Cultivar and Harvest Time of Almonds Affect Their Antioxidant and Nutritional Profile through Gut Microbiota Modifications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Morphological Measurements
2.4. Determination of Ash, Moisture and Protein Content
2.5. In Vitro Digestion and Fermentation
2.6. Antioxidant Assays
2.6.1. Trolox Equivalent Antioxidant Capacity against ABTS Radicals (TEACABTS) Assay
2.6.2. Trolox Equivalent Antioxidant Capacity Referred to Reducing Capacity (TEACFRAP) Assay
2.6.3. Trolox Equivalent Antioxidant Capacity against DPPH Radicals (TEACDPPH) Assay
2.6.4. Folin–Ciocalteu Assay
2.7. Ultra-High Performance Liquid Chromatography (UHPLC) Analysis
2.7.1. Analysis of Phenolic Compounds
2.7.2. Analysis of SCFAs, Lactic and Succinic Acids
2.8. Microbial Genomic DNA Isolation and High Throughput Sequencing
2.9. Statistical Analyses
3. Results and Discussion
3.1. Influence of Cultivar and Harvest Time on Ash, Moisture, Protein, and Morphology of Almonds
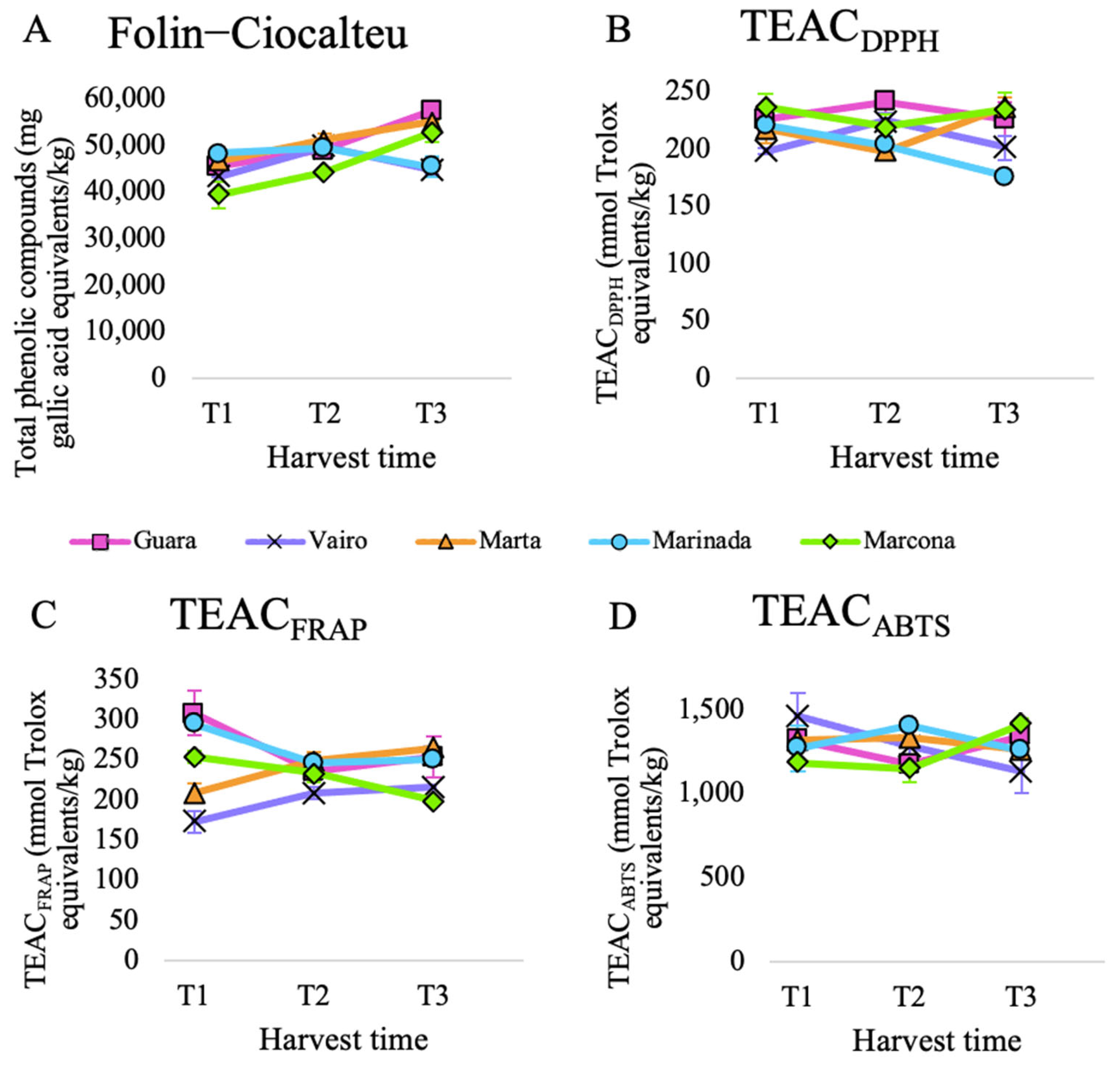
3.2. Antioxidant Capacity of the Samples Obtained after In Vitro Digestion and Fermentation
3.2.1. Evolution of the Total Antioxidant Capacity over Harvest Time
3.2.2. Contribution of In Vitro Digestion-Fermentation Fractions to Total Antioxidant Capacity
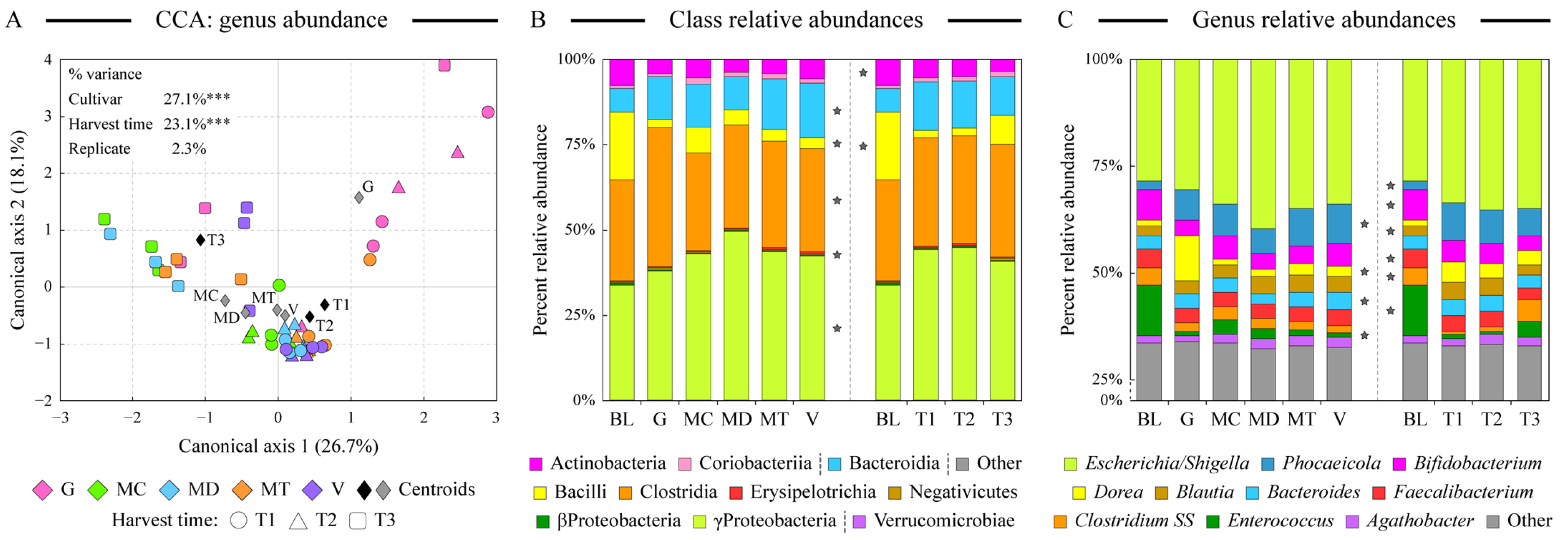
3.3. Microbiota Community Structure Supported by Fermentation of Digested Almond Samples
3.4. Phenolic Compounds, SCFAs and Lactic and Succinic Acids Measured after In Vitro Digestion and Fermentation
3.4.1. Phenolic Compounds
3.4.2. SCFAs, Lactic and Succinic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, İ.; et al. Almonds (Prunus dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Özcan, M.M. A Review on Some Properties of Almond: Impact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J. Food Sci. Technol. 2023, 60, 1493–1504. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M.; Juhaimi, F.A.; Adiamo, O.Q.; Alsawmahi, O.N.; Ghafoor, K.; Babiker, E.E. Effect of the Harvest Time on Oil Yield, Fatty Acid, Tocopherol and Sterol Contents of Developing Almond and Walnut Kernels. J. Oleo Sci. 2018, 67, 39–45. [Google Scholar] [CrossRef]
- Özcan, M.M.; Lemiasheuski, V. The Effect of Harvest Times on Mineral Contents of Almond and Walnut Kernels. Erwerbs-Obstbau 2020, 62, 455–458. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uslu, N. Effect of Variety on Bioactive Properties, Phytochemicals and Nutrients of Almond Kernels. Erwerbs-Obstbau 2023, 65, 981–988. [Google Scholar] [CrossRef]
