Light Pollution and Oxidative Stress: Effects on Retina and Human Health
Abstract
:1. Introduction
1.1. Light and Oxidative Stress
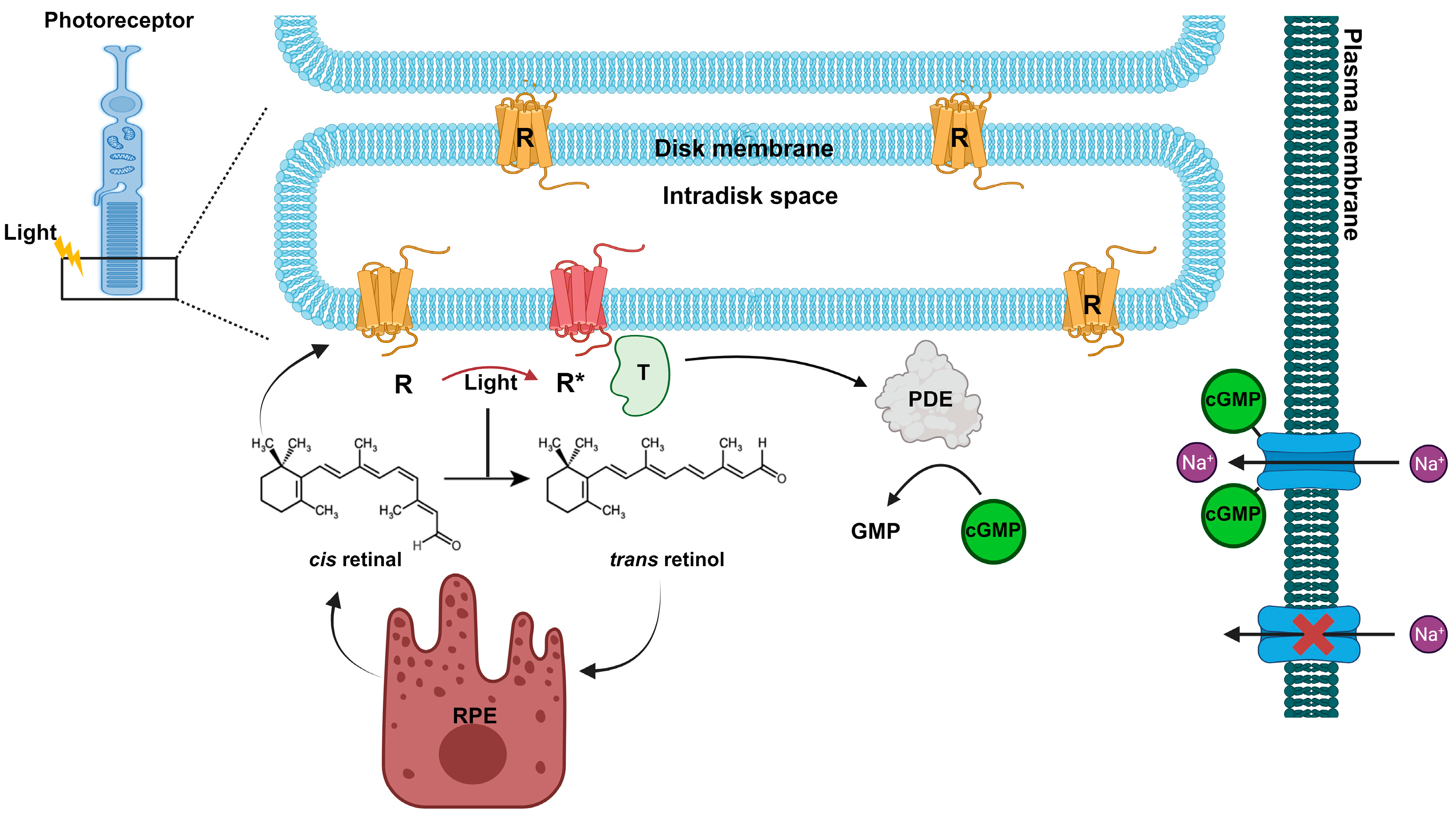
1.2. Effects of Light on Retina Photoreceptors
1.3. Impact of Light on Retinal Pigment Epithelium
1.4. Photosensitive Ganglion Cells and Physiological Rhythms
1.5. Effects of Light on the Skin and Uvea
2. Discussion
3. Conclusions
Future Research Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and Health: A Progress Update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Moyano, D.B.; Sola, Y.; González-Lezcano, R.A. Blue-Light Levels Emitted from Portable Electronic Devices Compared to Sunlight. Energies 2020, 13, 4276. [Google Scholar] [CrossRef]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal Light Toxicity. Eye 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free Radicals, Oxidative Stress, and Antioxidants in Human Health and Disease. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical Damage of the Retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B. Strategies for Reducing or Preventing the Generation of Oxidative Stress. Oxidative Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef]
- Tao, J.-X.; Zhou, W.-C.; Zhu, X.-G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxidative Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhang, C.X.; Chi, J.; Liang, Y.; Bao, X.L.; Cong, Y.Y.; Yu, B.; Li, X.; Li, G.Y. The Molecular Mechanism of Retina Light Injury Focusing on Damage from Short Wavelength Light. Oxidative Med. Cell. Longev. 2022, 2022, 8482149. [Google Scholar] [CrossRef] [PubMed]
- Cougnard-Gregoire, A.; Merle, B.M.J.; Aslam, T.; Seddon, J.M.; Aknin, I.; Klaver, C.C.W.; Garhöfer, G.; Layana, A.G.; Minnella, A.M.; Silva, R.; et al. Blue Light Exposure: Ocular Hazards and Prevention—A Narrative Review. Ophthalmol. Ther. 2023, 12, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Varela Perez, A.M. The Increasing Effects of Light Pollution on Professional and Amateur Astronomy. Science (1979) 2023, 380, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Kocifaj, M.; Wallner, S.; Barentine, J.C. Measuring and Monitoring Light Pollution: Current Approaches and Challenges. Science (1979) 2023, 380, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Taylor, M. Regulating Light Pollution: More than Just the Night Sky. Science (1979) 2023, 380, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- de Gálvez, E.N.; Aguilera, J.; Solis, A.; de Gálvez, M.V.; de Andrés, J.R.; Herrera-Ceballos, E.; Gago-Calderon, A. The Potential Role of UV and Blue Light from the Sun, Artificial Lighting, and Electronic Devices in Melanogenesis and Oxidative Stress. J. Photochem. Photobiol. B 2022, 228, 112405. [Google Scholar] [CrossRef]
- Zielinska-Dabkowska, K.M.; Schernhammer, E.S.; Hanifin, J.P.; Brainard, G.C. Reducing Nighttime Light Exposure in the Urban Environment to Benefit Human Health and Society. Science (1979) 2023, 380, 1130–1135. [Google Scholar] [CrossRef]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the Human Retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Zinn, K.M.; Marmor, M.F. (Eds.) The Retinal Pigment Epithelium; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Deeb, S.S.; Motulsky, A.G. Molecular Genetics of Human Color Vision. Behav. Genet. 1996, 26, 195–207. [Google Scholar] [CrossRef]
- Ingram, N.T.; Sampath, A.P.; Fain, G.L. Membrane Conductances of Mouse Cone Photoreceptors. J. Gen. Physiol. 2020, 152, e201912520. [Google Scholar] [CrossRef] [PubMed]
- Saari, J.C. Vitamin A Metabolism in Rod and Cone Visual Cycles. Annu. Rev. Nutr. 2012, 32, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Fishkin, N.; Yefidoff, R.; Gollipalli, D.R.; Rando, R.R. On the Mechanism of Isomerization of All-Trans-Retinol Esters to 11-Cis-Retinol in Retinal Pigment Epithelial Cells: 11-Fluoro-All-Trans-Retinol as Substrate/Inhibitor in the Visual Cycle. Bioorg. Med. Chem. 2005, 13, 5189–5194. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the Retinoid (Visual) Cycle. Chem. Rev. 2014, 114, 194–232. [Google Scholar] [CrossRef]
- Lamb, T.D.; Pugh, E.N. Dark Adaptation and the Retinoid Cycle of Vision. Prog. Retin. Eye Res. 2004, 23, 307–380. [Google Scholar] [CrossRef] [PubMed]
