Boron Reduced Copper Excess-Induced Oxidative Damage in Citrus sinensis by Modulating Reactive Oxygen Species and Methylglyoxal Formation and Their Detoxification Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seedling Culture and Treatments
2.2. Assays of HPR, SAPR, and Metabolites in Roots and Leaves
2.3. Assays of Enzyme Activities in Leaves and Roots
2.3.1. Antioxidant Enzymes
2.3.2. Sulfur Metabolism-Related Enzymes and Glyoxalases
2.4. Data Analysis
3. Results
3.1. Effects of Cu and B Treatments on HPR, SAPR, MG, and MDA in Leaves and Roots
3.2. Effects of Cu and B Treatments on ROS and MG Scavenging Enzyme Activities in Leaves and Roots
3.3. Effects of Cu and B Treatments on Antioxidant and S-Containing Compound Levels in Leaves and Roots
3.4. PCoA, PCA, and Regression Analysis for All the 31 Indexes in Leaves and Roots
4. Discussion
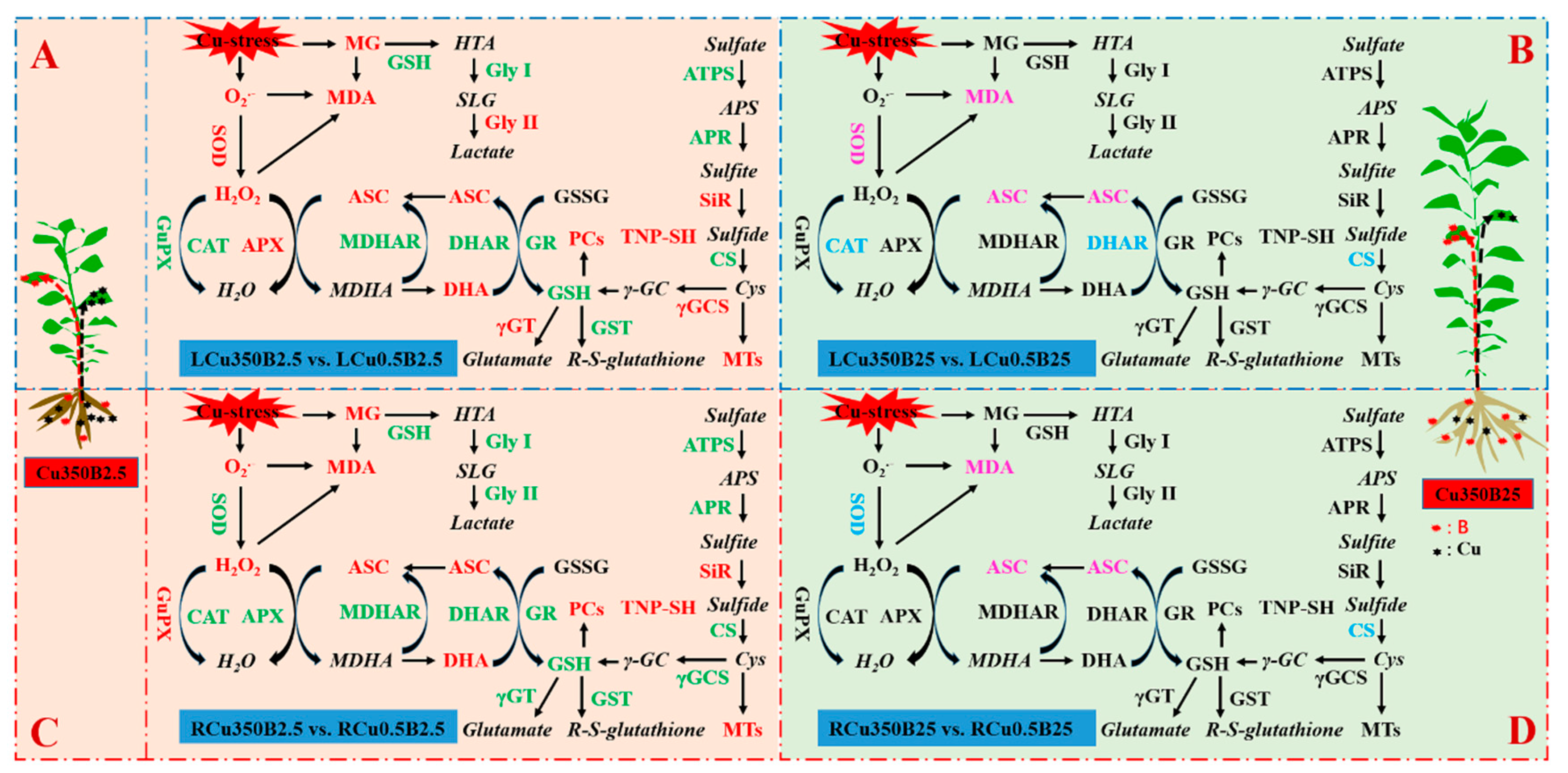
4.1. Cu and B Treatments Exhibited an Interactive Influence on ROS and MG Formation and Their Detoxification Systems in Leaves and Roots
4.2. B Addition Protected the Leaves and Roots of Cu-Exposed Seedlings from Oxidative Injury via the Coordinated Actions of ROS and MG Scavenging Systems
4.3. The Pathways Involved in the B-Mediated Amelioration of Oxidative Injury Caused by Cu Excess Differed between Leaves and Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, Y.F.; Zhang, C.B.; Wang, J.X.; Li, S.J.; Fu, X.Z.; Lin, L.L.; Cao, L.; Peng, L.Z. Review of research on copper in citrus. J. Fruit Sci. 2018, 35, 347–357. [Google Scholar]
- Wan, H.; Du, J.; He, J.; Lyu, D.; Li, H. Copper accumulation, subcellular partitioning and physiological and molecular responses in relation to different copper tolerance in apple rootstocks. Tree Physiol. 2019, 39, 1215–1234. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; He, Z.; Ma, L.Q.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soils Sediments 2011, 11, 348–639. [Google Scholar] [CrossRef]