- Piscopo, A.; Romeo, F.V.; Petrovicova, B.; Poiana, M. Effect of the Harvest Time on Kernel Quality of Several Almond Varieties (Prunus dulcis (Mill.) D.A. Webb). Sci. Hortic. 2010, 125, 41–46. [Google Scholar] [CrossRef]
- Beltrán Sanahuja, A.; Maestre Pérez, S.E.; Grané Teruel, N.; Valdés García, A.; Prats Moya, M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the Chemical and Nutritional Characteristics of Almonds (Prunus dulcis (Mill). D.A. Webb) as Influenced by Harvest Time and Cultivar. J. Sci. Food Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. 2–Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds and Nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2019; pp. 23–54. ISBN 978-0-12-814174-8. [Google Scholar]
- Thomson, C.; Garcia, A.L.; Edwards, C.A. Interactions between Dietary Fibre and the Gut Microbiota. Proc. Nutr. Soc. 2021, 80, 398–408. [Google Scholar] [CrossRef]
- Campos-Perez, W.; Martinez-Lopez, E. Effects of Short Chain Fatty Acids on Metabolic and Inflammatory Processes in Human Health. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut Microbiota-Derived Succinate: Friend or Foe in Human Metabolic Diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of Cooking Methods on the Antioxidant Capacity of Plant Foods Submitted to In Vitro Digestion–Fermentation. Antioxidants 2020, 9, 1312. [Google Scholar] [CrossRef]
- Garrido, I.; Urpi-Sarda, M.; Monagas, M.; Gómez-Cordovés, C.; Martín-álvarez, P.J.; Llorach, R.; Bartolomé, B.; Andrés-Lacueva, C. Targeted Analysis of Conjugated and Microbial-Derived Phenolic Metabolites in Human Urine After Consumption of an Almond Skin Phenolic Extract. J. Nutr. 2010, 140, 1799–1807. [Google Scholar] [CrossRef]
- Llorach, R.; Garrido, I.; Monagas, M.; Urpi-Sarda, M.; Tulipani, S.; Bartolome, B.; Andres-Lacueva, C. Metabolomics Study of Human Urinary Metabolome Modifications After Intake of Almond (Prunus dulcis (Mill.) D.A. Webb) Skin Polyphenols. J. Proteome Res. 2010, 9, 5859–5867. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Medina-Remón, A.; Andres-Lacueva, C.; Bartolomé, B. Profile of Plasma and Urine Metabolites after the Intake of Almond [Prunus dulcis (Mill.) D.A. Webb] Polyphenols in Humans. J. Agric. Food Chem. 2009, 57, 10134–10142. [Google Scholar] [CrossRef]