- Arshavsky, V.Y.; Burns, M.E. Photoreceptor Signaling: Supporting Vision across a Wide Range of Light Intensities. J. Biol. Chem. 2012, 287, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Arshavsky, V.Y.; Lamb, T.D.; Pugh, E.N. G Proteins and Phototransduction. Annu. Rev. Physiol. 2002, 64, 153–187. [Google Scholar] [CrossRef]
- Williams, T.P.; Webbers, J.P.P.; Giordano, L.; Henderson, R.P. Distribution of Photon Absorption Rates across the Rat Retina. J. Physiol. 1998, 508, 515–522. [Google Scholar] [CrossRef]
- Hunter, J.J.; Morgan, J.I.W.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The Susceptibility of the Retina to Photochemical Damage from Visible Light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal Damage Induced by Commercial Light Emitting Diodes (LEDs). Free. Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Thanos, S.; Heiduschka, P.; Romann, I. Exposure to a Solar Eclipse Causes Neuronal Death in the Retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 794–800. [Google Scholar] [CrossRef]
- Kutty, R.K.; Kutty, G.; Wiggert, B.; Chader, G.J.; Darrow, R.M.; Organisciak, D.T. Induction of Heme Oxygenase 1 in the Retina by Intense Visible Light: Suppression by the Antioxidant Dimethylthiourea. Proc. Natl. Acad. Sci. USA 1995, 92, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal Damage by Light in Rats. Investig. Ophthalmol. Vis. Sci. 1966, 5, 450–473. [Google Scholar]
- Noell, W.K. Effects of Environmental Lighting and Dietary Vitamin a on the Vulnerability of the Retina to Light Damage. Photochem. Photobiol. 1979, 29, 717–723. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal Light Damage: Mechanisms and Protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Darrow, R.M.; Barsalou, L.; Darrow, R.A.; Kutty, R.K.; Kutty, G.; Wiggert, B. Light History and Age-Related Changes in Retinal Light Damage. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1107–1116. [Google Scholar]
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-Induced Retinal Damage Using Different Light Sources, Protocols and Rat Strains Reveals LED Phototoxicity. Neuroscience 2016, 339, 296–307. [Google Scholar] [CrossRef]
- Algvere, P.V.; Marshall, J.; Seregard, S. Age-Related Maculopathy and the Impact of Blue Light Hazard. Acta Ophthalmol. Scand. 2006, 84, 4–15. [Google Scholar] [CrossRef]
- Gorn, R.A.; Kuwabara, T. Retinal Damage by Visible Light: A Physiologic Study. Arch. Ophthalmol. 1967, 77, 115–118. [Google Scholar] [CrossRef]
- Kuwabara, T.; Gorn, R.A. Retinal Damage by Visible Light: An Electron Microscopic Study. Arch. Ophthalmol. 1968, 79, 69–78. [Google Scholar] [CrossRef]
- Grignolo, A.; Orzalesi, N.; Castellazzo, R.; Vittone, P. Retinal Damage by Visible Light in Albino Rats. An Electron Microscope Study. Ophthalmologica 1969, 157, 43–59. [Google Scholar] [CrossRef]
- O’Steen, W.K.; Anderson, K.V. Photoreceptor Degeneration after Exposure of Rats to Incandescent Illumination. Z. Für Zellforsch. Und Mikrosk. Anat. 1972, 127, 306–313. [Google Scholar] [CrossRef]
- Nakamura, M.; Yako, T.; Kuse, Y.; Inoue, Y.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Exposure to Excessive Blue LED Light Damages Retinal Pigment Epithelium and Photoreceptors of Pigmented Mice. Exp. Eye Res. 2018, 177, 1–11. [Google Scholar] [CrossRef]
- Remé, C.E.; Grimm, C.; Hafezi, F.; Wenzel, A.; Williams, T.P. Apoptosis in the Retina: The Silent Death of Vision. News Physiol. Sci. 2000, 15, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Remé, C.E.; Grimm, C.; Hafezi, F.; Marti, A.; Wenzel, A. Apoptotic Cell Death in Retinal Degenerations. Prog. Retin. Eye Res. 1998, 17, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-Year Cumulative Incidence of Age-Related Macular Degeneration. The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef]
- Sui, G.Y.; Liu, G.C.; Liu, G.Y.; Gao, Y.Y.; Deng, Y.; Wang, W.Y.; Tong, S.H.; Wang, L. Is Sunlight Exposure a Risk Factor for Age-Related Macular Degeneration? A Systematic Review and Meta-Analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-Emitting Diodes (LED) for Domestic Lighting: Any Risks for the Eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef]