- Zambrosi, F.C.B.; Mesquita, G.L.; Tanaka, F.A.O.; Quaggio, J.A.; Mattos-Jr, D. Phosphorus availability and rootstock affect copper-induced damage to the root ultra-structure of citrus. Environ. Exp. Bot. 2013, 95, 25–33. [Google Scholar] [CrossRef]
- Chen, X.-F.; Hua, D.; Zheng, Z.-C.; Zhang, J.; Huang, W.-T.; Chen, H.-H.; Huang, Z.-R.; Yang, L.-T.; Ye, X.; Chen, L.-S. Boron-mediated amelioration of copper-toxicity in sweet orange [Citrus sinensis (L.) Osbeck cv. Xuegan] seedlings involved reduced damage to roots and improved nutrition and water status. Ecotoxicol. Environ. Saf. 2022, 234, 113423. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zheng, Z.-C.; Chen, W.-S.; Rao, R.-Y.; Chen, X.-F.; Ye, X.; Guo, J.; Yang, L.-T.; Chen, L.-S. Regulation on copper-tolerance in Citrus sinensis seedlings by boron addition: Insights from root exudates, related metabolism, and gene expression. J. Hazard. Mater. 2023, 459, 132277. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.-F.; Huang, W.-L.; Chen, H.-H.; Huang, Z.-R.; Ye, X.; Chen, L.-S. High pH alleviated sweet orange (Citrus sinensis) copper toxicity by enhancing the capacity to maintain a balance between formation and removal of reactive oxygen species and methylglyoxal in leaves and roots. Int. J. Mol. Sci. 2022, 23, 13896. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Sil, P.C. Arsenic-induced oxidative myocardial injury: Protective role of arjunolic acid. Arch. Toxicol. 2008, 82, 137–149. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; de Souza-Vieira, Y.; Lavaquial, L.C.; Cassol, D.; Galhego, V.; Bastos, G.A.; Felix-Cordeiro, T.; Corrêa, R.L.; Zámocký, M.; Margis-Pinheiro, M.; et al. Ascorbate-glutathione cycle genes families in Euphorbiaceae: Characterization and evolutionary analysis. Biology 2023, 12, 19. [Google Scholar] [CrossRef]