- Choo, J.M.; Tran, C.D.; Luscombe-Marsh, N.D.; Stonehouse, W.; Bowen, J.; Johnson, N.; Thompson, C.H.; Watson, E.-J.; Brinkworth, G.D.; Rogers, G.B. Almond Consumption Affects Fecal Microbiota Composition, Stool pH, and Stool Moisture in Overweight and Obese Adults with Elevated Fasting Blood Glucose: A Randomized Controlled Trial. Nutr. Res. 2021, 85, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Creedon, A.C.; Dimidi, E.; Hung, E.S.; Rossi, M.; Probert, C.; Grassby, T.; Miguens-Blanco, J.; Marchesi, J.R.; Scott, S.M.; Berry, S.E.; et al. The Impact of Almonds and Almond Processing on Gastrointestinal Physiology, Luminal Microbiology, and Gastrointestinal Symptoms: A Randomized Controlled Trial and Mastication Study. Am. J. Clin. Nutr. 2022, 116, 1790–1804. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential Prebiotic Properties of Almond (Amygdalus communis L.) Seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef]
- Şahin, M.; Arioglu-Tuncil, S.; Ünver, A.; Deemer, D.; Lindemann, S.R.; Tunçil, Y.E. Dietary Fibers of Tree Nuts Differ in Composition and Distinctly Impact the Fecal Microbiota and Metabolic Outcomes In Vitro. J. Agric. Food Chem. 2023, 71, 9762–9771. [Google Scholar] [CrossRef]
- Li, M.; Lu, P.; Wu, H.; de Souza, T.S.P.; Suleria, H.A.R. In Vitro Digestion and Colonic Fermentation of Phenolic Compounds and Their Bioaccessibility from Raw and Roasted Nut Kernels. Food Funct. 2023, 14, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rufián-Henares, J.A.; Pastoriza, S. Towards an Improved Global Antioxidant Response Method (GAR+): Physiological-Resembling in Vitro Digestion-Fermentation Method. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. An in Vitro Batch Fermentation Protocol for Studying the Contribution of Food to Gut Microbiota Composition and Functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Muñoz, A.E.; Álvarez, M.B.; Oliveras-López, M.-J.; Martínez, R.G.; Henares, J.Á.R.; Herrera, M.O. Determination of Polyphenolic Compounds by Ultra-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry and Antioxidant Capacity of Spanish Subtropical Fruits. Agric. Sci. 2018, 9, 180–199. [Google Scholar] [CrossRef]
- Panzella, L.; Pérez-Burillo, S.; Pastoriza, S.; Martín, M.Á.; Cerruti, P.; Goya, L.; Ramos, S.; Rufián-Henares, J.Á.; Napolitano, A.; d’Ischia, M. High Antioxidant Action and Prebiotic Activity of Hydrolyzed Spent Coffee Grounds (HSCG) in a Simulated Digestion–Fermentation Model: Toward the Development of a Novel Food Supplement. J. Agric. Food Chem. 2017, 65, 6452–6459. [Google Scholar] [CrossRef]
- Paliy, O.; Foy, B.D. Mathematical Modeling of 16S Ribosomal DNA Amplification Reveals Optimal Conditions for the Interrogation of Complex Microbial Communities with Phylogenetic Microarrays. Bioinformatics 2011, 27, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, S.; Freedman, D.A.; Sehgal, A.R.; Bui, X.; Paliy, O. Diet Quality and Body Mass Indices Show Opposite Associations with Distal Gut Microbiota in a Low-Income Cohort. SDRP-JFST 2019, 4, 846–851. [Google Scholar] [CrossRef]