- Fliesler, A.J.; Anderson, R.E. Chemistry and Metabolism of Lipids in the Vertebrate Retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Bazan, N.G.; Gordon, W.C.; Rodriguez de Turco, E.B. Docosahexaenoic Acid Uptake and Metabolism in Photoreceptors: Retinal Conservation by an Efficient Retinal Pigment Epithelial Cell-Mediated Recycling Process. Adv. Exp. Med. Biol. 1992, 318, 295–306. [Google Scholar]
- Wielgus, A.R.; Roberts, J.E. Retinal Photodamage by Endogenous and Xenobiotic Agents. Photochem. Photobiol. 2012, 88, 1320–1345. [Google Scholar] [CrossRef] [PubMed]
- Glickman, R.D. Phototoxicity to the Retina: Mechanisms of Damage. Int. J. Toxicol. 2002, 21, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Nagasaki, T.; Yoon, K.D.; Yamamoto, K.; Zhou, J. Fundus Autofluorescence and the Bisretinoids of Retina. Photochem. Photobiol. Sci. 2010, 9, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y.; Sakaguchi, H.; Hollyfield, J.G. Spectral Profiling of Autofluorescence Associated with Lipofuscin, Bruch’s Membrane, and Sub-RPE Deposits in Normal and AMD Eyes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2435–2441. [Google Scholar]
- Eldred, G.E.; Lasky, M.R. Retinal Age Pigments Generated by Self-Assembling Lysosomotropic Detergents. Nature 1993, 361, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.C.; Goger, D.G.; Dorey, C.K. Age-Related Accumulation and Spatial Distribution of Lipofuscin in RPE of Normal Subjects. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1855–1866. [Google Scholar]
- Boulton, M.; Docchio, F.; Dayhaw-Barker, P.; Ramponi, R.; Cubeddu, R. Age-Related Changes in the Morphology, Absorption and Fluorescence of Melanosomes and Lipofuscin Granules of the Retinal Pigment Epithelium. Vis. Res. 1990, 30, 1291–1303. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.; Yang, C.H.; Lee, L.L. White Light-Emitting Diodes (LEDs) at Domestic Lighting Levels and Retinal Injury in a Rat Model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [CrossRef]
- Benedetto, M.M.; Contin, M.A. Oxidative Stress in Retinal Degeneration Promoted by Constant LED Light. Front. Cell. Neurosci. 2019, 13, 139. [Google Scholar] [CrossRef]
- Rozanowska, M.B. Light-Induced Damage to the Retina: Current Understanding of the Mechanisms and Unresolved Questions: A Symposium-in-Print. Photochem. Photobiol. 2012, 88, 1303–1308. [Google Scholar] [CrossRef]
- Del Olmo-Aguado, S.; Manso, A.G.; Osborne, N.N. Light Might Directly Affect Retinal Ganglion Cell Mitochondria to Potentially Influence Function. Photochem. Photobiol. 2012, 88, 1346–1355. [Google Scholar] [CrossRef]
- Osborne, N.N.; Li, G.Y.; Ji, D.; Mortiboys, H.J.; Jackson, S. Light Affects Mitochondria to Cause Apoptosis to Cultured Cells: Possible Relevance to Ganglion Cell Death in Certain Optic Neuropathies. J. Neurochem. 2008, 105, 2013–2028. [Google Scholar] [CrossRef]
- Rong, R.; Yang, R.; Li, H.; You, M.; Liang, Z.; Zeng, Z.; Zhou, R.; Xia, X.; Ji, D. The Roles of Mitochondrial Dynamics and NLRP3 Inflammasomes in the Pathogenesis of Retinal Light Damage. Ann. N. Y. Acad. Sci. 2021, 1508, 78–91. [Google Scholar] [CrossRef]
- García, J.; Silva, E. Flavin-Sensitized Photooxidation of Amino Acids Present in a Parenteral Nutrition Infusate: Protection by Ascorbic Acid. J. Nutr. Biochem. 1997, 8, 341–345. [Google Scholar] [CrossRef]
- Silva, E.; González, T.; Edwards, A.M.; Zuloaga, F. Visible Light Induced Lipoperoxidation of a Parenteral Nutrition Fat Emulsion Sensitized by Flavins. J. Nutr. Biochem. 1998, 9, 149–154. [Google Scholar] [CrossRef]
- Hockberger, P.E.; Skimina, T.A.; Centonze, V.E.; Lavin, C.; Chu, S.; Dadras, S.; Reddy, J.K.; White, J.G. Activation of Flavin-Containing Oxidases Underlies Light-Induced Production of H2O2 in Mammalian Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6255–6260. [Google Scholar] [CrossRef]