- Li, Z.G. Methylglyoxal and glyoxalase system in plants: Old players, new concepts. Bot. Rev. 2016, 82, 183–203. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Hippler, F.W.R.; Petená, G.; Boaretto, R.M.; Quaggio, J.A.; Azevedo, R.A.; Mattos-Jr, D. Mechanisms of copper stress alleviation in citrus trees after metal uptake by leaves or roots. Environ. Sci. Pollut. Res. 2018, 25, 13134–13146. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Mostofa, M.G.; Polash, M.A.S.; Mahamud, A.G.M.S.U.; Afrin, S.; Hossain, M.A.; Hossain, M.A.; Murata, Y.; Tran, L.P. Comparative effects of ascobin and glutathione on copper homeostasis and oxidative stress metabolism in mitigation of copper toxicity in rice. Plant Biol. 2021, 23, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.M.; Alnusairi, M.H.A.; Almushhin, A.; Hasan, M.D.; Soliman, M.H. Alleviation of copper phytotoxicity by acetylsalicylic acid and nitric oxide application in mung bean involves the up-regulation of antioxidants, osmolytes and glyoxalase system. J. Plant Interact. 2021, 16, 201–212. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Qi, C.D.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.; Li, X.; Zhang, N.; Guo, Y.D. Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol. 2019, 60, 562–574. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef]
- Yang, L.-T.; Pan, J.-F.; Hu, N.-J.; Chen, H.-H.; Jiang, H.-X.; Lu, Y.-B.; Chen, L.-S. Citrus physiological and molecular response to boron stresses. Plants 2022, 11, 40. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, S.-Q.; Lin, W.-J.; Chen, H.-H.; Lin, F.; Zhu, D.-H.; Chen, L.-S.; Li, Y.; Guo, J.-X. Soil copper (Cu) nutrient status and its influencing factors in pomelo orchards in Pinghe county, Fujian Province. J. Fruit Sci. 2018, 35, 301–310. [Google Scholar]