- Paliy, O.; Shankar, V. Application of Multivariate Statistical Techniques in Microbial Ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef]
- Craig, M.P.; Rajakaruna, S.; Paliy, O.; Sajjad, M.; Madhavan, S.; Reddy, N.; Zhang, J.; Bottomley, M.; Agrawal, S.; Kadakia, M.P. Differential MicroRNA Signatures in the Pathogenesis of Barrett’s Esophagus. Clin. Transl. Gastroenterol. 2020, 11, e00125. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Morales-Corts, M.R. Agromorphological Characterization and Nutritional Value of Traditional Almond Cultivars Grown in the Central-Western Iberian Peninsula. Agronomy 2021, 11, 1238. [Google Scholar] [CrossRef]
- Cherif, A.; Sebei, K.; Boukhchina, S.; Kallel, H.; Belkacemi, K.; Arul, J. Kernel Fatty Acid and Triacylglycerol Composition for Three Almond Cultivars during Maturation. J. Am. Oil Chem. Soc. 2004, 81, 901–905. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Sánchez-Bel, P.; Domingo, R. The Effects of Contrasted Deficit Irrigation Strategies on the Fruit Growth and Kernel Quality of Mature Almond Trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Kazantzis, I.; Nanos, G.D.; Stavroulakis, G.G. Effect of Harvest Time and Storage Conditions on Almond Kernel Oil and Sugar Composition. J. Sci. Food Agric. 2003, 83, 354–359. [Google Scholar] [CrossRef]
- Nanos, G.D.; Kazantzis, I.; Kefalas, P.; Petrakis, C.; Stavroulakis, G.G. Irrigation and Harvest Time Affect Almond Kernel Quality and Composition. Sci. Hortic. 2002, 96, 249–256. [Google Scholar] [CrossRef]
- Soler, L.; Canellas, J.; Saura-Calixto, F. Changes in Carbohydrate and Protein Content and Composition of Developing Almond Seeds. J. Agric. Food Chem. 1989, 37, 1400–1404. [Google Scholar] [CrossRef]
- Hawker, J.S.; Buttrose, M.S. Development of the Almond Nut (Prunus dulcis (Mill.) D. A. Webb). Anatomy and Chemical Composition of Fruit Parts from Anthesis to Maturity. Ann. Bot. 1980, 46, 313–321. [Google Scholar] [CrossRef]
- Levent, O. A Detailed Comparative Study on Some Physicochemical Properties, Volatile Composition, Fatty Acid, and Mineral Profile of Different Almond (Prunus dulcis L.) Varieties. Horticulturae 2022, 8, 488. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant Activity and Bioactive Compounds of Ten Portuguese Regional and Commercial Almond Cultivars. Food Chem. Toxicol. 2008, 46, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol Content and Antioxidant Activity of California Almonds Depend on Cultivar and Harvest Year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and Fatty Acid Profiles, α-Tocopherol and Sucrose Contents, and Antioxidant Capacities of Understudied Portuguese Almond Cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef] [PubMed]