- Gao, M.L.; Deng, W.L.; Huang, N.; Wang, Y.Y.; Lei, X.L.; Xu, Z.Q.; Hu, D.N.; Cai, J.Q.; Lu, F.; Jin, Z.B. Upregulation of GADD45α in Light-Damaged Retinal Pigment Epithelial Cells. Cell Death Discov. 2016, 2, 16013. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wu, M.R.; Huang, W.J.; Chow, D.S.L.; Hsiao, G.; Cheng, Y.W. Low-Luminance Blue Light-Enhanced Phototoxicity in A2E-Laden RPE Cell Cultures and Rats. Int. J. Mol. Sci. 2019, 20, 1799. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.M.; Wang, G.S.; Sliney, D.H.; Yang, C.H.; Lee, L.L. Light-Emitting-Diode Induced Retinal Damage and Its Wavelength Dependency In Vivo. Int. J. Ophthalmol. 2017, 10, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Villalpando Rodriguez, G.; Boulenguez, P.; Carré, S.; Dassieni, I.; Lebon, C.; Chahory, S.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal Phototoxicity and the Evaluation of the Blue Light Hazard of a New Solid-State Lighting Technology. Sci. Rep. 2020, 10, 6733. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Sánchez-Chávez, G.; Salceda, R. Retinal Nrf2 Expression in Normal and Early Streptozotocin-Diabetic Rats. Neurochem. Int. 2021, 145, 105007. [Google Scholar] [CrossRef]
- Chen, W.J.; Wu, C.; Xu, Z.; Kuse, Y.; Hara, H.; Duh, E.J. Nrf2 Protects Photoreceptor Cells from Photo-Oxidative Stress Induced by Blue Light. Exp. Eye Res. 2017, 154, 151–158. [Google Scholar] [CrossRef]
- Yang, P.M.; Cheng, K.C.; Huang, J.Y.; Wang, S.Y.; Lin, Y.N.; Tseng, Y.T.; Hsieh, C.W.; Wung, B.S. Sulforaphane Inhibits Blue Light–Induced Inflammation and Apoptosis by Upregulating the SIRT1/PGC-1α/Nrf2 Pathway and Autophagy in Retinal Pigment Epithelial Cells. Toxicol. Appl. Pharmacol. 2021, 421, 115545. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.E.G.; Sundelin, S.P.; Wihlmark, U.; Brunk, U.T. Aging of Cultured Retinal Pigment Epithelial Cells: Oxidative Reactions, Lipofuscin Formation and Blue Light Damage. Doc. Ophthalmol. 2003, 106, 13–16. [Google Scholar] [CrossRef]
- Ooe, E.; Tsuruma, K.; Kuse, Y.; Kobayashi, S.; Shimazawa, M.; Hara, H. The Involvement of ATF4 and S-Opsin in Retinal Photoreceptor Cell Damage Induced by Blue LED Light. Mol. Vis. 2017, 23, 52–59. [Google Scholar] [PubMed]
- Chen, W.; Lin, R.; Xiao, K.; Yuan, K.; Chen, Z.; Huang, Y. Effects of Different Spectrum of LEDs on Retinal Degeneration Through Regulating Endoplasmic Reticulum Stress. Transl. Vis. Sci. Technol. 2023, 12, 16. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Sun, X.; Wang, F. Expression of Endoplasmic Reticulum Stress Markers GRP78 and CHOP Induced by Oxidative Stress in Blue Light-Mediated Damage of A2E-Containing Retinal Pigment Epithelium Cells. Ophthalmic Res. 2014, 52, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Bok, D. Processing and Transport of Retinoids by the Retinal Pigment Epithelium. Eye 1990, 4, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Saari, J.C.; Bredberg, D.L.; Noy, N. Control of Substrate Flow at a Branch in the Visual Cycle. Biochemistry 1994, 33, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, X.; Xia, Q.; Yao, K.; Chen, J.; Zhang, Y.; Naranmandura, H.; Liu, X.; Wu, Y. Involvement of Endoplasmic Reticulum Stress in All-Trans-Retinal-Induced Retinal Pigment Epithelium Degeneration. Toxicol. Sci. 2015, 143, 196–208. [Google Scholar] [CrossRef]
- Roechlecke, C.; Schaller, A.; Knels, L.; Funk, R.H.W. The Influence of Sublethal Blue Light Exposure on Human RPE Cells. Mol. Vis. 2009, 15, 1929–1938. [Google Scholar]
- Antemie, R.-G.; Samoilă, O.C.; Clichici, S.V. Blue Light—Ocular and Systemic Damaging Effects: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 5998. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.I.; Seo, Y.J.; Park, W.S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue Light Effect on Retinal Pigment Epithelial Cells by Display Devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Inoue, Y.; Iriyama, A.; Jang, W.-D. Effects of Yellow Intraocular Lenses on Light-Induced Upregulation of Vascular Endothelial Growth Factor. J. Cataract. Refract. Surg. 2006, 32, 1540–1544. [Google Scholar] [CrossRef]
- Núñez-Álvarez, C.; Suárez-Barrio, C.; del Olmo Aguado, S.; Osborne, N.N. Blue Light Negatively Affects the Survival of ARPE19 Cells through an Action on Their Mitochondria and Blunted by Red Light. Acta Ophthalmol. 2019, 97, E103–E115. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science (1979) 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Berson, D.M. Strange Vision: Ganglion Cells as Circadian Photoreceptors. Trends. Neurosci. 2003, 26, 314–320. [Google Scholar] [CrossRef]