- Chen, L.-S.; Qi, Y.-P.; Liu, X.-H. Effects of aluminum on light energy utilization and photoprotective systems in citrus leaves. Ann. Bot. 2005, 96, 35–41. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Garg, N.; Kaur, H. Response of antioxidant enzymes, phytochelatins and glutathione production towards Cd and Zn stresses in Cajanus cajan (L.) Millsp. genotypes colonized by arbuscular mycorrhizal fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Warrilow, A.G.S.; Hawkesford, M.J. Cysteine synthase (O-acetylserine (thiol) lyase) substrate specificities classify the mitochondrial isoform as a cyanoalanine synthase. J. Exp. Bot. 2000, 51, 985–993. [Google Scholar] [CrossRef]
- Trüper, H.G.; Rogers, L.A. Purification and properties of adenylyl sulfate reductase from the phototrophic sulfur bacterium, Thiocapsa roseopersicina. J. Bacteriol. 1971, 108, 1112–1121. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatase. Method Enzymol. 1966, 8, 115–118. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Fujita, M.; Hossain, M.Z. Modulation of pumpkin glutathione S-transferases by aldehydes and related compounds. Plant Cell Physiol. 2003, 44, 481–490. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Du, C.; Liu, Y.; Zeng, Y.; Jiang, C. Ameliorative role of boron to toxicity of aluminum in trifoliate orange roots. Ecotoxicol. Environ. Saf. 2019, 179, 212–221. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manag. 2018, 208, 149–158. [Google Scholar] [CrossRef]
- Qin, S.; Xu, Y.; Nie, Z.; Liu, H.; Gao, W.; Li, C.; Zhao, P. Metabolomic and antioxidant enzyme activity changes in response to cadmium stress under boron application of wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 34701–34713. [Google Scholar] [CrossRef]