- Maestri, D.; Martínez, M.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P.; Torres, M. Variability in Almond Oil Chemical Traits from Traditional Cultivars and Native Genetic Resources from Argentina. Food Chem. 2015, 170, 55–61. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Duarte, S.; Puchades, A.; Jiménez-Hernández, N.; Betoret, E.; Gosalbes, M.J.; Betoret, N. Almond (Prunus dulcis) Bagasse as a Source of Bioactive Compounds with Antioxidant Properties: An In Vitro Assessment. Antioxidants 2023, 12, 1229. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- D’hoe, K.; Conterno, L.; Fava, F.; Falony, G.; Vieira-Silva, S.; Vermeiren, J.; Tuohy, K.; Raes, J. Prebiotic Wheat Bran Fractions Induce Specific Microbiota Changes. Front. Microbiol. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Ates, U. Harvest Time Influences Quality Attributes and Phenolic Composition of Fig Fruit: Insights from Physicochemical Analysis and Antioxidant Activity Assessment. Erwerbs-Obstbau 2023, 65, 1627–1632. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of Harvest Time on Some in Vitro Functional Properties of Hop Polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an In Vitro Colonic Model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Schlörmann, W.; Birringer, M.; Lochner, A.; Lorkowski, S.; Richter, I.; Rohrer, C.; Glei, M. In Vitro Fermentation of Nuts Results in the Formation of Butyrate and C9,T11 Conjugated Linoleic Acid as Chemopreventive Metabolites. Eur. J. Nutr. 2016, 55, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.; Scharlau, D.; Schlörmann, W.; Birringer, M.; Glei, M. In Vitro Fermented Nuts Exhibit Chemopreventive Effects in HT29 Colon Cancer Cells. Br. J. Nutr. 2012, 108, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Zuelch, M.L.; Radtke, M.D.; Holt, R.R.; Basu, A.; Burton-Freeman, B.; Ferruzzi, M.G.; Li, Z.; Shay, N.F.; Shukitt-Hale, B.; Keen, C.L.; et al. Perspective: Challenges and Future Directions in Clinical Research with Nuts and Berries. Adv. Nutr. 2023, 14, 1005–1028. [Google Scholar] [CrossRef] [PubMed]


| Cultivar | Harvest Time | Length (cm) | Width (cm) | Thickness (cm) | Ash (%) | Moisture (%) | Protein (%) |