- Hannibal, J.; Christiansen, A.T.; Heegaard, S.; Fahrenkrug, J.; Kiilgaard, J.F. Melanopsin Expressing Human Retinal Ganglion Cells: Subtypes, Distribution, and Intraretinal Connectivity. J. Comp. Neurol. 2017, 525, 1934–1961. [Google Scholar] [CrossRef]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.-K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S. Intrinsically Photosensitive Retinal Ganglion Cells of the Human Retina. Front. Neurol. 2021, 12, 636330. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.M.; Wong, K.Y.; Shapiro, P.; Frederick, C.; Pattabiraman, K.; Berson, D.M. Melanopsin Ganglion Cells Use a Membrane-Associated Rhabdomeric Phototransduction Cascade. J. Neurophysiol. 2008, 99, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yue, W.W.S.; Chen, L.; Sheng, Y.; Yau, K.W. Cyclic-Nucleotide- and HCN-Channel-Mediated Phototransduction in Intrinsically Photosensitive Retinal Ganglion Cells. Cell 2018, 175, 652–664.e12. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Do, M.T.H.; Riccio, A.; Jiang, Z.; Hsieh, J.; Wang, H.C.; Merbs, S.L.; Welsbie, D.S.; Yoshioka, T.; Weissgerber, P.; et al. Melanopsin Signalling in Mammalian Iris and Retina. Nature 2011, 479, 67–73. [Google Scholar] [CrossRef]
- Hannibal, J.; Georg, B.; Hindersson, P.; Fahrenkrug, J. Light and Darkness Regulate Melanopsin in the Retinal Ganglion Cells of the Albino Wistar Rat. J. Mol. Neurosci. 2005, 27, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Mathes, A.; Engel, L.; Holthues, H.; Wolloscheck, T.; Spessert, R. Daily Profile in Melanopsin Transcripts Depends on Seasonal Lighting Conditions in the Rat Retina. J. Neuroendocr. 2007, 19, 952–957. [Google Scholar] [CrossRef]
- Wong, K.Y. A Retinal Ganglion Cell That Can Signal Irradiance Continuously for 10 Hours. J. Neurosci. 2012, 32, 11478–11485. [Google Scholar] [CrossRef]
- Hamm, H.E.; Menaker, M. Retinal Rhythms in Chicks: Circadian Variation in Melantonin and Serotonin N-Acetyltransferase Activity. Proc. Natl. Acad. Sci. USA 1980, 77, 4998–5002. [Google Scholar] [CrossRef]
- Ostrin, L.A. Ocular and Systemic Melatonin and the Influence of Light Exposure. Clin. Exp. Optom. 2019, 102, 99–108. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef]
- Do, A.; Li, V.W.; Huang, S.; Michalak, E.E.; Tam, E.M.; Chakrabarty, T.; Yatham, L.N.; Lam, R.W. Blue-Light Therapy for Seasonal and Non-Seasonal Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Psychiatry 2022, 67, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Lu, J.; Fischer, D.; Saper, C.B. A Broad Role for Melanopsin in Nonvisual Photoreception. J. Neurosci. 2003, 23, 7093–7106. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Gias, C.; Hatori, M.; Keding, S.R.; Semo, M.; Coffey, P.J.; Gigg, J.; Piggins, H.D.; Panda, S.; Lucas, R.J. Melanopsin Contributions to Irradiance Coding in the Thalamo-Cortical Visual System. PLoS Biol. 2010, 8, e1000558. [Google Scholar] [CrossRef] [PubMed]
- Delwig, A.; Larsen, D.D.; Yasumura, D.; Yang, C.F.; Shah, N.M.; Copenhagen, D.R. Retinofugal Projections from Melanopsin-Expressing Retinal Ganglion Cells Revealed by Intraocular Injections of Cre-Dependent Virus. PLoS ONE 2016, 11, e0149501. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.W.; Berson, D.M. Central Projections of Melanopsin-Expressing Retinal Ganglion Cells in the Mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.M.; Studholme, K.M. Retinofugal Projections in the Mouse. J. Comp. Neurol. 2014, 522, 3733–3753. [Google Scholar] [CrossRef]
- Quattrochi, L.E.; Stabio, M.E.; Kim, I.; Ilardi, M.C.; Michelle Fogerson, P.; Leyrer, M.L.; Berson, D.M. The M6 Cell: A Small-Field Bistratified Photosensitive Retinal Ganglion Cell. J. Comp. Neurol. 2019, 527, 297–311. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, W.D.; Pandi-Perumal, S.R.; Zakharia, R.; Bhatnagar, K.P.; Brzezinski, A. Melatonin and Human Reproduction: Shedding Light on the Darkness Hormone. Gynecol. Endocrinol. 2009, 25, 779–785. [Google Scholar] [CrossRef]
- Cajochen, C.; Kräuchi, K.; Wirz-Justice, A. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Paul, K.N.; Saafir, T.B.; Tosini, G. The Role of Retinal Photoreceptors in the Regulation of Circadian Rhythms. Rev. Endocr. Metab. Disord. 2009, 10, 271–278. [Google Scholar] [CrossRef]