- Chung, I.M.; Rekha, K.; Venkidasamy, B.; Thiruvengadam, M. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut. 2019, 230, 1–14. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Zhou, Y.; Núñez-Delgado, A.; Wang, X. Boron application mitigates Cd toxicity in leaves of rice by subcellular distribution, cell wall adsorption and antioxidant system. Ecotoxicol. Environ. Saf. 2021, 222, 112540. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Cheng, L. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca L. cv. Concord) leaves in responses to N limitation. J. Exp. Bot. 2003, 54, 2165–2175. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Bajguz, A.; Ahmad, P. The involvement of hydrogen sulphide in melatonin-induced tolerance to arsenic toxicity in pepper (Capsicum annuum L.) plants by regulating sequestration and subcellular distribution of arsenic, and antioxidant defense system. Chemosphere 2022, 309, 136678. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Zamocky, M.; Sachetto-Martins, G.; Margis-Pinheiro, M. Chloroplastic ascorbate peroxidases targeted to stroma or thylakoid membrane: The chicken or egg dilemma. FEBS Lett. 2022, 596, 2989–3004. [Google Scholar] [CrossRef]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef]
- Guo, P.; Li, Q.; Qi, Y.-P.; Yang, L.-T.; Ye, X.; Chen, H.-H.; Chen, L.-S. Sulfur mediated-alleviation of aluminum-toxicity in Citrus grandis seedlings. Int. J. Mol. Sci. 2017, 18, 2570. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, T.G.; Lee, J.; Chae, M.J.; Lee, E.J.; Kim, M.S.; Jung, G.B.; Emmanuel, A.; Jeon, S.; Lee, B.R. Foliar-applied glutathione mitigates cadmium-induced oxidative stress by modulating antioxidant-scavenging, redox-regulating, and hormone-balancing systems in Brassica napus. Front. Plant Sci. 2021, 12, 1954. [Google Scholar] [CrossRef]