|---|---|---|---|---|---|---|---|
| Guara | T1 | 2.47 ± 0.13 | 1.09 ± 0.10 a | 0.70 ± 0.09 a | 1.38 ± 0.12 a | 65.1 ± 1.10 | 11.2 ± 1.13 a |
| T2 | 2.51 ± 0.13 | 1.28 ± 0.08 | 0.70 ± 0.05 a | 1.40 ± 0.21 a | 47.9 ± 0.20 | 12.8 ± 0.62 a | |
| T3 | 2.41 ± 0.06 | 1.14 ± 0.14 a | 0.40 ± 0.05 | 5.55 ± 0.27 | 7.00 ± 0.10 | 24.6 ± 4.82 | |
| Significance | NS | * | * | NS | * | * | |
| Vairo | T1 | 2.24 ± 0.09 | 1.04 ± 0.09 | 0.56 ± 0.07 a | 1.61 ± 0.01 ab | 63.1 ± 0.6 | 14.8 ± 5.54 ab |
| T2 | 2.18 ± 0.11 | 1.11 ± 0.07 | 0.55 ± 0.05 a | 1.65 ± 0.14 a | 44.7 ± 0.8 | 13.8 ± 1.03 a | |
| T3 | 2.26 ± 0.10 | 1.11 ± 0.12 | 0.36 ± 0.05 | 3.63 ± 0.25 b | 6.00 ± 0.30 | 22.3 ± 0.72 b | |
| Significance | NS | NS | * | NS | * | * | |
| Marta | T1 | 2.34 ± 0.13 | 1.01 ± 0.12 ab | 0.73 ± 0.05 a | 1.37 ± 0.06 | 66.9 ± 1.1 a | 12.5 ± 3.28 |
| T2 | 2.27 ± 0.12 | 1.10 ± 0.07 a | 0.71 ± 0.07 a | 1.64 ± 0.04 | 55.1 ± 2.00 a | 9.36 ± 2.56 | |
| T3 | 2.25 ± 0.12 | 0.93 ± 0.09 b | 0.42 ± 0.06 | 4.56 ± 0.69 | 6.50 ± 0.00 | 22.6 ± 1.13 | |
| Significance | NS | * | * | NS | NS | * | |
| Marinada | T1 | 2.07 ± 0.12 a | 1.09 ± 0.10 ab | 0.71 ± 0.03 a | 1.14 ± 0.07 | 76.6 ± 0.30 | 6.96 ± 0.21 |
| T2 | 2.28 ± 0.18 b | 1.05 ± 0.08 a | 0.49 ± 0.06 a | 1.55 ± 0.02 | 47.7 ± 1.30 | 12.8 ± 1.74 | |
| T3 | 2.11 ± 0.14 ab | 1.20 ± 0.13 b | 0.69 ± 0.09 | 3.25 ± 1.08 | 8.80 ± 0.60 | 21.4 ± 1.54 | |
| Significance | * | * | * | NS | NS | * | |
| Marcona | T1 | 1.80 ± 0.08 | 1.20 ± 0.08 | 0.84 ± 0.05 | 1.41 ± 0.09 a | 54.6 ± 2.80 a | 10.2 ± 0.82 |
| T2 | 1.70 ± 0.12 | 1.14 ± 0.11 | 0.75 ± 0.07 | 1.63 ± 0.09 a | 50.3 ± 0.50 a | 11.8 ± 1.74 | |
| T3 | 1.74 ± 0.05 | 1.17 ± 0.05 | 0.62 ± 0.06 | 3.71 ± 0.15 | 9.30 ± 1.10 | 21.2 ± 5.33 | |
| Significance | NS | NS | * | NS | * | * | |
| Guara | Vairo | Marta | Marinada | Marcona | Significance | |
|---|---|---|---|---|---|---|
| (+)-Catechin | 104 ± 48.0 | 97.7 ± 55.6 | 73.4 ± 53.1 | 67.7 ± 63.3 | 83.0 ± 48.2 | NS |
| (-)-Epicatechin | 140 ± 65.8 ab | 90.1 ± 35.7 acd | 66.4 ± 18.9 bce | 38.2 ± 15.3 f | 49.0 ± 17.1 def | * |
| (-)-Epigallocatechin | 49.9 ± 9.33 abcd | 61.6 ± 9.71 aef | 43.2 ± 14.5 begh | 33.4 ± 15.8 cgi | 52.0 ± 15.2 dfhi | * |
| Ferulic acid | 0.002 ± 0 abcd | 0.005 ± 0.003 aefg | 0.002 ± 0 behi | 0.002 ± 0.001 cfhj | 0.002 ± 0 dgij | * |
| Kaempferol | 0.006 ± 0.002 | 0.005 ± 0.001 | 0.006 ± 0.002 | 0.004 ± 0.001 | 0.008 ± 0.003 | NS |
| Naringenin | 0.003 ± 0.001 | 0.004 ± 0 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | NS |
| Naringin | 0.003 ± 0.002 | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0 | 0.003 ± 0.001 | NS |