- Warren, E.J.; Allen, C.N.; Brown, R.L.; Robinson, D.W. The Light-Activated Signaling Pathway in SCN-Projecting Rat Retinal Ganglion Cells. Eur. J. Neurosci. 2006, 23, 2477–2487. [Google Scholar] [CrossRef]
- Johansson, A.; Nordin, S.; Heiden, M.; Sandström, M. Symptoms, Personality Traits, and Stress in People with Mobile Phone-Related Symptoms and Electromagnetic Hypersensitivity. J. Psychosom. Res. 2010, 68, 37–45. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Abbott, K.S.; Queener, H.M. Attenuation of Short Wavelengths Alters Sleep and the IpRGC Pupil Response. Ophthalmic Physiol. Opt. 2017, 37, 440–450. [Google Scholar] [CrossRef]
- Menet, J.S.; Rosbash, M. When Brain Clocks Lose Track of Time: Cause or Consequence of Neuropsychiatric Disorders. Curr. Opin. Neurobiol. 2011, 21, 849–857. [Google Scholar] [CrossRef]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Adverse Health Effects of Nighttime Lighting: Comments on American Medical Association Policy Statement. Am. J. Prev. Med. 2013, 45, 343–346. [Google Scholar] [CrossRef]
- Ward, E.M.; Germolec, D.; Kogevinas, M.; McCormick, D.; Vermeulen, R.; Anisimov, V.N. Carcinogenicity of Night Shift Work. Lancet Oncol. 2019, 20, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Arguelles-Prieto, R.; Martinez-Madrid, M.J.; Reiter, R.; Hardeland, R.; Rol, M.A.; Madrid, J.A. Protecting the Melatonin Rhythm through Circadian Healthy Light Exposure. Int. J. Mol. Sci. 2014, 15, 23448–23500. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Gronfier, C.; Van Gelder, R.N.; Bernstein, P.S.; Carreras, J.; Panda, S.; Marks, F.; Sliney, D.; Hunt, C.E.; Hirota, T.; et al. Global Rise of Potential Health Hazards Caused by Blue Light-Induced Circadian Disruption in Modern Aging Societies. NPJ Aging Mech. Dis. 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Zhu, Y. Electric Light, Particularly at Night, Disrupts Human Circadian Rhythmicity: Is That a Problem? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140120. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Damerow, S.; Kiessling, S.; Jakubcakova, V.; Abraham, D.; Tian, J.; Hoffmann, M.W.; Eichele, G. The Circadian Rhythm of Glucocorticoids Is Regulated by a Gating Mechanism Residing in the Adrenal Cortical Clock. Cell Metab. 2006, 4, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.K.; Kinrys, G. Treating Circadian Rhythm Disruption in Bipolar Disorder. Curr. Psychiatry Rep. 2019, 21, 14. [Google Scholar] [CrossRef]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.; Chmielewska-Krzesinska, M.; Vyniarska, A.; Sienkiewicz, W. Exposure to Blue Light Reduces Melanopsin Expression in Intrinsically Photoreceptive Retinal Ganglion Cells and Damages the Inner Retina in Rats. Investig. Opthalmol. Vis. Sci. 2022, 63, 26. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.; Lewczuk, B.; Szyryńska, N.; Rawicka, A.; Vyniarska, A. Low-Intensity Blue Light Exposure Reduces Melanopsin Expression in Intrinsically Photosensitive Retinal Ganglion Cells and Damages Mitochondria in Retinal Ganglion Cells in Wistar Rats. Cells 2023, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Paulis, L.; Simko, F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014, 15, 17920–17937. [Google Scholar] [CrossRef]
- Phelps, J. Dark Therapy for Bipolar Disorder Using Amber Lenses for Blue Light Blockade. Med. Hypotheses 2008, 70, 224–229. [Google Scholar] [CrossRef]
- Cibeira, N.; Maseda, A.; Lorenzo-López, L.; González-Abraldes, I.; López-López, R.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Bright Light Therapy in Older Adults with Moderate to Very Severe Dementia: Immediate Effects on Behavior, Mood, and Physiological Parameters. Healthcare 2021, 9, 1065. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Dim Blue Light at Night Induces Spatial Memory Impairment in Mice by Hippocampal Neuroinflammation and Oxidative Stress. Antioxidants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kubo, S.; Tsuji, K.; Sugiyama, D.; Hamano, H. Induction of Skin Cancer by Long-Term Blue Light Irradiation. Biomedicines 2023, 11, 2321. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; De Melo, W.C.M.A.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light Based Anti-Infectives: Ultraviolet C Irradiation, Photodynamic Therapy, Blue Light, and Beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef]