- Navarrete, A.; González, A.; Gómez, M.; Contreras, R.A.; Díaz, P.; Lobos, G.; Brown, M.T.; Sáez, C.A.; Moenne, A. Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa. Plant Physiol. Biochem. 2019, 135, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Trivedi, P.K. Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat. Toxicol. 2008, 86, 205–215. [Google Scholar] [CrossRef]
- Kováčik, J.; Dresler, S.; Peterková, V.; Babula, P. Metal-induced oxidative stress in terrestrial macrolichens. Chemosphere 2018, 203, 402–409. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.K.; Sopory, S.K. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Singla-Pareek, S.L.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 2006, 140, 613–623. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Duran-Hernandez, D.; Gutierrez-Miceli, F. Changes in the phenylalanine ammonia lyase activity, total phenolic compounds, and flavonoids in Prosopis glandulosa treated with cadmium and copper. An. Acad. Bras. Cienc. 2018, 90, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.M.; Zhang, Y.K.; Wu, X.B.; Liu, C.S. The investigation of the alleviated effect of copper toxicity by exogenous nitric oxide in tomato plants. Plant Soil Environ. 2010, 56, 274–281. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Juang, K.W.; Lo, Y.C.; Chen, T.H.; Chen, B.C. Effects of copper on root morphology, cations accumulation, and oxidative stress of grapevine seedlings. Bull. Environ. Contam. Toxicol. 2019, 102, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu excess leads to oxidative stress mediated alterations in MAPKinase transcript levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
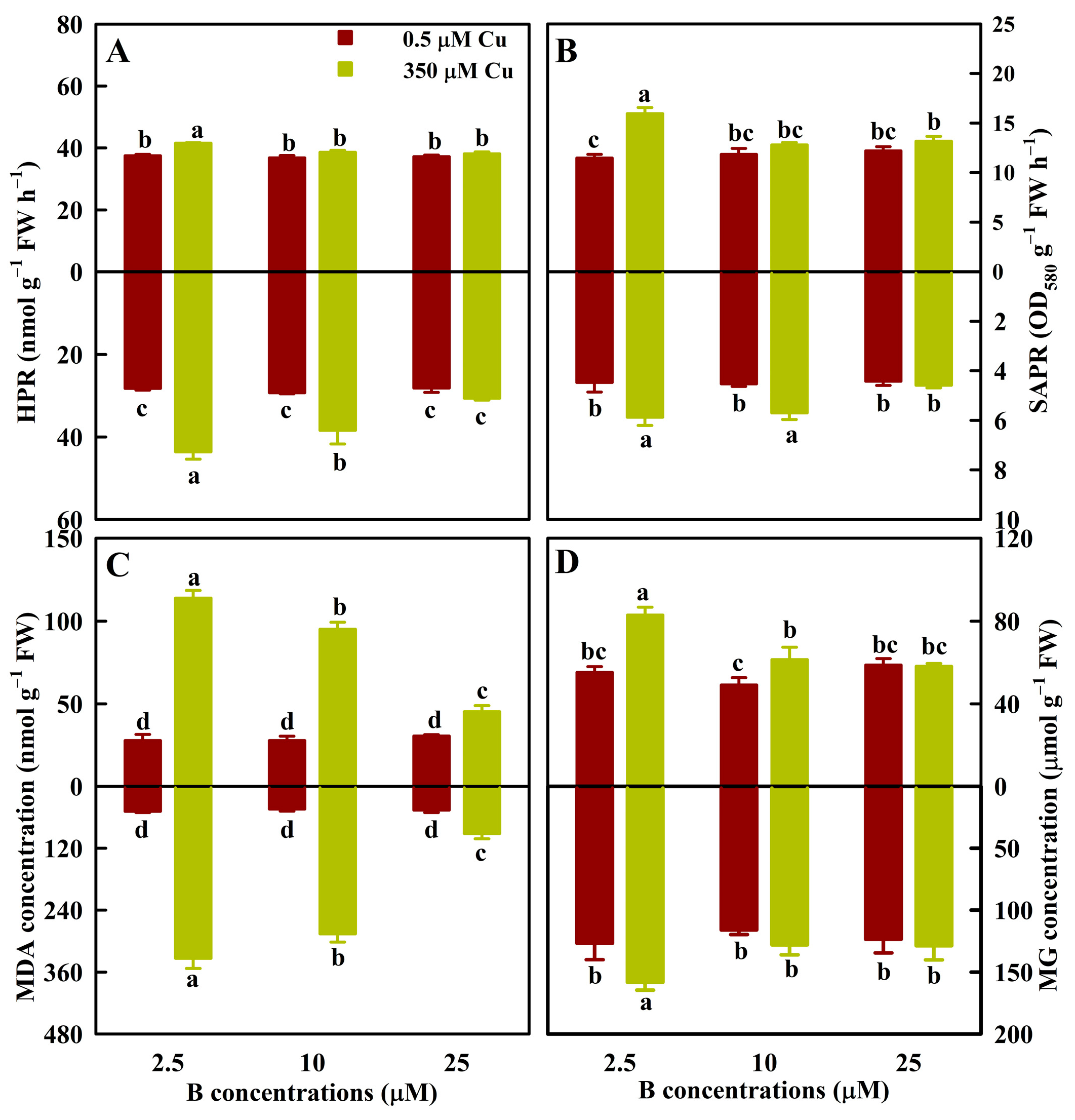