| p-Coumaric acid | 0.002 ± 0.001 abcd | 0.004 ± 0.003 aefg | 0.002 ± 0.002 behi | 0.001 ± 0 cfhj | 0.006 ± 0.006 dgij | * |
| Quercetin | 54.4 ± 16.2 | 61.8 ± 8.64 | 51.9 ± 10.5 | 51.8 ± 15.0 | 53.6 ± 13.8 | NS |
| Rutin | 84.0 ± 11.6 abcd | 80.7 ± 7.86 aefg | 75.6 ± 5.03 behi | 76.7 ± 2.28 cfh | 81.9 ± 3.63 dgi | * |
| 3,4-dihydroxyphenylacetic acid | 38.4 ± 14.3 | 35.4 ± 10.3 | 31.5 ± 13.4 | 23.7 ± 13.5 | 28.7 ± 5.84 | NS |
| 3-(3,4-dihydroxyphenyl)propionic acid | 269 ± 106 ab | 146 ± 73.4 acde | 84.3 ± 66.7 cfg | 87.8 ± 42.0 dfh | 161 ± 95.6 begh | * |
| 3-(3-hydroxyphenyl)propionic acid | 21.0 ± 7.28 abcd | 4.97 ± 11.6 aefg | 9.20 ± 8.17 behi | 11.7 ± 12.8 cfhj | 19.1 ± 11.7 dgij | * |
| 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 54.6 ± 57.0 ab | 183 ± 157 acde | 274 ± 115 cfg | 240 ± 113 dfh | 213 ± 134 begh | * |
| Phenol | 9.45 ± 2.79 | 8.41 ± 1.72 | 9.04 ± 4.11 | 10.6 ± 4.38 | 9.79 ± 4.42 | NS |
| Phloroglucinol | 207 ± 80.2 abcd | 259 ± 56.2 ae | 199 ± 34.4 befg | 168 ± 46.7 cfh | 186 ± 35.5 dgh | * |
| Urolithin A | 0.022 ± 0.011 | 0.026 ± 0.009 | 0.021 ± 0.008 | 0.017 ± 0.006 | 0.025 ± 0.016 | NS |
| Urolithin B | 0.002 ± 0 abc | 0.002 ± 0.001 adef | 0.001 ± 0 d | 0.002 ± 0 be | 0.004 ± 0.002 cf | * |
| Total | 1055 ± 39.2 | 1027 ± 40.9 | 921 ± 113 | 814 ± 24.0 | 944 ± 24.3 | NS |
| T1 | T2 | T3 | Significance | |
|---|---|---|---|---|
| (+)-Catechin | 97.3 ± 55.6 | 71.5 ± 53.9 | 85.6 ± 50.5 | NS |
| (-)-Epicatechin | 97.3 ± 56.9 | 78.7 ± 59.2 | 53.6 ± 18.7 | NS |
| (-)-Epigallocatechin | 54.1 ± 18.5 ab | 47.6 ± 13.3 ac | 40.2 ± 12.11 bc | * |
| Ferulic acid | 0.003 ± 0.002 | 0.002 ± 0.001 | 0.003 ± 0.002 | NS |
| Kaempferol | 0.006 ± 0.003 | 0.005 ± 0.002 | 0.007 ± 0.002 | NS |
| Naringenin | 0.003 ± 0.001 a | 0.003 ± 0.001 ab | 0.004 ± 0.001 b | * |
| Naringin | 0.002 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 | NS |
| p-Coumaric acid | 0.004 ± 0.005 | 0.002 ± 0.002 | 0.003 ± 0.002 | NS |
| Quercetin | 67.9 ± 5.92 | 48.0 ± 9.78 a | 48.2 ± 10.3 a | * |
| Rutin | 81.8 ± 10.0 | 81.1 ± 5.24 | 76.6 ± 4.77 | NS |
| 3,4-dihydroxyphenylacetic acid | 36.6 ± 12.4 | 32.6 ± 7.41 | 26.5 ± 14.7 | NS |
| 3-(3,4-dihydroxyphenyl)propionic acid | 162 ± 99.3 | 139 ± 70.3 | 142 ± 133 | NS |
| 3-(3-hydroxyphenyl)propionic acid | 16.2 ± 12.6 | 10.7 ± 8.78 | 13.6 ± 13.2 | NS |
| 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone | 214 ± 117 | 180 ± 138 | 185 ± 163 | NS |
| Phenol | 12.5 ± 2.86 | 8.77 ± 2.86 a | 7.08 ± 2.57 a | * |
| Phloroglucinol | 220 ± 69.2 | 204.7 ± 47.1 | 186 ± 59.3 | NS |