- Shen, J.; Tower, J. Effects of Light on Aging and Longevity. Ageing Res. Rev. 2019, 53, 100913. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Berneburg, M. Sun-Damaged Skin (Photoaging): What Is New? Hautarzt 2021, 72, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on Mechanisms of Skin Photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef]
- Dong, K.; Goyarts, E.C.; Pelle, E.; Trivero, J.; Pernodet, N. Blue Light Disrupts the Circadian Rhythm and Create Damage in Skin Cells. Int. J. Cosmet. Sci. 2019, 41, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sil, A.; Kumar, P.; Khan, I. Blue Light and Skin: What Is the Intriguing Link? Clin. Exp. Dermatol. 2023, 48, 968–977. [Google Scholar] [CrossRef]
- Lazzarini, R.; Tartaglione, M.F.; Ciarapica, V.; Piva, F.; Giulietti, M.; Fulgenzi, G.; Martelli, M.; Ledda, C.; Vitale, E.; Malavolta, M.; et al. Keratinocytes Exposed to Blue or Red Light: Proteomic Characterization Showed Cytoplasmic Thioredoxin Reductase 1 and Aldo-Keto Reductase Family 1 Member C3 Triggered Expression. Int. J. Mol. Sci. 2023, 24, 16189. [Google Scholar] [CrossRef]
- Chamayou-Robert, C.; DiGiorgio, C.; Brack, O.; Doucet, O. Blue Light Induces DNA Damage in Normal Human Skin Keratinocytes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 69–75. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Wu, F.; Cao, X.; Gou, K.; Wang, C.; Lin, C. Blue Light Induces Skin Apoptosis and Degeneration through Activation of the Endoplasmic Reticulum Stress-Autophagy Apoptosis Axis: Protective Role of Hydrogen Sulfide. J. Photochem. Photobiol. B 2022, 229, 112426. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef]
- Yang, R.; Deng, F.; Yang, Y.; Tian, Q.; Huangfu, S.; Yang, L.; Hou, J.; Yang, G.; Pang, W.; Lu, J.; et al. Blue Light Promotes Vitamin C-Mediated Ferroptosis of Melanoma through Specifically Upregulating Transporter SVCT2 and Generating Fe2+. Biomaterials 2023, 299, 122186. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, X.; Zhang, J.; Su, L.; Ma, M.; Ea, V.L.; Liu, X.; Wang, L.; Chang, J.; Li, X.; et al. Blue Light-Triggered Optogenetic System for Treating Uveal Melanoma. Oncogene 2020, 39, 2118–2124. [Google Scholar] [CrossRef]
- Alkahtani, S.; AL-Johani, N.S.; Alarifi, S.; Afzal, M. Cytotoxicity Mechanisms of Blue-Light-Activated Curcumin in T98G Cell Line: Inducing Apoptosis through ROS-Dependent Downregulation of MMP Pathways. Int. J. Mol. Sci. 2023, 24, 3842. [Google Scholar] [CrossRef]
- Papastefanou, V.P.; Cohen, V.M.L. Uveal Melanoma. J. Ski. Cancer 2011, 2011, 573974. [Google Scholar] [CrossRef]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current Molecular and Clinical Insights into Uveal Melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Logan, P.; Bernabeu, M.; Ferreira, A.; Burnier, M.N. Evidence for the Role of Blue Light in the Development of Uveal Melanoma. J. Ophthalmol. 2015, 2015, 386986. [Google Scholar] [CrossRef]
- Manning, W.S.; Greenlee, P.G.; Norton, J.N. Ocular Melanoma in a Long Evans Rat. Contemp. Top. Lab. Anim. Sci. 2004, 43, 44–46. [Google Scholar]
- Zhou, S.; Yamada, R.; Sakamoto, K. Low Energy Multiple Blue Light-Emitting Diode Light Irradiation Promotes Melanin Synthesis and Induces DNA Damage in B16F10 Melanoma Cells. PLoS ONE 2023, 18, e0281062. [Google Scholar] [CrossRef]
- Nishio, T.; Kishi, R.; Sato, K.; Sato, K. Blue Light Exposure Enhances Oxidative Stress, Causes DNA Damage, and Induces Apoptosis Signaling in B16F1 Melanoma Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 883–884, 503562. [Google Scholar] [CrossRef]
- Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. [Google Scholar] [CrossRef]
- Li, M.K.; Liu, C.; Hsu, J.T.S. The Use of Lasers and Light Devices in Acne Management: An Update. Am. J. Clin. Dermatol. 2021, 22, 785–800. [Google Scholar] [CrossRef]
- Siraji, M.A.; Spitschan, M.; Kalavally, V.; Haque, S. Light Exposure Behaviors Predict Mood, Memory and Sleep Quality. Sci. Rep. 2023, 13, 12425. [Google Scholar] [CrossRef]