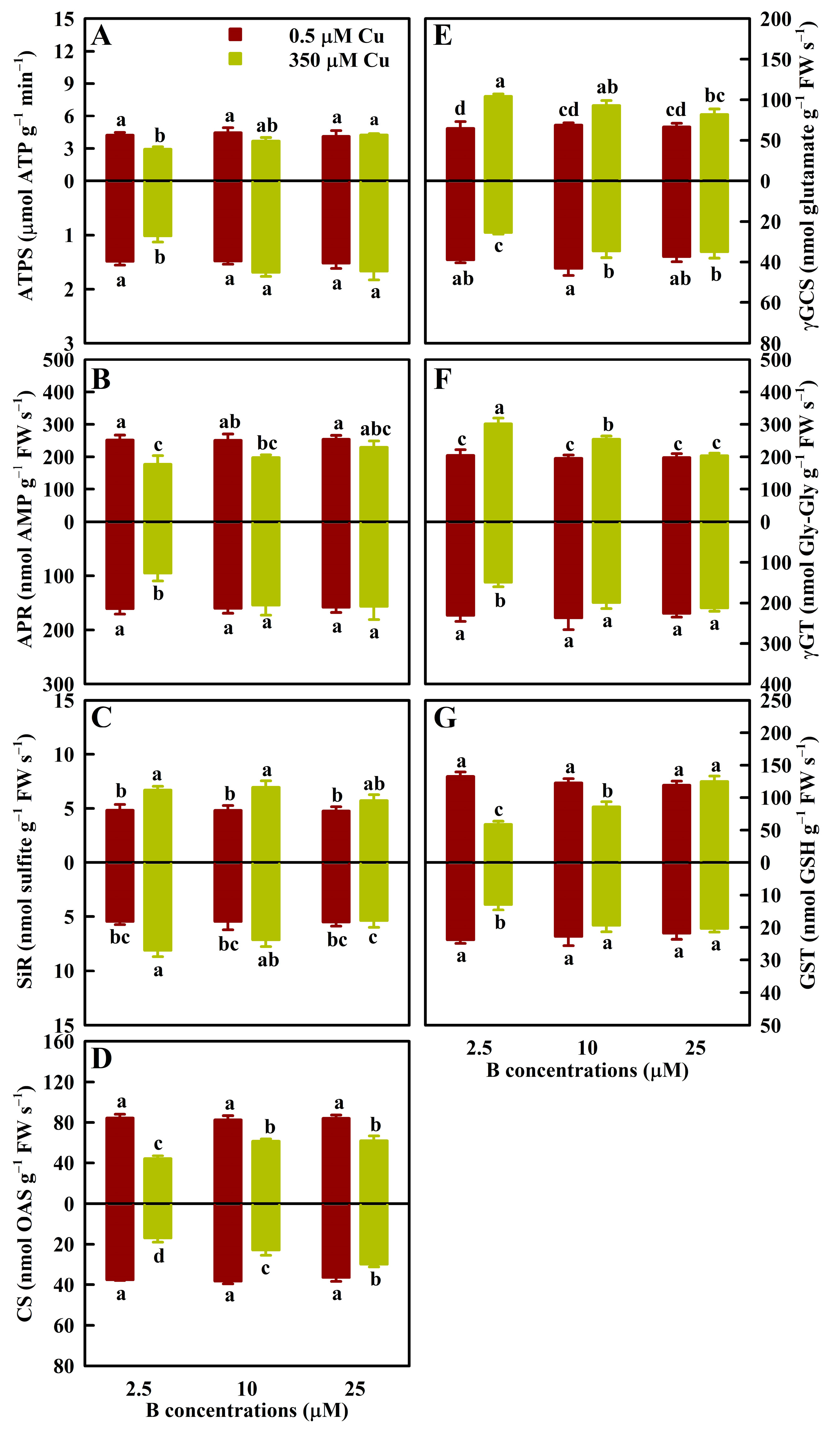
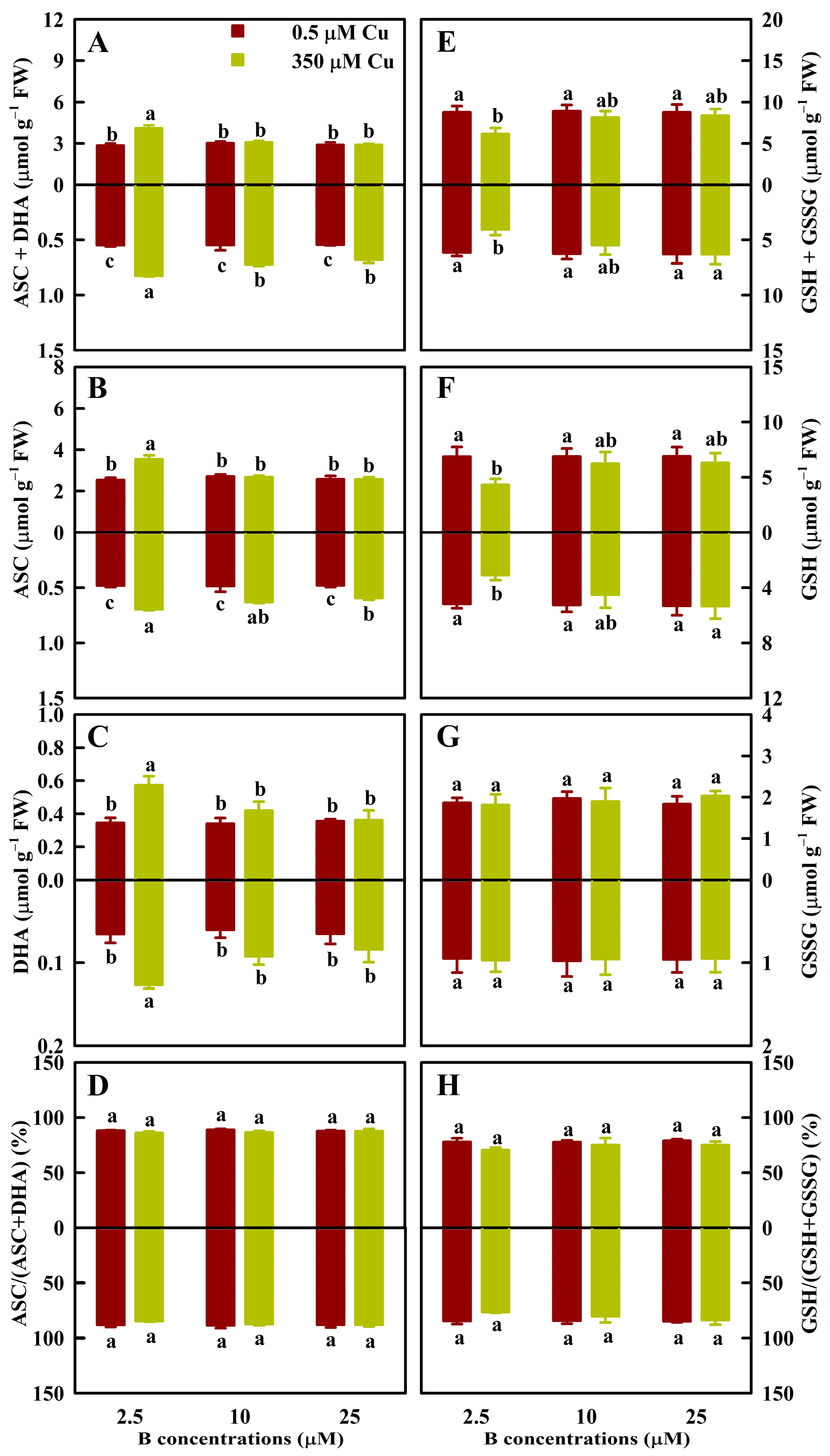


| Activities | Treatments | |||||
|---|---|---|---|---|---|---|
| Cu0.5B2.5 | Cu0.5B10 | Cu0.5B25 | Cu350B2.5 | Cu350B10 | Cu350B25 | |
| Leaves | ||||||
| Gly I (nmol MG g−1 FW s−1) | 133.9 ± 8.1 a | 117.3 ± 6.3 a | 125.9 ± 6.6 a | 92.6 ± 7.0 b | 129.5 ± 12.0 a | 120.9 ± 6.2 a |
| Gly II (nmol SLG g−1 FW s−1) | 17.7 ± 1.8 b | 17.5 ± 1.7 b | 16.7 ± 1.8 b | 23.0 ± 2.0 a | 20.0 ± 1.3 ab | 18.3 ± 1.5 ab |
| Roots | ||||||
| Gly I (nmol MG g−1 FW s−1) | 84.6 ± 4.6 a | 82.8 ± 5.8 a | 84.4 ± 3.8 a | 59.5 ± 7.1 b | 80.3 ± 7.9 a | 92.0 ± 6.8 a |
| Gly II (nmol SLG g−1 FW s−1) | 13.3 ± 0.7 a | 13.0 ± 1.1 a | 12.9 ± 1.1 a | 7.7 ± 0.7 b | 10.7 ± 0.5 a | 11.5 ± 0.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Chen, H.-H.; Huang, W.-L.; Huang, W.-T.; Huang, Z.-R.; Yang, L.-T.; Ye, X.; Chen, L.-S. Boron Reduced Copper Excess-Induced Oxidative Damage in Citrus sinensis by Modulating Reactive Oxygen Species and Methylglyoxal Formation and Their Detoxification Systems. Antioxidants 2024, 13, 268. https://doi.org/10.3390/antiox13030268
Chen X-F, Chen H-H, Huang W-L, Huang W-T, Huang Z-R, Yang L-T, Ye X, Chen L-S. Boron Reduced Copper Excess-Induced Oxidative Damage in Citrus sinensis by Modulating Reactive Oxygen Species and Methylglyoxal Formation and Their Detoxification Systems. Antioxidants. 2024; 13(3):268. https://doi.org/10.3390/antiox13030268
Chicago/Turabian StyleChen, Xu-Feng, Huan-Huan Chen, Wei-Lin Huang, Wei-Tao Huang, Zeng-Rong Huang, Lin-Tong Yang, Xin Ye, and Li-Song Chen. 2024. "Boron Reduced Copper Excess-Induced Oxidative Damage in Citrus sinensis by Modulating Reactive Oxygen Species and Methylglyoxal Formation and Their Detoxification Systems" Antioxidants 13, no. 3: 268. https://doi.org/10.3390/antiox13030268