| Urolithin A | 0.032 ± 0.011 | 0.018 ± 0.006 a | 0.016 ± 0.005 a | * |
| Urolithin B | 0.002 ± 0.002 | 0.002 ± 0.001 | 0.002 ± 0.001 | NS |
| Total | 1073 ± 49.4 | 921 ± 72.1 | 863 ± 159 | NS |
| Harvest Time | Acetic Acid | Propionic Acid | Butyric Acid | Lactic Acid | Succinic Acid | Total SCFAs |
|---|---|---|---|---|---|---|
| T1 | 12.0 ± 1.62 | 1.80 ± 0.38 ab | 0.44 ± 0.25 a | 7.13 ± 0.65 | 12.2 ± 0.22 ab | 14.3 ± 2.22 |
| T2 | 12.9 ± 2.61 | 3.51 ± 2.17 ac | 0.50 ± 0.31 a | 6.89 ± 0.58 | 8.62 ± 4.67 ac | 15.6 ± 4.65 |
| T3 | 14.5 ± 6.12 | 3.82 ± 3.05 bc | 2.35 ± 1.92 | 7.36 ± 1.00 | 12.2 ± 2.64 bc | 16.9 ± 10.9 |
| Significance | NS | * | * | NS | * | NS |
| Cultivar | Acetic acid | Propionic acid | Butyric acid | Lactic acid | Succinic acid | Total SCFAs |
| Guara | 19.5 ± 5.16 abc | 6.16 ± 3.2 abcd | 2.48 ± 2.29 abcd | 6.07 ± 0.19 a | 7.50 ± 3.76 abcd | 28.2 ± 10.4 abcd |
| Vairo | 12.6 ± 0.92 ade | 2.21 ± 0.57 aefg | 0.97 ± 1.00 aefg | 7.58 ± 0.10 bcd | 12.2 ± 0.62 aefg | 15.7 ± 2.42 aefg |
| Marta | 12.1 ± 0.38 bd | 2.25 ± 0.44 behi | 1.22 ± 1.35 behi | 7.49 ± 0.33 bef | 12.8 ± 0.56 behi | 15.6 ± 1.46 beh |
| Marinada | 11.1 ± 0.08 ce | 2.97 ± 1.94 cfhj | 0.33 ± 0.05 cfhj | 7.23 ± 0.53 ceg | 9.54 ± 5.10 cfhj | 14.4 ± 2.03 cfhi |
| Marcona | 10.6 ± 0.09 | 1.63 ± 0.18 dgij | 0.48 ± 0.24 dgij | 7.26 ± 1.09 adfg | 13.0 ± 0.74 dgij | 12.7 ± 0.36 dgi |
| Significance | * | * | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Osorio, A.; Navajas-Porras, B.; Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Toledano-Marín, Á.; Pastoriza de la Cueva, S.; Paliy, O.; Rufián-Henares, J.Á. Cultivar and Harvest Time of Almonds Affect Their Antioxidant and Nutritional Profile through Gut Microbiota Modifications. Antioxidants 2024, 13, 84. https://doi.org/10.3390/antiox13010084
Delgado-Osorio A, Navajas-Porras B, Pérez-Burillo S, Hinojosa-Nogueira D, Toledano-Marín Á, Pastoriza de la Cueva S, Paliy O, Rufián-Henares JÁ. Cultivar and Harvest Time of Almonds Affect Their Antioxidant and Nutritional Profile through Gut Microbiota Modifications. Antioxidants. 2024; 13(1):84. https://doi.org/10.3390/antiox13010084
Chicago/Turabian StyleDelgado-Osorio, Adriana, Beatriz Navajas-Porras, Sergio Pérez-Burillo, Daniel Hinojosa-Nogueira, Ángela Toledano-Marín, Silvia Pastoriza de la Cueva, Oleg Paliy, and José Ángel Rufián-Henares. 2024. "Cultivar and Harvest Time of Almonds Affect Their Antioxidant and Nutritional Profile through Gut Microbiota Modifications" Antioxidants 13, no. 1: 84. https://doi.org/10.3390/antiox13010084