- Félix Garza, Z.C.; Born, M.; Hilbers, P.A.J.; van Riel, N.A.W.; Liebmann, J. Visible Blue Light Therapy: Molecular Mechanisms and Therapeutic Opportunities. Curr. Med. Chem. 2017, 25, 5564–5577. [Google Scholar] [CrossRef]
- Félix Garza, Z.C.; Liebmann, J.; Born, M.; Hilbers, P.A.J.; van Riel, N.A.W. A Dynamic Model for Prediction of Psoriasis Management by Blue Light Irradiation. Front. Physiol. 2017, 8, 28. [Google Scholar] [CrossRef]
- Bapary, M.A.J.; Takano, J.-I.; Soma, S.; Sankai, T. Effect of Blue Light-Emitting Diode Light and Antioxidant Potential in a Somatic Cell. Cell Biol. Int. 2019, 43, 1296–1306. [Google Scholar] [CrossRef]
- Tolleson, W.H.; Cherng, S.H.; Xia, Q.; Boudreau, M.; Yin, J.J.; Wamer, W.G.; Howard, P.C.; Yu, H.; Fu, P.P. Photodecomposition and Phototoxicity of Natural Retinoids. Int. J. Environ. Res. Public Health 2005, 2, 147–155. [Google Scholar] [CrossRef]
- Yemanyi, F.; Bora, K.; Blomfield, A.K.; Chen, J. Retinoic Acid Receptor-Related Orphan Receptors (RORs) in Eye Development and Disease. Adv. Exp. Med. Biol. 2023, 1415, 327–332. [Google Scholar]
- Kumari, J.; Das, K.; Babaei, M.; Rokni, G.R.; Goldust, M. The Impact of Blue Light and Digital Screens on the Skin. J. Cosmet. Dermatol. 2023, 22, 1185–1190. [Google Scholar] [CrossRef]
- Gu, H.; Hu, P.; Zhao, Y.; Liu, Y.; Wang, Y.T.; Ahmed, A.A.; Liu, H.Y.; Cai, D. Nuclear Receptor RORα/γ: Exciting Modulators in Metabolic Syndrome and Related Disorders. Front. Nutr. 2022, 9, 925267. [Google Scholar] [CrossRef]
- Lodi, G.; Sannino, M.; Cannarozzo, G.; Giudice, A.; Del Duca, E.; Tamburi, F.; Bennardo, L.; Nisticò, S.P. Blue Light-Emitting Diodes in Hair Regrowth: The First Prospective Study. Lasers Med. Sci. 2021, 36, 1719–1723. [Google Scholar] [CrossRef]
- Dini, V.; Romanelli, M.; Oranges, T.; Davini, G.; Janowska, A. Blue Light Emission in the Management of Hard-to-Heal Wounds. Ital. J. Dermatol. Venereol. 2021, 156, 709–713. [Google Scholar] [CrossRef]
- Falchi, F.; Cinzano, P.; Elvidge, C.D.; Keith, D.M.; Haim, A. Limiting the Impact of Light Pollution on Human Health, Environment and Stellar Visibility. J. Environ. Manag. 2011, 92, 2714–2722. [Google Scholar] [CrossRef]
- Dresp-Langley, B.; Hutt, A. Digital Addiction and Sleep. Int. J. Environ. Res. Public Health 2022, 19, 6910. [Google Scholar] [CrossRef]
- Tahir, M.J.; Malik, N.I.; Ullah, I.; Khan, H.R.; Perveen, S.; Ramalho, R.; Siddiqi, A.R.; Waheed, S.; Mohamed Shalaby, M.M.; de Berardis, D.; et al. Internet Addiction and Sleep Quality among Medical Students during the COVID-19 Pandemic: A Multinational Cross-Sectional Survey. PLoS ONE 2021, 16, e0259594. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Chung, S.T.L.; Downie, L.E.; Guymer, R.H.; Vingrys, A.J. Age-Related Macular Degeneration: What’s New and on the Horizon. Optom. Vis. Sci. 2014, 91, 399–404. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of Blue Light-Induced Eye Hazard and Protective Measures: A Review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Theruveethi, N.; Bui, B.V.; Joshi, M.B.; Valiathan, M.; Ganeshrao, S.B.; Gopalakrishnan, S.; Kabekkodu, S.P.; Bhat, S.S.; Surendran, S. Blue Light-Induced Retinal Neuronal Injury and Amelioration by Commercially Available Blue Light-Blocking Lenses. Life 2022, 12, 243. [Google Scholar] [CrossRef]
- Riyad, Y.M.; Naumov, S.; Schastak, S.; Griebel, J.; Kahnt, A.; Häupl, T.; Neuhaus, J.; Abel, B.; Hermann, R. Chemical Modification of a Tetrapyrrole-Type Photosensitizer: Tuning Application and Photochemical Action beyond the Singlet Oxygen Channel. J. Phys. Chem. B 2014, 118, 11646–11658. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salceda, R. Light Pollution and Oxidative Stress: Effects on Retina and Human Health. Antioxidants 2024, 13, 362. https://doi.org/10.3390/antiox13030362
Salceda R. Light Pollution and Oxidative Stress: Effects on Retina and Human Health. Antioxidants. 2024; 13(3):362. https://doi.org/10.3390/antiox13030362
Chicago/Turabian StyleSalceda, Rocío. 2024. "Light Pollution and Oxidative Stress: Effects on Retina and Human Health" Antioxidants 13, no. 3: 362. https://doi.org/10.3390/antiox13030362




