/g METa) | 251.68 ± 6.80 |
| Flavonoids (mg QE/g METa) | 116.34 ± 0.43 |
| Antioxidant Assays | |
| DPPH (IC50 in µg METa/mL) | 22.89 ± 0.03 |
| FRAP (mg TE/g of METa) | 2.30 ± 0.25 |
| TEAC (mg TE/g of METa) | 0.85 ± 0.02 |
| Percentage ILP (at 100 µg METa/mL) | 61.74 ± 0.41 |
| Percentage ILP catechin (100 µg/mL) | 74.01 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, M.; Kruse, M.S.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Coirini, H. Ultra-High-Resolution Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Analysis of Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) and Antioxidant and Hypocholesterolemic Properties. Antioxidants 2024, 13, 50. https://doi.org/10.3390/antiox13010050
Rey M, Kruse MS, Gómez J, Simirgiotis MJ, Tapia A, Coirini H. Ultra-High-Resolution Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Analysis of Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) and Antioxidant and Hypocholesterolemic Properties. Antioxidants. 2024; 13(1):50. https://doi.org/10.3390/antiox13010050
Chicago/Turabian StyleRey, Mariana, María Sol Kruse, Jessica Gómez, Mario J. Simirgiotis, Alejandro Tapia, and Héctor Coirini. 2024. "Ultra-High-Resolution Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Analysis of Tessaria absinthioides (Hook. & Arn.) DC. (Asteraceae) and Antioxidant and Hypocholesterolemic Properties" Antioxidants 13, no. 1: 50. https://doi.org/10.3390/antiox13010050